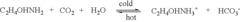Comparative evaluation of Solid sorbents for CO2 capture amine versus hybrid sorbents
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture and storage (CCS) has emerged as a critical technology in the global effort to mitigate climate change. The evolution of CO2 capture technologies has been driven by the urgent need to reduce greenhouse gas emissions while maintaining economic viability. Initially, CO2 capture focused on post-combustion technologies using liquid amine solvents, which have been commercially deployed since the 1930s for natural gas sweetening and later adapted for flue gas treatment.
The technological trajectory has progressed through several distinct phases. First-generation capture systems primarily utilized monoethanolamine (MEA) solutions, which despite their effectiveness, suffered from high regeneration energy requirements and solvent degradation issues. Second-generation technologies introduced advanced liquid solvents with improved properties, including sterically hindered amines and amino acid salts, which offered better energy efficiency and reduced degradation.
The current third-generation technologies represent a paradigm shift toward solid sorbents, which promise significant advantages over traditional liquid systems. This evolution has been accelerated by increasingly stringent emission regulations and the Paris Agreement's ambitious climate targets, which necessitate more efficient and cost-effective capture solutions.
Amine-functionalized solid sorbents emerged as promising candidates due to their high selectivity for CO2 and lower regeneration energy requirements compared to aqueous amine systems. These materials typically consist of amines immobilized on high-surface-area supports such as silica, activated carbon, or metal-organic frameworks (MOFs). Concurrently, hybrid sorbents have been developed, combining the advantages of different materials to enhance performance metrics.
The primary objectives of current CO2 capture technology development include: reducing the energy penalty associated with sorbent regeneration; improving sorbent capacity, selectivity, and stability under real operating conditions; minimizing production costs; and ensuring environmental sustainability throughout the lifecycle. Specifically for solid sorbents, objectives focus on optimizing the amine loading and distribution, enhancing mass transfer kinetics, and developing scalable synthesis methods.
Recent technological goals have expanded to include developing sorbents capable of operating in diverse industrial environments, from power plants to cement factories and direct air capture applications. The ideal sorbent would combine high CO2 capacity, fast adsorption/desorption kinetics, excellent stability over thousands of cycles, and low manufacturing costs—a combination that has proven challenging to achieve simultaneously.
The comparative evaluation of amine versus hybrid sorbents represents a critical research direction, as it aims to identify optimal materials that balance performance, cost, and environmental impact for next-generation carbon capture systems that can be deployed at the scale necessary to meet global climate objectives.
The technological trajectory has progressed through several distinct phases. First-generation capture systems primarily utilized monoethanolamine (MEA) solutions, which despite their effectiveness, suffered from high regeneration energy requirements and solvent degradation issues. Second-generation technologies introduced advanced liquid solvents with improved properties, including sterically hindered amines and amino acid salts, which offered better energy efficiency and reduced degradation.
The current third-generation technologies represent a paradigm shift toward solid sorbents, which promise significant advantages over traditional liquid systems. This evolution has been accelerated by increasingly stringent emission regulations and the Paris Agreement's ambitious climate targets, which necessitate more efficient and cost-effective capture solutions.
Amine-functionalized solid sorbents emerged as promising candidates due to their high selectivity for CO2 and lower regeneration energy requirements compared to aqueous amine systems. These materials typically consist of amines immobilized on high-surface-area supports such as silica, activated carbon, or metal-organic frameworks (MOFs). Concurrently, hybrid sorbents have been developed, combining the advantages of different materials to enhance performance metrics.
The primary objectives of current CO2 capture technology development include: reducing the energy penalty associated with sorbent regeneration; improving sorbent capacity, selectivity, and stability under real operating conditions; minimizing production costs; and ensuring environmental sustainability throughout the lifecycle. Specifically for solid sorbents, objectives focus on optimizing the amine loading and distribution, enhancing mass transfer kinetics, and developing scalable synthesis methods.
Recent technological goals have expanded to include developing sorbents capable of operating in diverse industrial environments, from power plants to cement factories and direct air capture applications. The ideal sorbent would combine high CO2 capacity, fast adsorption/desorption kinetics, excellent stability over thousands of cycles, and low manufacturing costs—a combination that has proven challenging to achieve simultaneously.
The comparative evaluation of amine versus hybrid sorbents represents a critical research direction, as it aims to identify optimal materials that balance performance, cost, and environmental impact for next-generation carbon capture systems that can be deployed at the scale necessary to meet global climate objectives.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market size has reached approximately 7.0 billion USD, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030. This growth trajectory is supported by governmental carbon pricing mechanisms and emissions trading schemes implemented across 45 countries.
Solid sorbents for CO2 capture represent a rapidly expanding segment within this market. Traditional amine-based sorbents currently dominate with roughly 65% market share due to their established technology and widespread industrial adoption. However, hybrid sorbents are gaining traction, showing a remarkable 27% year-over-year growth rate since 2020, albeit from a smaller base.
Geographically, North America leads the carbon capture market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. Within these markets, industrial applications constitute the largest demand segment at 48%, followed by power generation at 32%, and transportation at 15%.
Customer segmentation reveals three primary market categories: large industrial emitters (steel, cement, chemicals) representing 52% of demand; power generation facilities at 35%; and emerging applications in direct air capture and small-scale industrial operations at 13%. The purchasing decisions in these segments are primarily driven by regulatory compliance requirements, operational efficiency improvements, and corporate sustainability goals.
Key market trends include increasing interest in modular and scalable capture solutions, growing demand for technologies with lower energy penalties, and rising investment in integrated carbon capture and utilization systems. The market is also witnessing a shift toward service-based business models, with "Carbon Capture as a Service" offerings growing at 31% annually.
Competitive pricing analysis indicates that amine-based solutions currently offer the lowest capital expenditure but higher operational costs due to energy requirements and sorbent degradation. Hybrid sorbents, while commanding a 15-25% premium in initial investment, demonstrate 20-30% lower operational expenses over a typical 15-year facility lifespan, presenting a compelling total cost of ownership proposition for long-term operations.
Market barriers include high initial capital requirements, uncertain regulatory frameworks in developing economies, and infrastructure limitations for CO2 transportation and storage. Despite these challenges, market penetration of solid sorbent technologies is accelerating, with adoption rates increasing by 23% annually across industrial sectors.
Solid sorbents for CO2 capture represent a rapidly expanding segment within this market. Traditional amine-based sorbents currently dominate with roughly 65% market share due to their established technology and widespread industrial adoption. However, hybrid sorbents are gaining traction, showing a remarkable 27% year-over-year growth rate since 2020, albeit from a smaller base.
Geographically, North America leads the carbon capture market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. Within these markets, industrial applications constitute the largest demand segment at 48%, followed by power generation at 32%, and transportation at 15%.
Customer segmentation reveals three primary market categories: large industrial emitters (steel, cement, chemicals) representing 52% of demand; power generation facilities at 35%; and emerging applications in direct air capture and small-scale industrial operations at 13%. The purchasing decisions in these segments are primarily driven by regulatory compliance requirements, operational efficiency improvements, and corporate sustainability goals.
Key market trends include increasing interest in modular and scalable capture solutions, growing demand for technologies with lower energy penalties, and rising investment in integrated carbon capture and utilization systems. The market is also witnessing a shift toward service-based business models, with "Carbon Capture as a Service" offerings growing at 31% annually.
Competitive pricing analysis indicates that amine-based solutions currently offer the lowest capital expenditure but higher operational costs due to energy requirements and sorbent degradation. Hybrid sorbents, while commanding a 15-25% premium in initial investment, demonstrate 20-30% lower operational expenses over a typical 15-year facility lifespan, presenting a compelling total cost of ownership proposition for long-term operations.
Market barriers include high initial capital requirements, uncertain regulatory frameworks in developing economies, and infrastructure limitations for CO2 transportation and storage. Despite these challenges, market penetration of solid sorbent technologies is accelerating, with adoption rates increasing by 23% annually across industrial sectors.
Current Challenges in Solid Sorbent Technology
Despite significant advancements in solid sorbent technology for CO2 capture, several critical challenges persist that hinder widespread commercial implementation. The primary challenge remains the trade-off between CO2 adsorption capacity and selectivity versus regeneration energy requirements. Amine-functionalized sorbents demonstrate excellent CO2 affinity but often require substantial energy input for regeneration, reducing overall process efficiency. This energy penalty significantly impacts the economic viability of carbon capture systems in industrial settings.
Material stability presents another major obstacle, particularly for amine-based sorbents which suffer from degradation through oxidation, leaching, and urea formation during repeated adsorption-desorption cycles. This degradation progressively reduces capture capacity and necessitates frequent sorbent replacement, increasing operational costs. Hybrid sorbents, while showing promise in laboratory settings, often face accelerated degradation when scaled to industrial conditions with real flue gas contaminants such as SOx and NOx.
Moisture sensitivity remains problematic for many solid sorbents. While some amine-functionalized materials benefit from moderate humidity through the formation of bicarbonates that enhance CO2 uptake, excessive moisture can cause pore blockage and structural collapse. Conversely, some hybrid sorbents with hydrophobic components may perform poorly in humid conditions typical of industrial flue gas streams.
Manufacturing scalability presents significant technical barriers. Laboratory-scale synthesis methods for advanced hybrid sorbents often involve complex multi-step processes that are difficult to scale economically. The uniformity of functional group distribution and pore structure becomes increasingly challenging to maintain at industrial production scales, leading to performance inconsistencies.
Heat management during adsorption poses another engineering challenge. The exothermic nature of CO2 adsorption can cause temperature spikes within sorbent beds, potentially accelerating degradation and reducing working capacity. This is particularly problematic for amine-based sorbents where temperature control is critical for maintaining optimal performance.
Finally, there exists a significant knowledge gap in understanding the long-term performance of solid sorbents under real industrial conditions. Most research data comes from short-term laboratory studies using simulated flue gas, which fails to account for the complex interactions with trace contaminants and the effects of thermal, chemical, and mechanical stresses over thousands of cycles. This lack of comprehensive long-term performance data creates uncertainty for industrial adoption and complicates accurate techno-economic assessments.
Material stability presents another major obstacle, particularly for amine-based sorbents which suffer from degradation through oxidation, leaching, and urea formation during repeated adsorption-desorption cycles. This degradation progressively reduces capture capacity and necessitates frequent sorbent replacement, increasing operational costs. Hybrid sorbents, while showing promise in laboratory settings, often face accelerated degradation when scaled to industrial conditions with real flue gas contaminants such as SOx and NOx.
Moisture sensitivity remains problematic for many solid sorbents. While some amine-functionalized materials benefit from moderate humidity through the formation of bicarbonates that enhance CO2 uptake, excessive moisture can cause pore blockage and structural collapse. Conversely, some hybrid sorbents with hydrophobic components may perform poorly in humid conditions typical of industrial flue gas streams.
Manufacturing scalability presents significant technical barriers. Laboratory-scale synthesis methods for advanced hybrid sorbents often involve complex multi-step processes that are difficult to scale economically. The uniformity of functional group distribution and pore structure becomes increasingly challenging to maintain at industrial production scales, leading to performance inconsistencies.
Heat management during adsorption poses another engineering challenge. The exothermic nature of CO2 adsorption can cause temperature spikes within sorbent beds, potentially accelerating degradation and reducing working capacity. This is particularly problematic for amine-based sorbents where temperature control is critical for maintaining optimal performance.
Finally, there exists a significant knowledge gap in understanding the long-term performance of solid sorbents under real industrial conditions. Most research data comes from short-term laboratory studies using simulated flue gas, which fails to account for the complex interactions with trace contaminants and the effects of thermal, chemical, and mechanical stresses over thousands of cycles. This lack of comprehensive long-term performance data creates uncertainty for industrial adoption and complicates accurate techno-economic assessments.
Comparative Analysis of Amine and Hybrid Sorbents
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. These materials have exceptionally high surface areas and tunable pore sizes, making them effective for CO2 adsorption. MOFs can be designed with specific functional groups to enhance CO2 selectivity and capture efficiency. Their modular nature allows for customization of binding sites to optimize CO2 capture performance under various conditions, including temperature and pressure variations.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials that have shown exceptional CO2 capture efficiency due to their high surface area and tunable pore structures. These materials can be designed with specific metal centers and organic linkers to enhance CO2 selectivity and adsorption capacity. MOFs can be modified with functional groups to increase their affinity for CO2, resulting in improved capture efficiency even at low CO2 concentrations. Their regeneration typically requires less energy compared to traditional amine-based sorbents.
- Amine-functionalized solid sorbents: Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture with high efficiency. These materials combine the high CO2 affinity of amines with the structural advantages of solid supports such as silica, polymers, or carbon-based materials. The amine groups form chemical bonds with CO2 molecules, enabling selective capture even from dilute gas streams. Various amine types (primary, secondary, tertiary) and loading densities can be optimized to balance capture capacity, kinetics, and regeneration energy requirements.
- Zeolite-based CO2 capture systems: Zeolites are aluminosilicate materials with well-defined microporous structures that demonstrate significant potential for CO2 capture applications. Their molecular sieving properties allow for selective adsorption of CO2 from gas mixtures. The capture efficiency of zeolites can be enhanced through ion exchange, introducing cations that increase the affinity for CO2 molecules. Zeolites offer advantages including thermal stability, mechanical strength, and resistance to contaminants, making them suitable for industrial-scale carbon capture processes.
- Carbon-based adsorbents for CO2 capture: Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, offer promising CO2 capture efficiency due to their high surface area and pore volume. These materials can be functionalized or doped with nitrogen, oxygen, or metal particles to enhance CO2 selectivity and adsorption capacity. Carbon-based sorbents typically demonstrate good stability over multiple adsorption-desorption cycles and can be produced from renewable or waste resources, making them environmentally attractive options for carbon capture applications.
- Hybrid and composite sorbent materials: Hybrid and composite materials combine different types of sorbents to leverage their complementary properties for enhanced CO2 capture efficiency. These materials often integrate organic and inorganic components, such as polymer-inorganic composites, MOF-polymer blends, or amine-functionalized mesoporous silica. The synergistic effects between components can lead to improved adsorption capacity, selectivity, kinetics, and mechanical stability. These hybrid systems can be designed to operate effectively under various conditions, including different temperatures, pressures, and in the presence of contaminants.
02 Amine-functionalized sorbents for enhanced CO2 capture
Amine-functionalized solid sorbents utilize the chemical reaction between CO2 and amine groups to achieve high capture efficiency. These materials typically consist of a porous support structure (such as silica, alumina, or polymers) impregnated or grafted with various amine compounds. The amine groups form carbamates or bicarbonates upon reaction with CO2, enabling selective capture even at low CO2 concentrations. These sorbents demonstrate improved adsorption capacity, selectivity, and regeneration properties compared to conventional materials, making them suitable for post-combustion carbon capture applications.Expand Specific Solutions03 Zeolite-based materials for selective CO2 adsorption
Zeolites are microporous aluminosilicate minerals with well-defined crystalline structures that can be utilized as effective CO2 sorbents. Their uniform pore size distribution and high thermal stability make them suitable for pressure swing adsorption processes. Zeolites can be modified with various cations to enhance CO2 selectivity and adsorption capacity. The capture efficiency of zeolite-based materials depends on factors such as the Si/Al ratio, cation type, and framework structure, which can be optimized for specific CO2 capture conditions.Expand Specific Solutions04 Carbon-based sorbents for high-capacity CO2 capture
Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, offer advantages for CO2 capture due to their high surface area, tunable porosity, and relatively low cost. These materials can be functionalized with nitrogen-containing groups or metal particles to enhance CO2 binding affinity. The capture efficiency of carbon-based sorbents can be improved through physical and chemical activation processes that create optimal micropore structures. Their hydrophobic nature also provides advantages in humid conditions compared to other sorbent types.Expand Specific Solutions05 Regeneration methods and energy efficiency for CO2 sorbents
The overall efficiency of CO2 capture systems depends not only on adsorption capacity but also on the energy required for sorbent regeneration. Various regeneration methods, including temperature swing, pressure swing, and vacuum swing processes, can be optimized to reduce energy penalties. Advanced sorbent designs focus on achieving high working capacity with minimal regeneration energy requirements. Innovative approaches such as microwave-assisted regeneration, electrical swing adsorption, and hybrid regeneration techniques are being developed to improve the energy efficiency of the capture-regeneration cycle.Expand Specific Solutions
Leading Organizations in Carbon Capture Research
The CO2 capture market using solid sorbents is in a growth phase, with increasing attention on amine-based and hybrid sorbent technologies. The market is expanding rapidly due to global decarbonization efforts, with projections suggesting significant growth in the coming decade. Technologically, amine-based sorbents represent mature technology with established players like ExxonMobil and Global Thermostat leading commercial applications. Meanwhile, hybrid sorbents are emerging as promising alternatives, with research institutions (Tsinghua University, University of California, ETH Zurich) and specialized companies (Climeworks, Deep Carbon Technology) driving innovation. The competitive landscape features collaboration between academic institutions and industry partners, with companies like CSIRO and Lawrence Livermore National Security contributing to technological advancement through novel material development and process optimization.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed proprietary solid amine-functionalized sorbents for CO2 capture that utilize porous materials impregnated with or chemically bonded to amine groups. Their technology employs a temperature-swing adsorption process where CO2 is captured at lower temperatures (40-60°C) and released at higher temperatures (100-120°C). The company's approach focuses on reducing regeneration energy requirements through optimized sorbent formulations that balance CO2 capacity and selectivity. ExxonMobil's solid sorbents incorporate both primary and tertiary amines on silica supports, achieving CO2 capacities of 2-3 mmol/g under flue gas conditions. Their hybrid sorbent systems combine the high selectivity of amines with the thermal stability of inorganic supports, resulting in materials that maintain performance over thousands of adsorption-desorption cycles.
Strengths: High CO2 selectivity even at low concentrations; lower regeneration energy compared to liquid amine systems; reduced equipment corrosion issues. Weaknesses: Potential for amine leaching during extended cycling; higher production costs compared to conventional sorbents; thermal degradation concerns at higher regeneration temperatures.
Climeworks AG
Technical Solution: Climeworks has pioneered a direct air capture (DAC) technology using specialized solid sorbents based on amine-functionalized filter materials. Their proprietary sorbent consists of porous granulates modified with amines that selectively bind CO2 from ambient air. The technology operates through a cyclic process where ambient air passes through collectors containing the sorbent material, which captures CO2 even at very low atmospheric concentrations (approximately 400 ppm). Once saturated, the collectors are heated to 80-100°C using low-grade waste heat, releasing concentrated CO2 for storage or utilization. Climeworks' latest generation sorbents demonstrate improved working capacities of 1.5-2 mmol CO2/g sorbent under atmospheric conditions, with enhanced hydrothermal stability allowing for thousands of adsorption-desorption cycles. Their hybrid approach incorporates both physical adsorption properties of the porous substrate and chemical binding from the amine functionality.
Strengths: Highly selective for CO2 at ultra-low concentrations; modular system design allowing for scalability; ability to operate using low-grade waste heat for regeneration. Weaknesses: Higher energy requirements per ton of CO2 compared to point-source capture; limited by air contact efficiency; susceptibility to degradation from airborne contaminants over time.
Key Patents in Solid Sorbent Technology
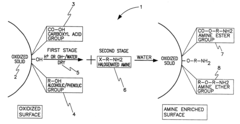
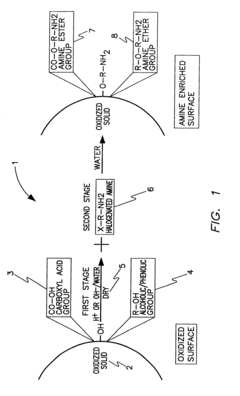
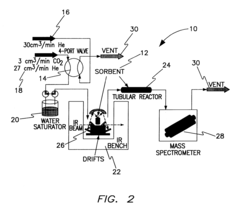
Amine enriched solid sorbents for carbon dioxide capture
PatentInactiveUS6547854B1
Innovation
- A two-step chemical treatment process incorporating amine functionalities onto oxidized solid substrates using metal hydroxides and substituted amine salts, eliminating the need for organic solvents and polymeric materials, and enabling CO2 capture through both physical and chemical adsorption over a range of temperatures.
Environmental Impact Assessment of Sorbent Technologies
The environmental impact assessment of CO2 capture technologies is crucial for determining their overall sustainability and viability in addressing climate change challenges. When comparing amine-based and hybrid sorbents, several environmental factors must be considered beyond their mere capture efficiency.
Traditional amine-based sorbents, while effective for CO2 capture, present significant environmental concerns. The production process of these materials typically involves energy-intensive manufacturing steps and the use of potentially harmful chemicals. During operation, amine degradation can lead to the release of toxic compounds such as nitrosamines and ammonia into the atmosphere, contributing to air pollution and potential health hazards for nearby communities.
Hybrid sorbents, which combine amines with inorganic supports or incorporate novel materials like metal-organic frameworks (MOFs), demonstrate promising environmental advantages. These materials often require lower regeneration temperatures, resulting in reduced energy consumption during the capture-release cycle. This energy efficiency translates directly to lower greenhouse gas emissions from the capture process itself, improving the net carbon reduction benefit.
Water consumption represents another critical environmental parameter. Amine-based systems typically demand substantial water resources for cooling and process operations. In contrast, many hybrid sorbent technologies exhibit lower water requirements, making them more suitable for deployment in water-stressed regions. This reduced water footprint becomes increasingly important as climate change exacerbates water scarcity globally.
Waste generation and disposal considerations also favor hybrid systems in many cases. Amine solutions require regular replacement due to thermal and oxidative degradation, creating liquid waste streams that necessitate specialized treatment. Solid hybrid sorbents generally demonstrate longer operational lifespans and, at end-of-life, can sometimes be recycled or repurposed, minimizing landfill impact.
Land use requirements differ significantly between technologies. Amine scrubbing systems typically demand larger physical footprints due to the need for extensive liquid handling equipment. Hybrid sorbent systems, particularly advanced solid sorbents, can often achieve comparable capture capacity with more compact installations, reducing habitat disruption and preserving natural landscapes.
Life cycle assessment (LCA) studies comparing these technologies reveal that hybrid sorbents generally demonstrate lower environmental impact across multiple categories, including global warming potential, acidification, and human toxicity. However, these advantages must be balanced against considerations of material availability, particularly for hybrid sorbents incorporating rare elements or complex synthesis pathways.
Traditional amine-based sorbents, while effective for CO2 capture, present significant environmental concerns. The production process of these materials typically involves energy-intensive manufacturing steps and the use of potentially harmful chemicals. During operation, amine degradation can lead to the release of toxic compounds such as nitrosamines and ammonia into the atmosphere, contributing to air pollution and potential health hazards for nearby communities.
Hybrid sorbents, which combine amines with inorganic supports or incorporate novel materials like metal-organic frameworks (MOFs), demonstrate promising environmental advantages. These materials often require lower regeneration temperatures, resulting in reduced energy consumption during the capture-release cycle. This energy efficiency translates directly to lower greenhouse gas emissions from the capture process itself, improving the net carbon reduction benefit.
Water consumption represents another critical environmental parameter. Amine-based systems typically demand substantial water resources for cooling and process operations. In contrast, many hybrid sorbent technologies exhibit lower water requirements, making them more suitable for deployment in water-stressed regions. This reduced water footprint becomes increasingly important as climate change exacerbates water scarcity globally.
Waste generation and disposal considerations also favor hybrid systems in many cases. Amine solutions require regular replacement due to thermal and oxidative degradation, creating liquid waste streams that necessitate specialized treatment. Solid hybrid sorbents generally demonstrate longer operational lifespans and, at end-of-life, can sometimes be recycled or repurposed, minimizing landfill impact.
Land use requirements differ significantly between technologies. Amine scrubbing systems typically demand larger physical footprints due to the need for extensive liquid handling equipment. Hybrid sorbent systems, particularly advanced solid sorbents, can often achieve comparable capture capacity with more compact installations, reducing habitat disruption and preserving natural landscapes.
Life cycle assessment (LCA) studies comparing these technologies reveal that hybrid sorbents generally demonstrate lower environmental impact across multiple categories, including global warming potential, acidification, and human toxicity. However, these advantages must be balanced against considerations of material availability, particularly for hybrid sorbents incorporating rare elements or complex synthesis pathways.
Cost-Benefit Analysis of Implementation Strategies
Implementing CO2 capture technologies requires careful economic analysis to determine the most cost-effective approach. When comparing amine-based and hybrid solid sorbents, several financial factors must be considered. Initial capital expenditure for amine-based systems typically ranges from $40-60 million for medium-scale industrial applications, while hybrid sorbent systems may require $45-70 million due to more complex material handling equipment and specialized reactors.
Operational costs present significant differences between these technologies. Amine-based systems incur higher energy penalties, typically consuming 2.5-3.5 GJ/ton CO2 captured, which translates to approximately $15-25 per ton in energy costs alone. Hybrid sorbents demonstrate improved energy efficiency with consumption rates of 1.8-2.8 GJ/ton CO2, reducing energy costs to $12-20 per ton. However, hybrid sorbents often require more frequent replacement, with average lifespans of 1-3 years compared to 3-5 years for conventional amine sorbents.
Maintenance requirements also differ substantially. Amine systems suffer from corrosion issues, necessitating annual maintenance costs of approximately 4-6% of capital investment. Hybrid systems typically require 3-5% of capital costs for maintenance but may need more specialized technical expertise for troubleshooting and optimization.
Scalability economics favor different technologies depending on implementation scale. For smaller applications (<100,000 tons CO2/year), hybrid sorbents often demonstrate better economics with lower minimum efficient scale. For large industrial applications (>500,000 tons CO2/year), amine-based systems benefit from economies of scale, potentially reducing costs by 15-25% compared to smaller installations.
Regulatory compliance costs must also be factored into the analysis. Amine systems face increasing scrutiny regarding potential atmospheric emissions of degradation products, with compliance costs estimated at $2-4 per ton CO2. Hybrid systems generally have lower environmental impact concerns but may face higher costs related to solid waste disposal, approximately $1-3 per ton CO2.
Return on investment timelines vary significantly based on carbon pricing mechanisms. At current carbon prices ($25-50/ton in developed markets), amine systems typically achieve ROI in 8-12 years, while hybrid systems may reach profitability in 6-10 years due to lower operational costs, despite higher initial investment. This advantage for hybrid systems becomes more pronounced as carbon prices increase, potentially shortening ROI timelines by 15-30% compared to conventional amine systems.
Operational costs present significant differences between these technologies. Amine-based systems incur higher energy penalties, typically consuming 2.5-3.5 GJ/ton CO2 captured, which translates to approximately $15-25 per ton in energy costs alone. Hybrid sorbents demonstrate improved energy efficiency with consumption rates of 1.8-2.8 GJ/ton CO2, reducing energy costs to $12-20 per ton. However, hybrid sorbents often require more frequent replacement, with average lifespans of 1-3 years compared to 3-5 years for conventional amine sorbents.
Maintenance requirements also differ substantially. Amine systems suffer from corrosion issues, necessitating annual maintenance costs of approximately 4-6% of capital investment. Hybrid systems typically require 3-5% of capital costs for maintenance but may need more specialized technical expertise for troubleshooting and optimization.
Scalability economics favor different technologies depending on implementation scale. For smaller applications (<100,000 tons CO2/year), hybrid sorbents often demonstrate better economics with lower minimum efficient scale. For large industrial applications (>500,000 tons CO2/year), amine-based systems benefit from economies of scale, potentially reducing costs by 15-25% compared to smaller installations.
Regulatory compliance costs must also be factored into the analysis. Amine systems face increasing scrutiny regarding potential atmospheric emissions of degradation products, with compliance costs estimated at $2-4 per ton CO2. Hybrid systems generally have lower environmental impact concerns but may face higher costs related to solid waste disposal, approximately $1-3 per ton CO2.
Return on investment timelines vary significantly based on carbon pricing mechanisms. At current carbon prices ($25-50/ton in developed markets), amine systems typically achieve ROI in 8-12 years, while hybrid systems may reach profitability in 6-10 years due to lower operational costs, despite higher initial investment. This advantage for hybrid systems becomes more pronounced as carbon prices increase, potentially shortening ROI timelines by 15-30% compared to conventional amine systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!