Research on Solid sorbents for CO2 capture for advanced porous and hybrid material development
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Background and Objectives
Carbon dioxide capture and storage (CCS) has emerged as a critical technology in the global effort to mitigate climate change. The atmospheric concentration of CO2 has increased dramatically since the industrial revolution, reaching levels unprecedented in human history. This increase is primarily attributed to anthropogenic activities, particularly the combustion of fossil fuels for energy production and industrial processes. As global energy demand continues to rise, especially in developing economies, the need for effective CO2 capture technologies becomes increasingly urgent.
The evolution of CO2 capture technologies has progressed through several generations. Early approaches focused on liquid amine-based absorption systems, which remain the most commercially mature technology. However, these systems suffer from significant drawbacks including high energy penalties for regeneration, corrosion issues, and amine degradation. This has driven research toward alternative capture methods, with solid sorbents emerging as a promising direction due to their potential for lower regeneration energy requirements and reduced environmental impact.
Solid sorbents for CO2 capture represent a diverse class of materials including activated carbons, zeolites, metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and various amine-functionalized porous materials. The development trajectory has moved from conventional materials toward increasingly sophisticated engineered structures with tailored properties for specific capture conditions. Recent advances in materials science and nanotechnology have accelerated innovation in this field, enabling precise control over pore architecture, surface chemistry, and functional group integration.
The primary technical objectives in solid sorbent development center around several key performance indicators: high CO2 selectivity over other flue gas components, substantial adsorption capacity, rapid adsorption/desorption kinetics, mechanical and chemical stability over multiple cycles, and cost-effective scalability. Additionally, researchers aim to develop materials that can operate effectively under realistic conditions, including the presence of moisture, contaminants, and varying temperature and pressure regimes.
Looking forward, the field is trending toward hybrid and composite materials that combine the advantages of different sorbent types. These advanced materials seek to overcome the traditional trade-offs between adsorption capacity and regeneration energy by incorporating multiple functional components within a single structured material. Computational modeling and high-throughput screening approaches are increasingly being employed to accelerate the discovery and optimization of novel sorbent materials.
The ultimate goal of this research direction is to develop next-generation solid sorbents that can significantly reduce the energy penalty and cost of CO2 capture, making CCS economically viable for widespread implementation across various industrial sectors and energy production facilities.
The evolution of CO2 capture technologies has progressed through several generations. Early approaches focused on liquid amine-based absorption systems, which remain the most commercially mature technology. However, these systems suffer from significant drawbacks including high energy penalties for regeneration, corrosion issues, and amine degradation. This has driven research toward alternative capture methods, with solid sorbents emerging as a promising direction due to their potential for lower regeneration energy requirements and reduced environmental impact.
Solid sorbents for CO2 capture represent a diverse class of materials including activated carbons, zeolites, metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and various amine-functionalized porous materials. The development trajectory has moved from conventional materials toward increasingly sophisticated engineered structures with tailored properties for specific capture conditions. Recent advances in materials science and nanotechnology have accelerated innovation in this field, enabling precise control over pore architecture, surface chemistry, and functional group integration.
The primary technical objectives in solid sorbent development center around several key performance indicators: high CO2 selectivity over other flue gas components, substantial adsorption capacity, rapid adsorption/desorption kinetics, mechanical and chemical stability over multiple cycles, and cost-effective scalability. Additionally, researchers aim to develop materials that can operate effectively under realistic conditions, including the presence of moisture, contaminants, and varying temperature and pressure regimes.
Looking forward, the field is trending toward hybrid and composite materials that combine the advantages of different sorbent types. These advanced materials seek to overcome the traditional trade-offs between adsorption capacity and regeneration energy by incorporating multiple functional components within a single structured material. Computational modeling and high-throughput screening approaches are increasingly being employed to accelerate the discovery and optimization of novel sorbent materials.
The ultimate goal of this research direction is to develop next-generation solid sorbents that can significantly reduce the energy penalty and cost of CO2 capture, making CCS economically viable for widespread implementation across various industrial sectors and energy production facilities.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market size for carbon capture technologies reached approximately $7.5 billion, with projections indicating growth to $15-20 billion by 2030. This represents a compound annual growth rate (CAGR) of 12-15%, significantly outpacing many other industrial technology sectors.
Solid sorbents for CO2 capture represent a particularly promising segment within this market. Traditional carbon capture methods using liquid amine solutions face challenges including high energy requirements for regeneration, equipment corrosion, and solvent degradation. These limitations have created substantial market demand for advanced solid sorbent materials that offer improved efficiency and reduced operational costs.
Industrial sectors with the highest demand for carbon capture solutions include power generation (particularly coal and natural gas plants), cement production, steel manufacturing, and chemical processing. These industries collectively account for approximately 65% of global CO2 emissions from industrial sources. The power generation sector alone represents about 40% of the current market for carbon capture technologies.
Geographically, North America and Europe lead in carbon capture technology adoption, accounting for approximately 60% of global market share. However, the Asia-Pacific region, particularly China and India, is expected to show the fastest growth rate over the next decade due to their heavy reliance on fossil fuels coupled with increasing environmental regulations.
Government policies and incentives are significantly shaping market dynamics. The implementation of carbon pricing mechanisms in over 40 countries has created economic incentives for carbon capture adoption. In the United States, the 45Q tax credit provides up to $85 per metric ton for captured and sequestered CO2, substantially improving the economic viability of these technologies.
Customer requirements in this market emphasize several key factors: energy efficiency of the capture process, CO2 selectivity, material durability, scalability, and total cost of ownership. Advanced porous materials and hybrid sorbents are increasingly valued for their potential to address these requirements more effectively than conventional technologies.
Market analysis indicates that partnerships between material science companies, engineering firms, and end-users are becoming the dominant business model in this sector. This collaborative approach helps bridge the gap between laboratory-scale material development and commercial-scale implementation, addressing one of the key barriers to market growth.
Solid sorbents for CO2 capture represent a particularly promising segment within this market. Traditional carbon capture methods using liquid amine solutions face challenges including high energy requirements for regeneration, equipment corrosion, and solvent degradation. These limitations have created substantial market demand for advanced solid sorbent materials that offer improved efficiency and reduced operational costs.
Industrial sectors with the highest demand for carbon capture solutions include power generation (particularly coal and natural gas plants), cement production, steel manufacturing, and chemical processing. These industries collectively account for approximately 65% of global CO2 emissions from industrial sources. The power generation sector alone represents about 40% of the current market for carbon capture technologies.
Geographically, North America and Europe lead in carbon capture technology adoption, accounting for approximately 60% of global market share. However, the Asia-Pacific region, particularly China and India, is expected to show the fastest growth rate over the next decade due to their heavy reliance on fossil fuels coupled with increasing environmental regulations.
Government policies and incentives are significantly shaping market dynamics. The implementation of carbon pricing mechanisms in over 40 countries has created economic incentives for carbon capture adoption. In the United States, the 45Q tax credit provides up to $85 per metric ton for captured and sequestered CO2, substantially improving the economic viability of these technologies.
Customer requirements in this market emphasize several key factors: energy efficiency of the capture process, CO2 selectivity, material durability, scalability, and total cost of ownership. Advanced porous materials and hybrid sorbents are increasingly valued for their potential to address these requirements more effectively than conventional technologies.
Market analysis indicates that partnerships between material science companies, engineering firms, and end-users are becoming the dominant business model in this sector. This collaborative approach helps bridge the gap between laboratory-scale material development and commercial-scale implementation, addressing one of the key barriers to market growth.
Current Status and Challenges in Solid Sorbent Technology
The global landscape of solid sorbent technology for CO2 capture has witnessed significant advancements in recent years, with research institutions and industrial players across North America, Europe, and Asia making substantial contributions. Current state-of-the-art solid sorbents include metal-organic frameworks (MOFs), zeolites, activated carbons, amine-functionalized materials, and hybrid composites. These materials have demonstrated promising CO2 capture capacities ranging from 1-8 mmol/g under various operating conditions, representing a marked improvement over conventional liquid amine scrubbing technologies.
Despite these advancements, several critical challenges persist in the development and implementation of solid sorbent technologies. Thermal and chemical stability remains a significant concern, particularly for MOFs and amine-functionalized materials that often degrade after multiple adsorption-desorption cycles. Current research indicates that most advanced sorbents maintain only 70-85% of their initial capacity after 100 cycles, falling short of the industrial requirement of thousands of stable cycles.
Scalability presents another formidable challenge, as many high-performing materials synthesized in laboratory settings utilize expensive precursors and complex synthesis procedures. The translation from milligram-scale production to ton-scale manufacturing introduces inconsistencies in material properties and performance metrics. Recent techno-economic analyses suggest that material costs must decrease by at least an order of magnitude to achieve economic viability in large-scale applications.
Energy requirements for regeneration continue to be a limiting factor, with most current solid sorbents requiring 2.5-4.0 GJ/ton CO2 for the desorption process. This energy penalty significantly impacts the overall efficiency and economic feasibility of carbon capture systems. Additionally, the presence of contaminants in real flue gas streams, including SOx, NOx, and moisture, substantially reduces the selectivity and capacity of many promising materials.
The geographic distribution of research expertise shows concentration in specific regions, with the United States, China, and the European Union leading in patent filings and high-impact publications. Japan and South Korea have established strong positions in specialized hybrid materials, while emerging contributions from India, Australia, and Middle Eastern countries are increasingly significant, particularly in low-cost synthesis methods and biomass-derived sorbents.
Water stability represents a particular challenge for many advanced materials, especially MOFs and certain zeolites, which can lose structural integrity or adsorption capacity when exposed to moisture. Recent developments in hydrophobic modifications and composite structures have shown promise but have yet to achieve the necessary balance between water resistance and CO2 selectivity required for practical applications.
Despite these advancements, several critical challenges persist in the development and implementation of solid sorbent technologies. Thermal and chemical stability remains a significant concern, particularly for MOFs and amine-functionalized materials that often degrade after multiple adsorption-desorption cycles. Current research indicates that most advanced sorbents maintain only 70-85% of their initial capacity after 100 cycles, falling short of the industrial requirement of thousands of stable cycles.
Scalability presents another formidable challenge, as many high-performing materials synthesized in laboratory settings utilize expensive precursors and complex synthesis procedures. The translation from milligram-scale production to ton-scale manufacturing introduces inconsistencies in material properties and performance metrics. Recent techno-economic analyses suggest that material costs must decrease by at least an order of magnitude to achieve economic viability in large-scale applications.
Energy requirements for regeneration continue to be a limiting factor, with most current solid sorbents requiring 2.5-4.0 GJ/ton CO2 for the desorption process. This energy penalty significantly impacts the overall efficiency and economic feasibility of carbon capture systems. Additionally, the presence of contaminants in real flue gas streams, including SOx, NOx, and moisture, substantially reduces the selectivity and capacity of many promising materials.
The geographic distribution of research expertise shows concentration in specific regions, with the United States, China, and the European Union leading in patent filings and high-impact publications. Japan and South Korea have established strong positions in specialized hybrid materials, while emerging contributions from India, Australia, and Middle Eastern countries are increasingly significant, particularly in low-cost synthesis methods and biomass-derived sorbents.
Water stability represents a particular challenge for many advanced materials, especially MOFs and certain zeolites, which can lose structural integrity or adsorption capacity when exposed to moisture. Recent developments in hydrophobic modifications and composite structures have shown promise but have yet to achieve the necessary balance between water resistance and CO2 selectivity required for practical applications.
Existing Solid Sorbent Solutions and Implementations
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated to organic ligands. These materials have shown exceptional CO2 capture efficiency due to their high surface area, tunable pore size, and chemical functionality. MOFs can be designed with specific binding sites for CO2, enhancing selectivity and adsorption capacity. Their modular nature allows for customization to optimize capture performance under various conditions, making them promising candidates for industrial carbon capture applications.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They exhibit high surface areas, tunable pore sizes, and chemical functionality, making them effective solid sorbents for CO2 capture. MOFs can be designed with specific binding sites for CO2 molecules, enhancing selectivity and capture efficiency. Their modular nature allows for customization to optimize CO2 adsorption capacity and kinetics under various operating conditions.
- Amine-functionalized sorbents for enhanced CO2 capture: Amine-functionalized solid sorbents leverage the chemical affinity between amine groups and CO2 molecules to achieve high capture efficiency. These materials typically consist of a porous support structure (such as silica, alumina, or polymers) impregnated or grafted with various amine compounds. The amine groups form carbamates or carbonates upon reaction with CO2, enabling selective capture even at low CO2 concentrations. These sorbents demonstrate improved adsorption capacity, selectivity, and regenerability compared to conventional materials, making them suitable for post-combustion carbon capture applications.
- Zeolite-based materials for selective CO2 adsorption: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that enable molecular sieving properties for CO2 capture. Their high thermal stability, chemical resistance, and tunable pore sizes make them effective solid sorbents. Zeolites can be modified through ion exchange, dealumination, or incorporation of functional groups to enhance CO2 selectivity and adsorption capacity. These materials operate through physical adsorption mechanisms and can be regenerated using temperature or pressure swing processes, offering cost-effective solutions for carbon capture applications.
- Carbon-based sorbents with enhanced surface properties: Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, serve as effective CO2 sorbents due to their high surface area, pore volume, and surface functionality. These materials can be modified through chemical activation, nitrogen doping, or incorporation of metal particles to enhance CO2 binding affinity and selectivity. The tunable pore structure allows for optimization of diffusion kinetics and adsorption capacity. Carbon-based sorbents offer advantages including low cost, high thermal stability, and resistance to moisture, making them promising candidates for industrial-scale carbon capture applications.
- Hybrid and composite sorbents for optimized capture efficiency: Hybrid and composite sorbents combine multiple materials to leverage complementary properties for enhanced CO2 capture performance. These materials typically integrate components such as MOFs, amines, zeolites, or carbon-based materials with supporting matrices to overcome limitations of single-component sorbents. The synergistic effects result in improved adsorption capacity, selectivity, mechanical stability, and regenerability. Advanced fabrication techniques enable precise control over morphology and component distribution, optimizing mass transfer and reaction kinetics. These hybrid systems can be tailored for specific operating conditions, offering versatile solutions for various carbon capture scenarios.
02 Amine-functionalized sorbents for enhanced CO2 capture
Amine-functionalized solid sorbents utilize the chemical reaction between CO2 and amine groups to achieve high capture efficiency. These materials typically consist of amines grafted onto high-surface-area supports such as silica, activated carbon, or polymeric substrates. The amine groups form carbamates or bicarbonates upon reaction with CO2, enabling selective capture even at low CO2 concentrations. These sorbents demonstrate improved adsorption capacity, selectivity, and regenerability compared to conventional materials, making them suitable for post-combustion carbon capture applications.Expand Specific Solutions03 Zeolite-based materials for selective CO2 adsorption
Zeolites are crystalline aluminosilicate materials with well-defined pore structures that enable molecular sieving for selective CO2 capture. Their high thermal stability and mechanical strength make them suitable for industrial applications. Zeolites can be modified through ion exchange, impregnation with functional groups, or incorporation of metals to enhance CO2 adsorption capacity and selectivity. These materials operate through physisorption mechanisms and can be tailored to specific operating conditions by adjusting the silicon-to-aluminum ratio and pore architecture.Expand Specific Solutions04 Carbon-based sorbents for high-capacity CO2 capture
Carbon-based sorbents, including activated carbons, carbon nanotubes, and graphene derivatives, offer advantages for CO2 capture due to their high surface area, tunable porosity, and relatively low cost. These materials can be functionalized with nitrogen-containing groups or metal nanoparticles to enhance CO2 binding affinity. The hydrophobic nature of carbon materials can provide advantages in humid conditions, reducing competition from water molecules. Advanced carbon materials demonstrate good regenerability and stability over multiple adsorption-desorption cycles, making them promising for practical applications.Expand Specific Solutions05 Hybrid and composite sorbents for optimized capture efficiency
Hybrid and composite sorbents combine different materials to leverage complementary properties and overcome limitations of single-component systems. These may include MOF-polymer composites, amine-functionalized mesoporous silica, or zeolite-carbon hybrids. The synergistic effects between components can enhance CO2 adsorption capacity, selectivity, kinetics, and stability. These materials often demonstrate improved performance under challenging conditions such as presence of moisture, contaminants, or temperature fluctuations. The modular design approach allows for tailoring sorbent properties to specific capture requirements and process conditions.Expand Specific Solutions
Leading Organizations in Solid Sorbent Development
The solid sorbents for CO2 capture market is in a growth phase, driven by increasing global focus on carbon reduction technologies. The market size is expanding rapidly, with projections indicating significant growth as carbon capture becomes essential for climate change mitigation strategies. Technologically, the field shows varying maturity levels across different sorbent types. Leading players include established energy corporations like ExxonMobil and Sinopec, which leverage their industrial scale and R&D capabilities, alongside specialized innovators such as CarbonCapture Inc. and Hot Lime Labs focusing on direct air capture applications. Academic institutions including Rice University, Norwegian University of Science & Technology, and West Virginia University are advancing fundamental research, while research organizations like CSIRO and Empa bridge the gap between laboratory discoveries and commercial implementation.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed proprietary Metal-Organic Framework (MOF) based solid sorbents for CO2 capture with exceptional selectivity and capacity. Their technology utilizes specially engineered porous materials with tailored pore sizes and functionalized surfaces that can selectively adsorb CO2 from flue gas streams. The company's approach involves a temperature/pressure swing adsorption process where the sorbent captures CO2 at lower temperatures and releases it when heated or when pressure is reduced, allowing for continuous operation cycles. ExxonMobil has demonstrated this technology at pilot scale, showing potential for 90% CO2 capture efficiency with significantly lower energy penalties compared to conventional amine scrubbing. Their research focuses on enhancing the stability of these materials under industrial conditions while maintaining high CO2 selectivity in the presence of water vapor and other contaminants.
Strengths: Superior CO2 selectivity and capacity compared to conventional materials; lower regeneration energy requirements; robust performance in industrial environments. Weaknesses: Higher initial manufacturing costs for specialized MOF materials; potential for performance degradation over multiple adsorption-desorption cycles; scaling challenges for very large industrial applications.
China Petroleum & Chemical Corp.
Technical Solution: Sinopec has developed advanced hierarchical porous materials for CO2 capture, focusing on mesoporous silica and carbon-based sorbents with tailored surface chemistry. Their technology utilizes a dual-functionality approach where primary amine groups provide high CO2 selectivity while secondary pore structures enhance diffusion kinetics and overall capacity. Sinopec's solid sorbents demonstrate CO2 capture capacities exceeding 3.5 mmol/g under flue gas conditions with fast adsorption-desorption kinetics. Their manufacturing process leverages existing petrochemical infrastructure, enabling cost-effective production at industrial scale. Sinopec has implemented this technology in pilot demonstrations at several refineries, showing potential for integration with existing processes where waste heat can be utilized for sorbent regeneration. Recent developments include composite materials incorporating alkali metal oxides that enhance working capacity while maintaining mechanical stability over multiple cycles. Their research also addresses the challenge of sorbent poisoning by SOx and NOx through protective surface modifications that extend operational lifetime in real industrial environments.
Strengths: Extensive industrial implementation experience; cost-effective manufacturing leveraging existing infrastructure; demonstrated integration with refinery and petrochemical processes. Weaknesses: Materials show some sensitivity to water vapor in flue gas; regeneration energy requirements remain higher than theoretical minimums; performance degradation in the presence of certain industrial contaminants.
Key Innovations in Advanced Porous and Hybrid Materials
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
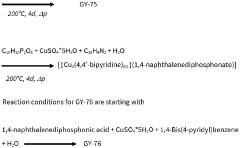
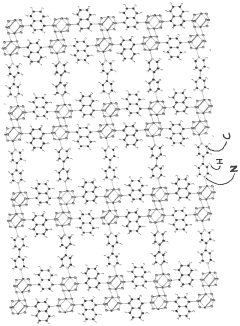
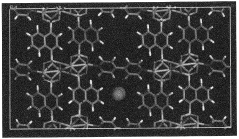
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Environmental Impact Assessment of Sorbent Technologies
The environmental impact of solid sorbent technologies for CO2 capture extends beyond their primary function of reducing greenhouse gas emissions. These technologies must be evaluated across their entire lifecycle to ensure they deliver net environmental benefits. Current assessment methodologies indicate that while solid sorbents offer significant advantages over traditional liquid amine systems, they still present environmental challenges that require careful consideration.
Life cycle assessment (LCA) studies reveal that solid sorbents typically demonstrate lower environmental footprints compared to conventional capture technologies. Metal-organic frameworks (MOFs), zeolites, and activated carbon-based sorbents generally require less energy for regeneration, resulting in reduced indirect emissions from power consumption. However, the production phase of advanced materials like MOFs often involves energy-intensive synthesis processes and potentially toxic precursors, creating environmental trade-offs that must be quantified.
Water usage represents another critical environmental parameter. Solid sorbents generally demonstrate superior performance in this regard, with significantly lower water requirements compared to aqueous amine systems. This advantage becomes particularly valuable in water-stressed regions where conventional capture technologies might exacerbate local resource scarcity. Nevertheless, certain hybrid materials may still require water during synthesis or regeneration cycles, necessitating regional impact assessments.
Land use impacts vary considerably among different sorbent technologies. The compact nature of solid sorbent systems typically results in smaller physical footprints compared to liquid absorption columns. However, mining operations for raw materials used in sorbent production can cause significant land disturbance and habitat fragmentation, particularly for materials requiring rare earth elements or specialized minerals.
Waste generation and management present ongoing challenges. While solid sorbents generally demonstrate longer operational lifespans than liquid amines, their eventual disposal or recycling pathways remain underdeveloped. Current research indicates that some advanced porous materials may release potentially harmful degradation products or nanoparticles during their lifecycle, raising concerns about long-term environmental persistence and bioaccumulation.
Air quality impacts beyond CO2 reduction must also be considered. Most solid sorbents demonstrate favorable profiles regarding criteria pollutants and hazardous air pollutants compared to amine systems, which can release ammonia and other volatile compounds. However, particulate emissions during handling of powdered sorbents require mitigation strategies, particularly for nanoporous materials where particle size may present unique environmental and health considerations.
Life cycle assessment (LCA) studies reveal that solid sorbents typically demonstrate lower environmental footprints compared to conventional capture technologies. Metal-organic frameworks (MOFs), zeolites, and activated carbon-based sorbents generally require less energy for regeneration, resulting in reduced indirect emissions from power consumption. However, the production phase of advanced materials like MOFs often involves energy-intensive synthesis processes and potentially toxic precursors, creating environmental trade-offs that must be quantified.
Water usage represents another critical environmental parameter. Solid sorbents generally demonstrate superior performance in this regard, with significantly lower water requirements compared to aqueous amine systems. This advantage becomes particularly valuable in water-stressed regions where conventional capture technologies might exacerbate local resource scarcity. Nevertheless, certain hybrid materials may still require water during synthesis or regeneration cycles, necessitating regional impact assessments.
Land use impacts vary considerably among different sorbent technologies. The compact nature of solid sorbent systems typically results in smaller physical footprints compared to liquid absorption columns. However, mining operations for raw materials used in sorbent production can cause significant land disturbance and habitat fragmentation, particularly for materials requiring rare earth elements or specialized minerals.
Waste generation and management present ongoing challenges. While solid sorbents generally demonstrate longer operational lifespans than liquid amines, their eventual disposal or recycling pathways remain underdeveloped. Current research indicates that some advanced porous materials may release potentially harmful degradation products or nanoparticles during their lifecycle, raising concerns about long-term environmental persistence and bioaccumulation.
Air quality impacts beyond CO2 reduction must also be considered. Most solid sorbents demonstrate favorable profiles regarding criteria pollutants and hazardous air pollutants compared to amine systems, which can release ammonia and other volatile compounds. However, particulate emissions during handling of powdered sorbents require mitigation strategies, particularly for nanoporous materials where particle size may present unique environmental and health considerations.
Scalability and Industrial Integration Considerations
The scalability of solid sorbents for CO2 capture represents a critical factor in their transition from laboratory-scale demonstrations to industrial implementation. Current manufacturing processes for advanced porous materials and hybrid sorbents often involve complex synthesis routes that are challenging to scale up while maintaining consistent performance characteristics. Batch-to-batch variations in porosity, surface area, and functional group distribution can significantly impact CO2 capture efficiency when production volumes increase.
Industrial integration of these materials requires careful consideration of process engineering aspects. The mechanical stability of sorbents under industrial conditions presents a significant challenge, as materials must withstand thousands of adsorption-desorption cycles without substantial degradation. Attrition resistance becomes particularly important in fluidized bed systems, where particle integrity directly impacts operational efficiency and maintenance requirements.
Cost considerations remain paramount for widespread adoption. While laboratory-scale synthesis may utilize expensive precursors and energy-intensive processes, industrial implementation demands cost-effective production routes. Economic analyses indicate that material costs must typically fall below $10-15 per kg for competitive deployment in power plants and industrial facilities, a threshold many advanced materials currently exceed by an order of magnitude.
Energy requirements for regeneration represent another critical integration factor. The parasitic energy load for sorbent regeneration directly impacts the overall efficiency of carbon capture systems. Materials with lower regeneration temperatures (below 100°C) offer significant advantages for waste heat utilization but must maintain sufficient working capacity to remain economically viable.
Compatibility with existing infrastructure presents both challenges and opportunities. Retrofit applications require sorbent systems that can be integrated with minimal modifications to existing plants, while greenfield implementations allow for more optimized designs. The physical footprint of sorbent-based systems compared to traditional amine scrubbing technologies must be evaluated within spatial constraints of industrial facilities.
Water tolerance and contaminant resistance significantly impact real-world performance. Flue gases contain various impurities including SOx, NOx, and water vapor that can permanently deactivate sensitive sorbent materials. Developing materials with selective CO2 adsorption in the presence of these contaminants remains crucial for practical deployment without extensive pre-treatment requirements.
Industrial integration of these materials requires careful consideration of process engineering aspects. The mechanical stability of sorbents under industrial conditions presents a significant challenge, as materials must withstand thousands of adsorption-desorption cycles without substantial degradation. Attrition resistance becomes particularly important in fluidized bed systems, where particle integrity directly impacts operational efficiency and maintenance requirements.
Cost considerations remain paramount for widespread adoption. While laboratory-scale synthesis may utilize expensive precursors and energy-intensive processes, industrial implementation demands cost-effective production routes. Economic analyses indicate that material costs must typically fall below $10-15 per kg for competitive deployment in power plants and industrial facilities, a threshold many advanced materials currently exceed by an order of magnitude.
Energy requirements for regeneration represent another critical integration factor. The parasitic energy load for sorbent regeneration directly impacts the overall efficiency of carbon capture systems. Materials with lower regeneration temperatures (below 100°C) offer significant advantages for waste heat utilization but must maintain sufficient working capacity to remain economically viable.
Compatibility with existing infrastructure presents both challenges and opportunities. Retrofit applications require sorbent systems that can be integrated with minimal modifications to existing plants, while greenfield implementations allow for more optimized designs. The physical footprint of sorbent-based systems compared to traditional amine scrubbing technologies must be evaluated within spatial constraints of industrial facilities.
Water tolerance and contaminant resistance significantly impact real-world performance. Flue gases contain various impurities including SOx, NOx, and water vapor that can permanently deactivate sensitive sorbent materials. Developing materials with selective CO2 adsorption in the presence of these contaminants remains crucial for practical deployment without extensive pre-treatment requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!