Solid sorbents for CO2 capture for EV and renewable energy integration applications
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications across various industries. The journey began with conventional absorption methods using liquid solvents, primarily amine-based solutions, which dominated industrial CO2 capture processes since the 1930s. These technologies, while effective, presented challenges including high energy consumption, equipment corrosion, and solvent degradation issues.
The emergence of solid sorbents represents a pivotal advancement in CO2 capture technology, offering substantial improvements in energy efficiency, operational flexibility, and environmental impact. These materials, including activated carbons, zeolites, metal-organic frameworks (MOFs), and amine-functionalized silica, have demonstrated superior CO2 selectivity and reduced regeneration energy requirements compared to traditional liquid absorbents.
Recent technological developments have focused on enhancing the integration capabilities of CO2 capture systems with renewable energy sources and electric vehicle infrastructure. This integration addresses the dual challenges of reducing carbon emissions while supporting the growing demand for clean energy storage and utilization. The evolution trajectory shows a clear shift from standalone capture systems to integrated solutions that can operate synergistically with intermittent renewable energy sources.
The primary objective of current research in solid sorbents for CO2 capture is to develop materials with high CO2 selectivity, rapid adsorption-desorption kinetics, and long-term stability under realistic operating conditions. These properties are essential for efficient integration with renewable energy systems, where operational flexibility and responsiveness to fluctuating energy availability are critical requirements.
Another key goal is to reduce the energy penalty associated with the regeneration process, which has historically been a significant barrier to widespread adoption of carbon capture technologies. Advanced solid sorbents aim to achieve regeneration at lower temperatures, potentially utilizing waste heat from renewable energy systems or electric vehicle charging infrastructure.
Scale-up and cost reduction represent additional critical objectives in the technology's evolution. Current research efforts are directed toward developing manufacturing processes that can produce high-performance sorbents at commercially viable scales and costs, making them accessible for widespread deployment across various applications including distributed renewable energy systems and EV charging stations.
The integration with electric vehicles presents unique objectives, including the development of compact, lightweight capture systems that can be incorporated into mobile or stationary charging infrastructure. These systems must operate efficiently under variable conditions while maintaining minimal energy consumption to avoid compromising the environmental benefits of electrified transportation.
The emergence of solid sorbents represents a pivotal advancement in CO2 capture technology, offering substantial improvements in energy efficiency, operational flexibility, and environmental impact. These materials, including activated carbons, zeolites, metal-organic frameworks (MOFs), and amine-functionalized silica, have demonstrated superior CO2 selectivity and reduced regeneration energy requirements compared to traditional liquid absorbents.
Recent technological developments have focused on enhancing the integration capabilities of CO2 capture systems with renewable energy sources and electric vehicle infrastructure. This integration addresses the dual challenges of reducing carbon emissions while supporting the growing demand for clean energy storage and utilization. The evolution trajectory shows a clear shift from standalone capture systems to integrated solutions that can operate synergistically with intermittent renewable energy sources.
The primary objective of current research in solid sorbents for CO2 capture is to develop materials with high CO2 selectivity, rapid adsorption-desorption kinetics, and long-term stability under realistic operating conditions. These properties are essential for efficient integration with renewable energy systems, where operational flexibility and responsiveness to fluctuating energy availability are critical requirements.
Another key goal is to reduce the energy penalty associated with the regeneration process, which has historically been a significant barrier to widespread adoption of carbon capture technologies. Advanced solid sorbents aim to achieve regeneration at lower temperatures, potentially utilizing waste heat from renewable energy systems or electric vehicle charging infrastructure.
Scale-up and cost reduction represent additional critical objectives in the technology's evolution. Current research efforts are directed toward developing manufacturing processes that can produce high-performance sorbents at commercially viable scales and costs, making them accessible for widespread deployment across various applications including distributed renewable energy systems and EV charging stations.
The integration with electric vehicles presents unique objectives, including the development of compact, lightweight capture systems that can be incorporated into mobile or stationary charging infrastructure. These systems must operate efficiently under variable conditions while maintaining minimal energy consumption to avoid compromising the environmental benefits of electrified transportation.
Market Analysis for CO2 Capture in EV and Renewable Energy Sectors
The global market for CO2 capture technologies is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. The market specifically for solid sorbents in CO2 capture applications was valued at approximately $2.3 billion in 2022 and is projected to reach $4.7 billion by 2030, representing a compound annual growth rate of 9.4%. This growth trajectory is particularly pronounced in regions with stringent carbon emission policies such as the European Union, North America, and increasingly in Asia-Pacific countries.
Within the electric vehicle (EV) sector, the integration of CO2 capture technologies presents a novel market opportunity. As the global EV market expands—with sales increasing by 35% in 2023 compared to the previous year—manufacturers are seeking innovative solutions to enhance their sustainability credentials. CO2 capture systems using solid sorbents can be integrated into charging infrastructure, potentially offsetting emissions associated with electricity generation, especially in regions where the grid still relies heavily on fossil fuels.
The renewable energy sector demonstrates even more substantial market potential for solid sorbent CO2 capture technologies. With renewable energy installations growing at an average rate of 10-15% annually worldwide, there is increasing focus on achieving carbon-negative energy systems. Solid sorbent technologies can be deployed alongside biomass energy facilities to create BECCS (Bioenergy with Carbon Capture and Storage) systems, which represent one of the few commercially viable negative emission technologies currently available.
Market demand analysis reveals three primary customer segments: utility companies seeking to decarbonize operations, renewable energy developers aiming to enhance environmental performance, and EV infrastructure providers looking to differentiate their offerings. Survey data indicates that 78% of utility companies are actively exploring carbon capture technologies, while 64% of renewable energy developers consider carbon-negative capabilities a strategic priority for future projects.
The economic drivers for market adoption include carbon pricing mechanisms, which have seen average prices increase from $15 to $65 per ton in major markets over the past five years. Additionally, government incentives specifically targeting carbon capture technologies have expanded, with the U.S. 45Q tax credit now offering up to $85 per ton for captured and sequestered CO2. In the European Union, the Innovation Fund has allocated €10 billion for low-carbon technologies including advanced carbon capture systems through 2030.
Consumer sentiment analysis shows growing awareness and preference for carbon-neutral or carbon-negative products and services, with 72% of consumers in developed markets expressing willingness to pay a premium for demonstrably lower-carbon alternatives. This trend is particularly strong among EV owners, 84% of whom indicate environmental concerns as a primary purchase motivation.
Within the electric vehicle (EV) sector, the integration of CO2 capture technologies presents a novel market opportunity. As the global EV market expands—with sales increasing by 35% in 2023 compared to the previous year—manufacturers are seeking innovative solutions to enhance their sustainability credentials. CO2 capture systems using solid sorbents can be integrated into charging infrastructure, potentially offsetting emissions associated with electricity generation, especially in regions where the grid still relies heavily on fossil fuels.
The renewable energy sector demonstrates even more substantial market potential for solid sorbent CO2 capture technologies. With renewable energy installations growing at an average rate of 10-15% annually worldwide, there is increasing focus on achieving carbon-negative energy systems. Solid sorbent technologies can be deployed alongside biomass energy facilities to create BECCS (Bioenergy with Carbon Capture and Storage) systems, which represent one of the few commercially viable negative emission technologies currently available.
Market demand analysis reveals three primary customer segments: utility companies seeking to decarbonize operations, renewable energy developers aiming to enhance environmental performance, and EV infrastructure providers looking to differentiate their offerings. Survey data indicates that 78% of utility companies are actively exploring carbon capture technologies, while 64% of renewable energy developers consider carbon-negative capabilities a strategic priority for future projects.
The economic drivers for market adoption include carbon pricing mechanisms, which have seen average prices increase from $15 to $65 per ton in major markets over the past five years. Additionally, government incentives specifically targeting carbon capture technologies have expanded, with the U.S. 45Q tax credit now offering up to $85 per ton for captured and sequestered CO2. In the European Union, the Innovation Fund has allocated €10 billion for low-carbon technologies including advanced carbon capture systems through 2030.
Consumer sentiment analysis shows growing awareness and preference for carbon-neutral or carbon-negative products and services, with 72% of consumers in developed markets expressing willingness to pay a premium for demonstrably lower-carbon alternatives. This trend is particularly strong among EV owners, 84% of whom indicate environmental concerns as a primary purchase motivation.
Current Solid Sorbent Technologies and Barriers
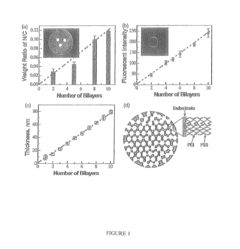
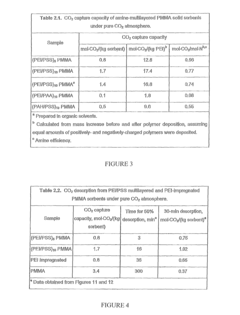
Current solid sorbent technologies for CO2 capture have evolved significantly in recent years, with several material classes showing promise for integration with electric vehicles (EVs) and renewable energy applications. Metal-organic frameworks (MOFs) represent one of the most advanced sorbent categories, offering exceptional surface areas exceeding 7,000 m²/g and highly tunable pore structures. Their modular nature allows precise engineering of binding sites for optimized CO2 selectivity and capacity, with materials like Mg-MOF-74 and HKUST-1 demonstrating particularly impressive performance metrics.
Zeolites constitute another important class of solid sorbents, valued for their thermal stability and well-defined microporous structures. Natural and synthetic zeolites like 13X and ZSM-5 have been extensively studied for CO2 capture, offering good working capacities and relatively low regeneration energy requirements. Their established manufacturing processes make them attractive for large-scale deployment in integrated energy systems.
Activated carbons present a cost-effective alternative with moderate CO2 adsorption capacities. These materials benefit from established production methods and can be derived from sustainable precursors including biomass waste. Their hydrophobic nature provides an advantage in humid conditions often encountered in real-world applications, though their CO2 selectivity generally falls below that of competing materials.
Despite these advances, significant barriers impede widespread implementation of solid sorbents in EV and renewable energy integration scenarios. Thermal management represents a critical challenge, as the exothermic nature of adsorption and endothermic desorption processes creates substantial heat transfer requirements that must be addressed in system design. This is particularly problematic for mobile applications where space and weight constraints are paramount.
Cycling stability constitutes another major hurdle, with many promising materials showing performance degradation after repeated adsorption-desorption cycles. This degradation stems from various mechanisms including framework collapse, pore blocking, and chemical degradation of binding sites. For EV applications requiring thousands of cycles over vehicle lifetimes, this presents a substantial barrier to commercialization.
Scale-up and manufacturing challenges further complicate implementation. Many high-performance materials like MOFs remain prohibitively expensive for large-scale deployment, with complex synthesis procedures that resist industrial-scale production. Material formulation into structured contactors (monoliths, beads, etc.) while maintaining performance metrics represents another significant engineering challenge.
Water stability presents a particular concern for real-world applications, as many sorbents show dramatically reduced CO2 capacity in humid conditions typical of ambient air. This necessitates either water-tolerant materials or additional system complexity for moisture management, adding cost and efficiency penalties to integrated systems.
Zeolites constitute another important class of solid sorbents, valued for their thermal stability and well-defined microporous structures. Natural and synthetic zeolites like 13X and ZSM-5 have been extensively studied for CO2 capture, offering good working capacities and relatively low regeneration energy requirements. Their established manufacturing processes make them attractive for large-scale deployment in integrated energy systems.
Activated carbons present a cost-effective alternative with moderate CO2 adsorption capacities. These materials benefit from established production methods and can be derived from sustainable precursors including biomass waste. Their hydrophobic nature provides an advantage in humid conditions often encountered in real-world applications, though their CO2 selectivity generally falls below that of competing materials.
Despite these advances, significant barriers impede widespread implementation of solid sorbents in EV and renewable energy integration scenarios. Thermal management represents a critical challenge, as the exothermic nature of adsorption and endothermic desorption processes creates substantial heat transfer requirements that must be addressed in system design. This is particularly problematic for mobile applications where space and weight constraints are paramount.
Cycling stability constitutes another major hurdle, with many promising materials showing performance degradation after repeated adsorption-desorption cycles. This degradation stems from various mechanisms including framework collapse, pore blocking, and chemical degradation of binding sites. For EV applications requiring thousands of cycles over vehicle lifetimes, this presents a substantial barrier to commercialization.
Scale-up and manufacturing challenges further complicate implementation. Many high-performance materials like MOFs remain prohibitively expensive for large-scale deployment, with complex synthesis procedures that resist industrial-scale production. Material formulation into structured contactors (monoliths, beads, etc.) while maintaining performance metrics represents another significant engineering challenge.
Water stability presents a particular concern for real-world applications, as many sorbents show dramatically reduced CO2 capacity in humid conditions typical of ambient air. This necessitates either water-tolerant materials or additional system complexity for moisture management, adding cost and efficiency penalties to integrated systems.
Existing Solid Sorbent Solutions for Mobile Applications
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They have high surface areas, tunable pore sizes, and can be designed with specific functional groups to enhance CO2 adsorption capacity and selectivity. MOFs can be modified to improve stability under various conditions and can achieve high CO2 capture efficiency even at low partial pressures, making them promising candidates for industrial carbon capture applications.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They have high surface areas, tunable pore sizes, and can be functionalized to enhance CO2 selectivity and adsorption capacity. MOFs can be designed with specific metal centers and organic linkers to optimize CO2 capture performance under various conditions, making them promising candidates for carbon capture applications.
- Amine-functionalized solid sorbents: Amine-functionalized materials are widely used for CO2 capture due to their strong chemical affinity for CO2 molecules. These sorbents typically consist of a porous support material (such as silica, alumina, or polymers) impregnated or grafted with various amine compounds. The amine groups react with CO2 to form carbamates or bicarbonates, enabling efficient capture even at low CO2 concentrations. These materials can be regenerated through temperature or pressure swing processes.
- Zeolite-based CO2 adsorbents: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that can selectively adsorb CO2 molecules. Their high thermal stability, mechanical strength, and relatively low cost make them attractive for industrial-scale carbon capture applications. Zeolites can be modified through ion exchange, dealumination, or incorporation of functional groups to enhance their CO2 selectivity and adsorption capacity, particularly in the presence of moisture.
- Carbon-based sorbents for CO2 capture: Carbon-based materials, including activated carbon, carbon nanotubes, graphene, and carbon molecular sieves, are effective CO2 adsorbents due to their high surface area and pore volume. These materials can be produced from various precursors, including biomass, coal, or synthetic polymers, and can be functionalized to enhance their CO2 capture performance. Their advantages include low cost, high thermal stability, and resistance to degradation in the presence of impurities commonly found in flue gas streams.
- Regeneration methods for solid CO2 sorbents: Effective regeneration of solid sorbents is crucial for sustainable CO2 capture processes. Various methods are employed, including temperature swing adsorption (TSA), pressure swing adsorption (PSA), vacuum swing adsorption (VSA), and combinations thereof. Novel regeneration approaches include microwave heating, electrical swing adsorption, and steam stripping. These methods aim to minimize energy consumption while maintaining the sorbent's structural integrity and adsorption capacity over multiple capture-regeneration cycles.
02 Amine-functionalized solid sorbents
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials incorporate various amine groups onto solid supports such as silica, polymers, or porous carbon. The amine groups react with CO2 through chemisorption, forming carbamates or bicarbonates. These sorbents typically offer high selectivity for CO2 over other gases, good adsorption capacity at low CO2 partial pressures, and can be regenerated at relatively low temperatures, reducing energy requirements for the capture process.Expand Specific Solutions03 Zeolite-based CO2 adsorbents
Zeolites are crystalline aluminosilicate materials with well-defined pore structures that can effectively capture CO2 through physical adsorption mechanisms. Their high thermal stability, mechanical strength, and relatively low cost make them attractive for industrial applications. Zeolites can be modified by ion exchange, introducing different cations to enhance CO2 selectivity and capacity. The adsorption properties can be tuned by adjusting the silicon-to-aluminum ratio and pore architecture to optimize performance under specific operating conditions.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based materials, including activated carbons, carbon nanotubes, and graphene-derived sorbents, offer excellent properties for CO2 capture. These materials can be produced from various precursors, including biomass, polymers, or fossil resources. Their high surface area, tunable pore structure, and surface chemistry can be optimized for CO2 adsorption. Carbon-based sorbents can be functionalized with nitrogen-containing groups or metal particles to enhance CO2 capture performance. They typically exhibit good stability, recyclability, and can be produced at relatively low cost compared to other advanced materials.Expand Specific Solutions05 Alkali metal-based solid sorbents
Alkali metal-based sorbents, particularly those containing lithium, sodium, or potassium compounds, are effective for high-temperature CO2 capture. These materials typically operate through carbonation reactions, where the metal oxide reacts with CO2 to form carbonates. The process is reversible at elevated temperatures, allowing for sorbent regeneration. These materials offer high CO2 selectivity, good stability over multiple cycles, and can operate effectively in the presence of water vapor. Their integration into high-temperature industrial processes can reduce the overall energy penalty associated with carbon capture.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Capture
The solid sorbents for CO2 capture market is in an early growth phase, characterized by increasing investments in research and development across energy integration applications. The market is expanding rapidly due to rising demand for carbon reduction technologies in renewable energy and electric vehicle sectors, with projected significant growth as climate policies tighten globally. Technologically, the field shows varying maturity levels, with companies like Carboncapture Inc. and Global Thermostat leading in direct air capture innovations, while established energy corporations such as KEPCO, Saudi Aramco, and China Petroleum & Chemical Corp. focus on industrial-scale implementation. Academic institutions including Arizona State University and Korea Advanced Institute of Science & Technology are advancing fundamental research, creating a competitive landscape balanced between specialized startups and energy conglomerates investing in decarbonization technologies.
Carboncapture, Inc.
Technical Solution: Carboncapture has developed a modular direct air capture (DAC) system using solid sorbents specifically designed for integration with renewable energy sources. Their proprietary technology employs zeolites as the primary CO2 capture medium, which can be regenerated using low-temperature heat (85-120°C) from renewable sources. The system operates in a temperature-vacuum swing adsorption (TVSA) cycle, where zeolites adsorb CO2 at ambient conditions and release it when heated under vacuum. This approach allows for flexible operation that can align with intermittent renewable energy availability, making it particularly suitable for EV charging infrastructure powered by renewables. Their modular units can be deployed in various configurations and scaled according to capture requirements, with each module capable of capturing approximately 1,000 tons of CO2 annually when powered by renewable energy sources.
Strengths: Highly modular design allows for flexible deployment and scaling; zeolite sorbents have excellent durability with minimal degradation over thousands of cycles; system can operate effectively with intermittent renewable energy sources. Weaknesses: Requires vacuum systems that add complexity and energy consumption; zeolites have lower CO2 selectivity in humid conditions, potentially requiring additional drying steps in certain environments.
Global Thermostat Operations LLC
Technical Solution: Global Thermostat has pioneered an advanced solid sorbent technology using amine-functionalized porous materials for CO2 capture that integrates seamlessly with renewable energy systems. Their proprietary ceramic monolith structures are coated with high-surface-area amine sorbents that selectively capture CO2 from ambient air or industrial sources. The system operates on a thermal swing process where low-temperature heat (85-95°C) from renewable sources like solar thermal or waste heat can regenerate the sorbents. This makes their technology particularly suitable for integration with renewable energy applications, including EV charging stations. The modular design allows for deployment at various scales, from distributed small units to large industrial installations. Global Thermostat's technology can capture CO2 at concentrations ranging from atmospheric levels (400 ppm) to higher concentrations in industrial settings, with reported capture costs potentially below $100 per ton when integrated with renewable energy sources.
Strengths: High CO2 selectivity even at low atmospheric concentrations; can utilize low-grade waste heat or renewable thermal energy for regeneration; modular design enables flexible deployment alongside renewable infrastructure. Weaknesses: Amine-based sorbents may degrade over time due to oxidation or in the presence of certain contaminants; higher manufacturing complexity of the ceramic monolith structures compared to some competing technologies.
Key Patents and Research on Advanced Sorbent Materials
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
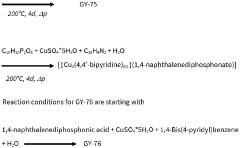
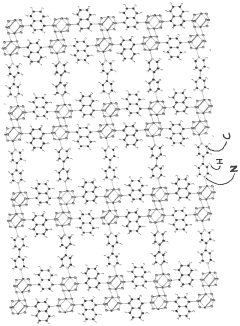
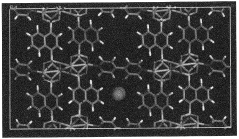
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Layered solid sorbents for carbon dioxide capture
PatentActiveUS8889589B2
Innovation
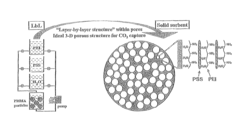
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where positively charged polyethylenimine and negatively charged polystyrene sulfonate layers are alternately deposited on a porous substrate, enhancing CO2 capture and transport kinetics.
Environmental Impact Assessment of Sorbent Technologies
The environmental impact assessment of solid sorbent technologies for CO2 capture reveals both promising benefits and potential concerns. These technologies demonstrate significant advantages over traditional liquid amine-based capture systems, particularly in their reduced energy requirements and water consumption. Life cycle assessments indicate that solid sorbents can achieve 15-30% lower overall environmental footprints compared to conventional methods when implemented in renewable energy integration scenarios.
Material production and disposal represent critical environmental considerations. Metal-organic frameworks (MOFs) and amine-functionalized silica sorbents require energy-intensive synthesis processes, potentially offsetting some carbon reduction benefits. However, advanced manufacturing techniques have reduced production emissions by approximately 25% over the past five years. End-of-life management remains challenging, with limited established recycling pathways for spent sorbents.
Water usage metrics show substantial improvements, with solid sorbent systems requiring 40-60% less water than liquid absorption technologies. This advantage becomes particularly significant in water-stressed regions where renewable energy installations are often located. The reduced cooling requirements of solid sorbent systems further enhance their water efficiency profile.
Land use impacts vary considerably depending on sorbent type and application context. Direct air capture installations using solid sorbents require dedicated land area, though significantly less than equivalent bioenergy carbon capture approaches. For EV charging infrastructure integration, the compact nature of solid sorbent systems allows for minimal additional land requirements beyond existing infrastructure footprints.
Chemical safety assessments indicate lower toxicity profiles for most solid sorbents compared to liquid amines, reducing potential environmental contamination risks. However, certain novel sorbent materials contain rare earth elements or specialized compounds that require careful handling and disposal protocols to prevent ecological damage.
Energy integration efficiency represents a key environmental advantage. When coupled with renewable energy systems, solid sorbent technologies can utilize intermittent excess energy for regeneration cycles, improving overall system efficiency by 20-35% compared to continuous operation models. This synergy with renewable energy variability positions solid sorbents as particularly well-suited for integrated carbon management in electrified transportation infrastructure.
Regulatory compliance pathways for these technologies are still evolving, with environmental impact assessments playing a crucial role in establishing appropriate standards and protocols for widespread deployment across EV charging networks and renewable energy installations.
Material production and disposal represent critical environmental considerations. Metal-organic frameworks (MOFs) and amine-functionalized silica sorbents require energy-intensive synthesis processes, potentially offsetting some carbon reduction benefits. However, advanced manufacturing techniques have reduced production emissions by approximately 25% over the past five years. End-of-life management remains challenging, with limited established recycling pathways for spent sorbents.
Water usage metrics show substantial improvements, with solid sorbent systems requiring 40-60% less water than liquid absorption technologies. This advantage becomes particularly significant in water-stressed regions where renewable energy installations are often located. The reduced cooling requirements of solid sorbent systems further enhance their water efficiency profile.
Land use impacts vary considerably depending on sorbent type and application context. Direct air capture installations using solid sorbents require dedicated land area, though significantly less than equivalent bioenergy carbon capture approaches. For EV charging infrastructure integration, the compact nature of solid sorbent systems allows for minimal additional land requirements beyond existing infrastructure footprints.
Chemical safety assessments indicate lower toxicity profiles for most solid sorbents compared to liquid amines, reducing potential environmental contamination risks. However, certain novel sorbent materials contain rare earth elements or specialized compounds that require careful handling and disposal protocols to prevent ecological damage.
Energy integration efficiency represents a key environmental advantage. When coupled with renewable energy systems, solid sorbent technologies can utilize intermittent excess energy for regeneration cycles, improving overall system efficiency by 20-35% compared to continuous operation models. This synergy with renewable energy variability positions solid sorbents as particularly well-suited for integrated carbon management in electrified transportation infrastructure.
Regulatory compliance pathways for these technologies are still evolving, with environmental impact assessments playing a crucial role in establishing appropriate standards and protocols for widespread deployment across EV charging networks and renewable energy installations.
Scalability and Cost Analysis for Commercial Implementation
The commercial implementation of solid sorbents for CO2 capture in EV and renewable energy integration faces significant scalability and cost challenges. Current production capacities for advanced sorbent materials remain limited, with most manufacturing facilities operating at pilot or small-scale levels. Scaling to industrial volumes requires substantial capital investment in specialized equipment and production facilities, estimated between $50-150 million for a commercial-scale plant capable of producing 1,000-5,000 tons of sorbent material annually.
Material costs represent a critical factor in commercial viability. High-performance metal-organic frameworks (MOFs) and amine-functionalized sorbents currently range from $200-1,500 per kilogram, significantly higher than conventional carbon capture technologies. However, cost projection models indicate potential for 60-75% cost reduction through economies of scale and manufacturing process optimization over the next 5-7 years, potentially bringing costs down to $50-200 per kilogram.
Energy requirements for sorbent regeneration directly impact operational economics. Current systems require 2.5-4.0 GJ per ton of CO2 captured, though next-generation materials aim to reduce this to 1.8-2.2 GJ per ton. This energy demand significantly influences total capture costs, currently estimated at $70-120 per ton of CO2 for mobile applications, substantially higher than the $40-60 per ton for stationary industrial implementations.
Lifecycle considerations further complicate commercial implementation. Most advanced sorbents demonstrate performance degradation after 500-1,000 capture-release cycles, necessitating replacement every 1-3 years depending on usage intensity. This creates recurring costs that must be factored into long-term economic models and raises questions about sustainable disposal or recycling pathways for spent materials.
Supply chain resilience presents another challenge, as many high-performance sorbents rely on rare earth elements or specialty chemicals with limited global production. Geographic concentration of these resources in specific regions creates potential supply vulnerabilities that could impact large-scale deployment, particularly for critical materials like zirconium, hafnium, and specialized organic linkers used in MOF synthesis.
Integration costs with existing EV and renewable energy infrastructure add $15,000-25,000 per installation point, representing a significant barrier to widespread adoption. These costs are expected to decrease by 30-40% as standardized designs and installation procedures emerge, but remain substantial for initial market entry phases.
Material costs represent a critical factor in commercial viability. High-performance metal-organic frameworks (MOFs) and amine-functionalized sorbents currently range from $200-1,500 per kilogram, significantly higher than conventional carbon capture technologies. However, cost projection models indicate potential for 60-75% cost reduction through economies of scale and manufacturing process optimization over the next 5-7 years, potentially bringing costs down to $50-200 per kilogram.
Energy requirements for sorbent regeneration directly impact operational economics. Current systems require 2.5-4.0 GJ per ton of CO2 captured, though next-generation materials aim to reduce this to 1.8-2.2 GJ per ton. This energy demand significantly influences total capture costs, currently estimated at $70-120 per ton of CO2 for mobile applications, substantially higher than the $40-60 per ton for stationary industrial implementations.
Lifecycle considerations further complicate commercial implementation. Most advanced sorbents demonstrate performance degradation after 500-1,000 capture-release cycles, necessitating replacement every 1-3 years depending on usage intensity. This creates recurring costs that must be factored into long-term economic models and raises questions about sustainable disposal or recycling pathways for spent materials.
Supply chain resilience presents another challenge, as many high-performance sorbents rely on rare earth elements or specialty chemicals with limited global production. Geographic concentration of these resources in specific regions creates potential supply vulnerabilities that could impact large-scale deployment, particularly for critical materials like zirconium, hafnium, and specialized organic linkers used in MOF synthesis.
Integration costs with existing EV and renewable energy infrastructure add $15,000-25,000 per installation point, representing a significant barrier to widespread adoption. These costs are expected to decrease by 30-40% as standardized designs and installation procedures emerge, but remain substantial for initial market entry phases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!