Research on Solid sorbents for CO2 capture for advanced porous materials and MOFs
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change and reduce greenhouse gas emissions. The journey began with conventional absorption methods using liquid amines in the 1930s, primarily for natural gas sweetening rather than climate concerns. By the 1990s, as climate change awareness grew, research expanded to explore more efficient and less energy-intensive capture technologies, marking the first generation of purposeful CO2 capture systems.
The early 2000s witnessed a paradigm shift with the emergence of solid sorbents as promising alternatives to liquid-based systems. These materials offered advantages including lower regeneration energy, reduced corrosion issues, and greater operational flexibility. This period saw the initial exploration of zeolites, activated carbons, and early metal-organic frameworks (MOFs) for CO2 capture applications.
The field experienced revolutionary advancement with the rapid development of MOFs starting around 2005. These crystalline porous materials, composed of metal nodes connected by organic linkers, offered unprecedented surface areas exceeding 7,000 m²/g and highly tunable pore structures. Their exceptional CO2 selectivity and capacity positioned them as game-changers in adsorption-based capture technology.
Current research objectives focus on addressing several critical challenges in solid sorbent technology. Primary goals include enhancing CO2 selectivity under realistic flue gas conditions, improving hydrothermal stability for long-term operation, reducing synthesis costs for industrial-scale production, and optimizing regeneration energy requirements. Researchers aim to develop sorbents that maintain performance under the challenging conditions of moisture, contaminants, and temperature fluctuations typical in industrial settings.
The technical objectives specifically for advanced porous materials and MOFs include designing structures with optimized pore sizes (typically 0.7-0.9 nm) that maximize CO2 adsorption while minimizing competitive adsorption of other gases. Engineering functionalized materials with tailored chemical binding sites, particularly nitrogen-rich functional groups, remains a priority for enhancing CO2 affinity without prohibitively increasing regeneration energy.
Looking forward, the field aims to bridge the gap between laboratory discoveries and industrial implementation through scalable synthesis methods and process integration. The ultimate objective is developing next-generation solid sorbents capable of reducing the energy penalty of carbon capture to below 1.0 GJ/ton CO2, making carbon capture economically viable across various industrial sectors and supporting global decarbonization efforts.
The early 2000s witnessed a paradigm shift with the emergence of solid sorbents as promising alternatives to liquid-based systems. These materials offered advantages including lower regeneration energy, reduced corrosion issues, and greater operational flexibility. This period saw the initial exploration of zeolites, activated carbons, and early metal-organic frameworks (MOFs) for CO2 capture applications.
The field experienced revolutionary advancement with the rapid development of MOFs starting around 2005. These crystalline porous materials, composed of metal nodes connected by organic linkers, offered unprecedented surface areas exceeding 7,000 m²/g and highly tunable pore structures. Their exceptional CO2 selectivity and capacity positioned them as game-changers in adsorption-based capture technology.
Current research objectives focus on addressing several critical challenges in solid sorbent technology. Primary goals include enhancing CO2 selectivity under realistic flue gas conditions, improving hydrothermal stability for long-term operation, reducing synthesis costs for industrial-scale production, and optimizing regeneration energy requirements. Researchers aim to develop sorbents that maintain performance under the challenging conditions of moisture, contaminants, and temperature fluctuations typical in industrial settings.
The technical objectives specifically for advanced porous materials and MOFs include designing structures with optimized pore sizes (typically 0.7-0.9 nm) that maximize CO2 adsorption while minimizing competitive adsorption of other gases. Engineering functionalized materials with tailored chemical binding sites, particularly nitrogen-rich functional groups, remains a priority for enhancing CO2 affinity without prohibitively increasing regeneration energy.
Looking forward, the field aims to bridge the gap between laboratory discoveries and industrial implementation through scalable synthesis methods and process integration. The ultimate objective is developing next-generation solid sorbents capable of reducing the energy penalty of carbon capture to below 1.0 GJ/ton CO2, making carbon capture economically viable across various industrial sectors and supporting global decarbonization efforts.
Market Analysis for Carbon Capture Solutions
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.5 billion, with projections indicating a compound annual growth rate of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade. This growth trajectory is supported by substantial government investments, with the US Inflation Reduction Act allocating $369 billion for climate and energy initiatives, including carbon capture technologies.
The demand for advanced solid sorbents, particularly Metal-Organic Frameworks (MOFs) and other porous materials, represents a rapidly expanding segment within this market. These materials offer superior CO2 selectivity and capacity compared to traditional liquid amine scrubbing technologies, which currently dominate approximately 70% of the commercial carbon capture installations.
Industrial sectors constitute the primary market for these technologies, with power generation, cement production, and steel manufacturing collectively accounting for over 60% of the potential application base. The cement industry alone contributes roughly 8% of global CO2 emissions, creating an urgent need for effective capture solutions. Early adopters of solid sorbent technologies include European and North American industrial conglomerates seeking to meet stringent emission reduction targets.
Regional analysis reveals that North America currently leads the carbon capture market with a 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate in the coming years due to rapid industrialization in China and India, coupled with increasing environmental regulations in these countries.
The economic viability of solid sorbent technologies continues to improve, with current capture costs ranging from $50-90 per ton of CO2 for advanced MOF-based systems, compared to $65-120 for conventional amine scrubbing. This cost advantage is driving increased commercial interest, particularly as carbon pricing mechanisms become more widespread globally. The EU Emissions Trading System price has stabilized above €80 per ton of CO2, creating a favorable economic environment for these technologies.
Market barriers include high initial capital requirements, with typical industrial-scale installations requiring investments of $300-500 million, and technological maturity concerns. Despite these challenges, venture capital funding for advanced carbon capture technologies reached $1.9 billion in 2022, a 50% increase from the previous year, indicating strong investor confidence in the sector's future growth potential.
The demand for advanced solid sorbents, particularly Metal-Organic Frameworks (MOFs) and other porous materials, represents a rapidly expanding segment within this market. These materials offer superior CO2 selectivity and capacity compared to traditional liquid amine scrubbing technologies, which currently dominate approximately 70% of the commercial carbon capture installations.
Industrial sectors constitute the primary market for these technologies, with power generation, cement production, and steel manufacturing collectively accounting for over 60% of the potential application base. The cement industry alone contributes roughly 8% of global CO2 emissions, creating an urgent need for effective capture solutions. Early adopters of solid sorbent technologies include European and North American industrial conglomerates seeking to meet stringent emission reduction targets.
Regional analysis reveals that North America currently leads the carbon capture market with a 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate in the coming years due to rapid industrialization in China and India, coupled with increasing environmental regulations in these countries.
The economic viability of solid sorbent technologies continues to improve, with current capture costs ranging from $50-90 per ton of CO2 for advanced MOF-based systems, compared to $65-120 for conventional amine scrubbing. This cost advantage is driving increased commercial interest, particularly as carbon pricing mechanisms become more widespread globally. The EU Emissions Trading System price has stabilized above €80 per ton of CO2, creating a favorable economic environment for these technologies.
Market barriers include high initial capital requirements, with typical industrial-scale installations requiring investments of $300-500 million, and technological maturity concerns. Despite these challenges, venture capital funding for advanced carbon capture technologies reached $1.9 billion in 2022, a 50% increase from the previous year, indicating strong investor confidence in the sector's future growth potential.
Current Solid Sorbents Landscape and Challenges
The current landscape of solid sorbents for CO2 capture is characterized by a diverse array of materials with varying properties and performance metrics. Traditional sorbents such as activated carbons, zeolites, and silica-based materials have been extensively studied and deployed in industrial settings. These materials offer advantages including relatively low cost, established manufacturing processes, and reasonable stability. However, they typically suffer from limited CO2 selectivity, moderate adsorption capacities, and energy-intensive regeneration requirements.
Advanced porous materials, particularly Metal-Organic Frameworks (MOFs), have emerged as promising alternatives due to their exceptional porosity, tunable pore structures, and high surface areas often exceeding 6,000 m²/g. Notable MOFs such as Mg-MOF-74, HKUST-1, and ZIF-8 have demonstrated CO2 uptake capacities significantly higher than conventional sorbents under ambient conditions. The record-holding MOF-210 exhibits a surface area of approximately 6,240 m²/g, highlighting the remarkable potential of these materials.
Despite these advantages, several critical challenges impede the widespread implementation of advanced sorbents for CO2 capture. Stability issues remain paramount, with many high-performing MOFs exhibiting degradation under humid conditions, thermal cycling, or exposure to impurities in flue gas streams. This hydrolytic instability particularly affects MOFs with metal-carboxylate bonds, limiting their practical application in real-world scenarios.
Scalability presents another significant hurdle. While laboratory synthesis of MOFs has been well-established, industrial-scale production faces challenges in maintaining consistent quality, reducing production costs, and developing environmentally benign synthesis routes. Current production methods often involve expensive precursors and harmful solvents, contradicting the sustainability goals of carbon capture technologies.
The regeneration energy requirement remains a critical limitation across all solid sorbents. The energy penalty associated with temperature or pressure swing processes for sorbent regeneration can account for 70-80% of the total energy consumption in carbon capture systems. This significantly impacts the overall efficiency and economic viability of the technology.
Selectivity in mixed gas environments represents another challenge, particularly for flue gas applications where CO2 concentrations typically range from 4-15% alongside nitrogen, oxygen, water vapor, and trace contaminants. Many sorbents that perform well in pure CO2 environments show diminished performance in these complex mixtures.
The geographic distribution of research and development in this field shows concentration in North America, Europe, and East Asia, with emerging contributions from Middle Eastern research institutions leveraging their expertise in hydrocarbon processing. This global research landscape reflects both the universal importance of carbon capture technologies and the varying regional approaches to addressing climate challenges.
Advanced porous materials, particularly Metal-Organic Frameworks (MOFs), have emerged as promising alternatives due to their exceptional porosity, tunable pore structures, and high surface areas often exceeding 6,000 m²/g. Notable MOFs such as Mg-MOF-74, HKUST-1, and ZIF-8 have demonstrated CO2 uptake capacities significantly higher than conventional sorbents under ambient conditions. The record-holding MOF-210 exhibits a surface area of approximately 6,240 m²/g, highlighting the remarkable potential of these materials.
Despite these advantages, several critical challenges impede the widespread implementation of advanced sorbents for CO2 capture. Stability issues remain paramount, with many high-performing MOFs exhibiting degradation under humid conditions, thermal cycling, or exposure to impurities in flue gas streams. This hydrolytic instability particularly affects MOFs with metal-carboxylate bonds, limiting their practical application in real-world scenarios.
Scalability presents another significant hurdle. While laboratory synthesis of MOFs has been well-established, industrial-scale production faces challenges in maintaining consistent quality, reducing production costs, and developing environmentally benign synthesis routes. Current production methods often involve expensive precursors and harmful solvents, contradicting the sustainability goals of carbon capture technologies.
The regeneration energy requirement remains a critical limitation across all solid sorbents. The energy penalty associated with temperature or pressure swing processes for sorbent regeneration can account for 70-80% of the total energy consumption in carbon capture systems. This significantly impacts the overall efficiency and economic viability of the technology.
Selectivity in mixed gas environments represents another challenge, particularly for flue gas applications where CO2 concentrations typically range from 4-15% alongside nitrogen, oxygen, water vapor, and trace contaminants. Many sorbents that perform well in pure CO2 environments show diminished performance in these complex mixtures.
The geographic distribution of research and development in this field shows concentration in North America, Europe, and East Asia, with emerging contributions from Middle Eastern research institutions leveraging their expertise in hydrocarbon processing. This global research landscape reflects both the universal importance of carbon capture technologies and the varying regional approaches to addressing climate challenges.
State-of-the-Art MOF and Porous Material Solutions
01 Metal-Organic Frameworks (MOFs) for CO2 Capture
Metal-Organic Frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated to organic ligands, forming one-, two-, or three-dimensional structures. These materials have exceptionally high surface areas and tunable pore sizes, making them highly effective for CO2 capture applications. The metal centers in MOFs can be designed to have specific interactions with CO2 molecules, enhancing selectivity and adsorption capacity. Various modifications to the organic linkers and metal nodes can optimize the CO2 capture performance under different conditions.- Metal-Organic Frameworks (MOFs) for CO2 capture: Metal-Organic Frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated to organic ligands, forming one-, two-, or three-dimensional structures. These materials have exceptionally high surface areas, tunable pore sizes, and chemical functionality, making them highly effective for selective CO2 adsorption. The metal centers and organic linkers can be modified to enhance CO2 binding affinity and selectivity, while maintaining good regeneration properties under mild conditions.
- Advanced porous materials with functionalized surfaces: Porous materials with chemically modified surfaces can significantly improve CO2 capture performance. These materials include activated carbons, silicas, and polymeric frameworks whose surfaces are functionalized with amine groups, metal sites, or other CO2-philic moieties. The functionalization enhances the interaction between the sorbent surface and CO2 molecules, increasing adsorption capacity and selectivity. These materials often exhibit good stability under repeated adsorption-desorption cycles and can operate effectively at various temperature and pressure conditions.
- Composite and hybrid sorbent materials: Composite and hybrid materials combine different types of sorbents to leverage their complementary properties for enhanced CO2 capture. These materials may integrate MOFs with polymers, incorporate nanoparticles into porous matrices, or combine different classes of porous materials. The resulting composites often show improved mechanical stability, heat transfer properties, and resistance to degradation while maintaining or enhancing CO2 adsorption capacity. These hybrid approaches can overcome limitations of individual material types and provide synergistic effects for CO2 capture applications.
- Process optimization for solid sorbent CO2 capture systems: Process engineering and system design play crucial roles in maximizing the efficiency of solid sorbent-based CO2 capture technologies. This includes optimizing operating parameters such as temperature, pressure, gas flow rates, and cycle times. Advanced process configurations like pressure swing adsorption (PSA), temperature swing adsorption (TSA), and vacuum swing adsorption (VSA) can be tailored to specific sorbent properties. Heat integration strategies and novel regeneration methods are also developed to reduce energy consumption and improve the overall economics of CO2 capture systems.
- Novel manufacturing methods for advanced sorbent materials: Innovative manufacturing techniques are being developed to produce advanced porous materials with controlled structures and properties for CO2 capture. These methods include template-assisted synthesis, hydrothermal/solvothermal approaches, microwave-assisted synthesis, and 3D printing of structured sorbents. These techniques enable precise control over pore size distribution, morphology, and surface chemistry, which are critical for optimizing CO2 adsorption performance. Additionally, scalable production methods are being developed to facilitate the transition from laboratory-scale synthesis to industrial-scale manufacturing of these advanced materials.
02 Advanced Porous Materials for CO2 Adsorption
Advanced porous materials beyond MOFs, such as zeolites, activated carbons, and porous polymers, offer significant potential for CO2 capture. These materials feature high surface areas, controlled pore structures, and can be functionalized to enhance CO2 selectivity. The diverse range of porous materials allows for tailoring specific properties like hydrophobicity, thermal stability, and regeneration capacity. Some advanced materials incorporate amine-functionalization to improve CO2 binding through chemical adsorption mechanisms, while others rely on physical adsorption through optimized pore structures.Expand Specific Solutions03 Composite and Hybrid Sorbent Materials
Composite and hybrid materials combine different types of sorbents to leverage the advantages of each component while mitigating their individual limitations. These materials often integrate MOFs with polymers, inorganic substrates, or other porous materials to create synergistic effects for enhanced CO2 capture. Hybrid materials can offer improved mechanical stability, better heat transfer properties, and enhanced resistance to degradation under industrial conditions. Some composites incorporate both physical and chemical adsorption mechanisms to maximize CO2 uptake across varying temperature and pressure conditions.Expand Specific Solutions04 Process Integration and System Design for CO2 Capture
Effective integration of solid sorbents into practical CO2 capture systems requires specialized process designs and engineering solutions. This includes developing efficient methods for sorbent regeneration, managing heat transfer during adsorption/desorption cycles, and optimizing flow dynamics in fixed or fluidized bed configurations. Advanced system designs incorporate pressure swing adsorption (PSA), temperature swing adsorption (TSA), or vacuum swing adsorption (VSA) techniques to maximize the efficiency of the capture process. Some systems also address challenges related to sorbent degradation, gas contaminants, and energy consumption to make CO2 capture more economically viable.Expand Specific Solutions05 Functionalization and Surface Modification of Sorbents
Chemical functionalization and surface modification of porous materials significantly enhance their CO2 capture performance. Common approaches include amine-grafting, incorporation of open metal sites, and introduction of polar functional groups that increase affinity for CO2. These modifications can be tailored to optimize selectivity for CO2 over other gases like nitrogen or methane, which is crucial for applications such as flue gas treatment or natural gas purification. Advanced functionalization techniques also focus on improving the kinetics of adsorption and desorption, as well as enhancing the stability of the sorbent materials under repeated cycling conditions.Expand Specific Solutions
Leading Organizations in Advanced Porous Materials Research
The solid sorbents for CO2 capture market is in a growth phase, driven by increasing environmental regulations and decarbonization efforts. The global market size is expanding rapidly, with projections indicating substantial growth as carbon capture technologies become essential for climate goals. Technologically, the field is advancing from early commercial applications to more sophisticated solutions, with companies like Carboncapture Inc. and Aspen Aerogels leading commercial deployment. Academic institutions including Rice University, KAUST, and Columbia University are driving fundamental research in advanced porous materials and MOFs, while industrial players such as ExxonMobil, SINOPEC, and Saudi Aramco are scaling up technologies for industrial implementation. The ecosystem demonstrates a healthy balance of academic innovation and industrial application, with increasing cross-sector collaboration accelerating technology maturation.
Carboncapture, Inc.
Technical Solution: CarbonCapture Inc. has developed a modular direct air capture (DAC) system using advanced solid sorbents based on zeolites. Their proprietary technology employs specially engineered molecular sieves with optimized pore structures that selectively capture CO2 from ambient air. The company's approach involves a temperature-vacuum swing adsorption (TVSA) process where ambient air passes through their sorbent materials, which capture CO2 at near-ambient conditions. Once saturated, the modules are heated and placed under vacuum to release concentrated CO2 for permanent storage or utilization. Their system architecture allows for continuous operation through parallel processing of modules in different cycle stages, enabling scalable deployment with minimal downtime[1][2]. CarbonCapture's technology stands out for its use of commercially available zeolites that have been modified to enhance CO2 selectivity while maintaining stability through thousands of adsorption-desorption cycles.
Strengths: Uses commercially available zeolites that are already manufactured at scale, reducing production barriers; modular design allows for flexible deployment and scaling; sorbents demonstrate excellent durability over thousands of cycles. Weaknesses: Zeolites typically require significant energy for regeneration compared to some MOF alternatives; system may have higher capital costs than competing technologies due to the need for vacuum equipment.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed proprietary Metal-Organic Framework (MOF) materials specifically engineered for industrial-scale carbon capture applications. Their technology focuses on hybrid MOF structures that combine exceptional CO2 selectivity with mechanical and chemical stability under real-world industrial conditions. ExxonMobil's approach involves precise control of pore architecture and surface chemistry to maximize CO2 adsorption capacity while minimizing energy requirements for regeneration. Their MOF materials are designed to operate effectively in the presence of moisture and contaminants typically found in flue gas streams. The company has integrated these advanced sorbents into pressure swing adsorption (PSA) and temperature swing adsorption (TSA) systems optimized for different capture scenarios, from natural gas processing to post-combustion capture at power plants[5]. ExxonMobil has also developed innovative manufacturing techniques to produce these materials at scale, addressing one of the key barriers to commercial deployment of MOF-based capture technologies. Their research includes carbon capture solutions for hard-to-abate sectors like cement and steel production[6].
Strengths: Extensive industrial expertise and infrastructure for scaling and implementing carbon capture technologies; significant financial resources for R&D and commercialization; integrated approach addressing the entire carbon capture value chain. Weaknesses: As a fossil fuel company, faces potential conflicts between carbon capture investments and core business; proprietary nature of technology may limit broader scientific collaboration and advancement in the field.
Breakthrough Patents in Solid Sorbent Technology
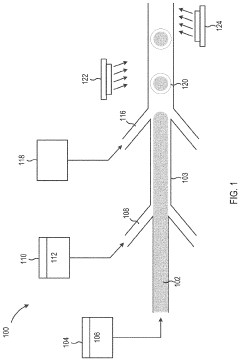
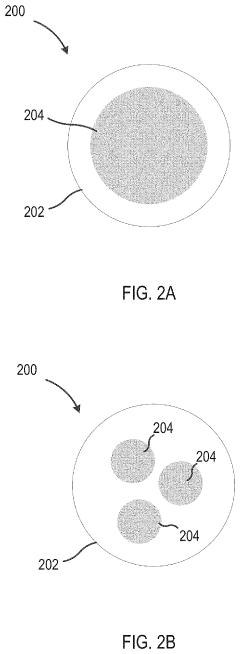
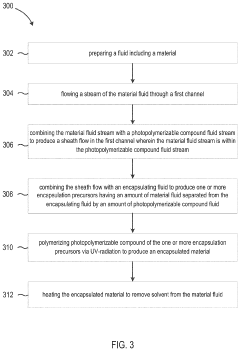
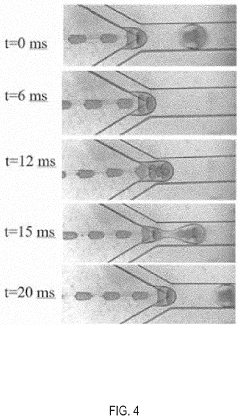
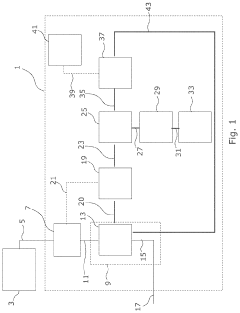
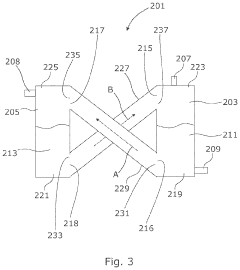
Methods, systems, and apparatus for encapsulating a sequestration medium
PatentInactiveUS20210060512A1
Innovation
- An apparatus and method for encapsulating carbon sequestration media, such as nanoparticle organic hybrid materials and MOFs, using a multi-channel system with photopolymerizable compounds and UV-radiation to create a permeable polymer shell, allowing for efficient CO2 capture and handling of fine particulate matter.
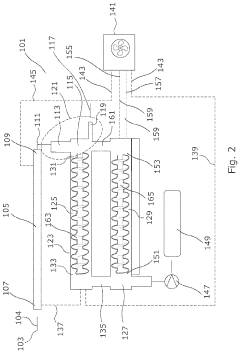
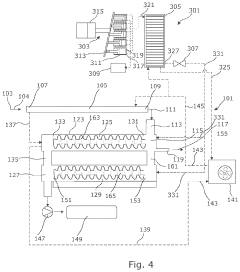
Carbon capture device
PatentPendingUS20240066461A1
Innovation
- A carbon capture device with a flue gas input and output, featuring a carbon adsorption zone with CO2 sorbent material and a desorption zone, where CO2-rich sorbent material is transported between zones using an actuator, allowing for continuous CO2 capture and reuse, reducing the need for new infrastructure and battery dependency.
Environmental Impact Assessment of Capture Technologies
The environmental impact of CO2 capture technologies extends far beyond their primary function of reducing greenhouse gas emissions. When evaluating solid sorbents like advanced porous materials and Metal-Organic Frameworks (MOFs), a comprehensive assessment reveals both benefits and potential concerns across multiple environmental dimensions.
The primary environmental benefit of these capture technologies is their potential to significantly reduce atmospheric CO2 concentrations, directly addressing climate change mitigation. Studies indicate that MOF-based capture systems can achieve up to 90% CO2 removal efficiency under optimal conditions, substantially reducing emissions from point sources such as power plants and industrial facilities.
However, the environmental footprint of manufacturing these advanced materials warrants careful consideration. The synthesis of MOFs typically involves solvothermal processes requiring significant energy inputs and potentially hazardous solvents like dimethylformamide (DMF). Life cycle assessments indicate that the energy-intensive production of MOFs can partially offset their carbon reduction benefits, particularly when renewable energy sources are not utilized in their manufacture.
Water consumption represents another critical environmental consideration. While some solid sorbents demonstrate excellent water stability, others may require substantial water resources for regeneration cycles or cooling systems. This poses particular challenges in water-stressed regions where deployment of these technologies might exacerbate existing resource pressures.
Land use impacts vary significantly between different capture technologies. Solid sorbent systems generally offer more compact footprints compared to traditional amine scrubbing technologies, potentially reducing habitat disruption and land transformation. MOF-based systems, in particular, can achieve higher capture densities per unit area, minimizing spatial requirements.
The fate of captured CO2 also influences the overall environmental assessment. When coupled with geological sequestration, these technologies can achieve near-permanent carbon removal. However, potential risks of leakage and induced seismicity must be monitored. Alternatively, utilizing captured CO2 for enhanced oil recovery may partially undermine climate benefits through continued fossil fuel extraction.
Waste management considerations include the disposal or recycling of spent sorbent materials. The potential toxicity and degradation pathways of novel MOFs remain under investigation, with some materials demonstrating promising recyclability while others may present end-of-life management challenges. Recent advances in green synthesis approaches and biodegradable MOF designs show promise for reducing these environmental concerns.
The primary environmental benefit of these capture technologies is their potential to significantly reduce atmospheric CO2 concentrations, directly addressing climate change mitigation. Studies indicate that MOF-based capture systems can achieve up to 90% CO2 removal efficiency under optimal conditions, substantially reducing emissions from point sources such as power plants and industrial facilities.
However, the environmental footprint of manufacturing these advanced materials warrants careful consideration. The synthesis of MOFs typically involves solvothermal processes requiring significant energy inputs and potentially hazardous solvents like dimethylformamide (DMF). Life cycle assessments indicate that the energy-intensive production of MOFs can partially offset their carbon reduction benefits, particularly when renewable energy sources are not utilized in their manufacture.
Water consumption represents another critical environmental consideration. While some solid sorbents demonstrate excellent water stability, others may require substantial water resources for regeneration cycles or cooling systems. This poses particular challenges in water-stressed regions where deployment of these technologies might exacerbate existing resource pressures.
Land use impacts vary significantly between different capture technologies. Solid sorbent systems generally offer more compact footprints compared to traditional amine scrubbing technologies, potentially reducing habitat disruption and land transformation. MOF-based systems, in particular, can achieve higher capture densities per unit area, minimizing spatial requirements.
The fate of captured CO2 also influences the overall environmental assessment. When coupled with geological sequestration, these technologies can achieve near-permanent carbon removal. However, potential risks of leakage and induced seismicity must be monitored. Alternatively, utilizing captured CO2 for enhanced oil recovery may partially undermine climate benefits through continued fossil fuel extraction.
Waste management considerations include the disposal or recycling of spent sorbent materials. The potential toxicity and degradation pathways of novel MOFs remain under investigation, with some materials demonstrating promising recyclability while others may present end-of-life management challenges. Recent advances in green synthesis approaches and biodegradable MOF designs show promise for reducing these environmental concerns.
Scalability and Industrial Implementation Strategies
The transition from laboratory-scale research to industrial implementation represents a critical challenge for solid sorbents in CO2 capture applications, particularly for advanced porous materials and MOFs. Current industrial deployment remains limited despite promising laboratory results, primarily due to scalability constraints in material synthesis and system integration.
Manufacturing scale-up of MOFs and advanced porous materials faces significant hurdles in maintaining consistent quality, structural integrity, and adsorption performance. Traditional batch synthesis methods that work effectively at gram-scale in laboratories often encounter efficiency losses and quality control issues when scaled to kilogram or ton quantities required for industrial implementation. Recent advancements in continuous flow synthesis and mechanochemical approaches show promise for overcoming these limitations, potentially reducing production costs by 30-40% while maintaining material performance.
System engineering considerations present another critical dimension for industrial implementation. The integration of solid sorbents into existing power plants or industrial facilities requires careful design of adsorption columns, heat management systems, and regeneration processes. Fixed-bed, fluidized-bed, and moving-bed configurations each offer distinct advantages depending on the specific application environment and sorbent characteristics. Pressure-swing adsorption (PSA) and temperature-swing adsorption (TSA) systems must be optimized for the unique properties of advanced porous materials to maximize CO2 capture efficiency while minimizing energy penalties.
Economic viability remains a decisive factor in industrial adoption. Current cost estimates for MOF-based capture systems range from $50-80 per ton of CO2 captured, which exceeds the economically viable threshold for many industries. Pathways to cost reduction include optimizing sorbent lifetime through enhanced hydrothermal stability, reducing regeneration energy requirements, and developing more efficient synthesis routes. Multi-cycle stability under real industrial conditions represents a particular challenge, with performance degradation over thousands of cycles still requiring significant improvement.
Regulatory frameworks and carbon pricing mechanisms will substantially influence implementation timelines. Regions with established carbon markets or stringent emissions regulations provide more favorable conditions for early industrial adoption. Strategic partnerships between material developers, engineering firms, and end-users have proven essential for successful technology transfer, as demonstrated by pilot projects in Europe and East Asia that have achieved capture capacities of 1-10 tons CO2 per day using advanced sorbent technologies.
Manufacturing scale-up of MOFs and advanced porous materials faces significant hurdles in maintaining consistent quality, structural integrity, and adsorption performance. Traditional batch synthesis methods that work effectively at gram-scale in laboratories often encounter efficiency losses and quality control issues when scaled to kilogram or ton quantities required for industrial implementation. Recent advancements in continuous flow synthesis and mechanochemical approaches show promise for overcoming these limitations, potentially reducing production costs by 30-40% while maintaining material performance.
System engineering considerations present another critical dimension for industrial implementation. The integration of solid sorbents into existing power plants or industrial facilities requires careful design of adsorption columns, heat management systems, and regeneration processes. Fixed-bed, fluidized-bed, and moving-bed configurations each offer distinct advantages depending on the specific application environment and sorbent characteristics. Pressure-swing adsorption (PSA) and temperature-swing adsorption (TSA) systems must be optimized for the unique properties of advanced porous materials to maximize CO2 capture efficiency while minimizing energy penalties.
Economic viability remains a decisive factor in industrial adoption. Current cost estimates for MOF-based capture systems range from $50-80 per ton of CO2 captured, which exceeds the economically viable threshold for many industries. Pathways to cost reduction include optimizing sorbent lifetime through enhanced hydrothermal stability, reducing regeneration energy requirements, and developing more efficient synthesis routes. Multi-cycle stability under real industrial conditions represents a particular challenge, with performance degradation over thousands of cycles still requiring significant improvement.
Regulatory frameworks and carbon pricing mechanisms will substantially influence implementation timelines. Regions with established carbon markets or stringent emissions regulations provide more favorable conditions for early industrial adoption. Strategic partnerships between material developers, engineering firms, and end-users have proven essential for successful technology transfer, as demonstrated by pilot projects in Europe and East Asia that have achieved capture capacities of 1-10 tons CO2 per day using advanced sorbent technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!