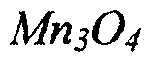Analysis of Spray Pyrolysis in Pharmaceutical Particle Design
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spray Pyrolysis Evolution and Objectives
Spray pyrolysis has evolved significantly since its inception in the 1950s, initially developed for ceramic and metal oxide powder production. The technique has undergone substantial refinement over subsequent decades, transitioning from basic laboratory applications to sophisticated industrial processes. By the 1980s, researchers began exploring its potential in pharmaceutical applications, recognizing its unique ability to create particles with controlled morphology and composition.
The evolution of spray pyrolysis in pharmaceutical particle design has been driven by increasing demands for advanced drug delivery systems with enhanced bioavailability and targeted release profiles. Traditional pharmaceutical manufacturing methods often produce particles with inconsistent size distribution and suboptimal dissolution properties, limiting therapeutic efficacy. Spray pyrolysis emerged as a promising alternative due to its capacity to generate uniform, spherical particles with tailored characteristics.
Recent technological advancements have significantly expanded the capabilities of spray pyrolysis in pharmaceutical applications. The integration of precise temperature control systems, advanced atomization technologies, and real-time monitoring tools has enabled unprecedented control over particle formation mechanisms. These developments have facilitated the production of complex pharmaceutical formulations, including sustained-release medications, inhalable therapies, and nanostructured drug carriers.
The primary objective of spray pyrolysis in pharmaceutical particle design is to develop a versatile, scalable manufacturing platform capable of producing drug particles with precisely controlled physical and chemical properties. This includes achieving narrow particle size distributions, specific surface characteristics, and predetermined crystallinity levels—all critical factors affecting drug performance in vivo.
Another key objective is to enhance the bioavailability of poorly water-soluble drugs, which constitute approximately 40% of approved pharmaceuticals and nearly 90% of developmental pipeline compounds. Spray pyrolysis offers unique advantages in addressing this challenge through the creation of amorphous solid dispersions and nanostructured formulations that significantly improve dissolution rates.
Process optimization represents a crucial objective, focusing on increasing production efficiency while maintaining strict quality standards. This involves developing robust process parameters that ensure batch-to-batch consistency and comply with stringent regulatory requirements for pharmaceutical manufacturing.
Looking forward, the field aims to establish spray pyrolysis as a platform technology for personalized medicine, enabling on-demand production of customized pharmaceutical formulations tailored to individual patient needs. This ambitious goal requires further refinement of process controls and deeper understanding of structure-property relationships in spray-pyrolyzed pharmaceutical particles.
The evolution of spray pyrolysis in pharmaceutical particle design has been driven by increasing demands for advanced drug delivery systems with enhanced bioavailability and targeted release profiles. Traditional pharmaceutical manufacturing methods often produce particles with inconsistent size distribution and suboptimal dissolution properties, limiting therapeutic efficacy. Spray pyrolysis emerged as a promising alternative due to its capacity to generate uniform, spherical particles with tailored characteristics.
Recent technological advancements have significantly expanded the capabilities of spray pyrolysis in pharmaceutical applications. The integration of precise temperature control systems, advanced atomization technologies, and real-time monitoring tools has enabled unprecedented control over particle formation mechanisms. These developments have facilitated the production of complex pharmaceutical formulations, including sustained-release medications, inhalable therapies, and nanostructured drug carriers.
The primary objective of spray pyrolysis in pharmaceutical particle design is to develop a versatile, scalable manufacturing platform capable of producing drug particles with precisely controlled physical and chemical properties. This includes achieving narrow particle size distributions, specific surface characteristics, and predetermined crystallinity levels—all critical factors affecting drug performance in vivo.
Another key objective is to enhance the bioavailability of poorly water-soluble drugs, which constitute approximately 40% of approved pharmaceuticals and nearly 90% of developmental pipeline compounds. Spray pyrolysis offers unique advantages in addressing this challenge through the creation of amorphous solid dispersions and nanostructured formulations that significantly improve dissolution rates.
Process optimization represents a crucial objective, focusing on increasing production efficiency while maintaining strict quality standards. This involves developing robust process parameters that ensure batch-to-batch consistency and comply with stringent regulatory requirements for pharmaceutical manufacturing.
Looking forward, the field aims to establish spray pyrolysis as a platform technology for personalized medicine, enabling on-demand production of customized pharmaceutical formulations tailored to individual patient needs. This ambitious goal requires further refinement of process controls and deeper understanding of structure-property relationships in spray-pyrolyzed pharmaceutical particles.
Pharmaceutical Market Needs for Advanced Particle Engineering
The pharmaceutical industry is experiencing a paradigm shift in drug formulation and delivery systems, creating an urgent demand for advanced particle engineering technologies. Traditional manufacturing methods often struggle to achieve precise control over particle properties such as size distribution, morphology, crystallinity, and surface characteristics - all critical factors affecting drug bioavailability, stability, and efficacy. This limitation has created a significant market gap that spray pyrolysis technology is uniquely positioned to address.
Recent market analyses indicate that pharmaceutical companies are increasingly seeking technologies that can produce particles with tailored characteristics to enhance drug performance. The growing prevalence of poorly water-soluble active pharmaceutical ingredients (APIs) presents a particular challenge, with approximately 70% of new chemical entities exhibiting low solubility. This has intensified the need for particle engineering approaches that can improve dissolution rates and bioavailability without compromising stability.
The rise of targeted and personalized medicine has further amplified demand for precise particle design capabilities. Delivery systems requiring specific release profiles, tissue targeting, or cellular uptake characteristics necessitate unprecedented control over particle properties at the micro and nano scales. Spray pyrolysis offers the potential to meet these requirements through its versatility in producing particles with customizable attributes.
Regulatory pressures are also driving market needs for more consistent and reproducible manufacturing processes. Quality by Design (QbD) initiatives from regulatory bodies worldwide emphasize the importance of understanding how manufacturing parameters influence final product characteristics. Technologies like spray pyrolysis that offer precise control and scalability align well with these regulatory expectations.
The global market for advanced drug delivery systems is projected to grow substantially, creating opportunities for innovative particle engineering technologies. Particular growth is anticipated in areas such as pulmonary drug delivery, where particle size and aerodynamic properties directly impact therapeutic efficacy. The inhalation drug delivery market segment specifically values technologies that can produce respirable particles with controlled characteristics.
Cost considerations remain significant, with pharmaceutical manufacturers seeking technologies that can reduce production costs while maintaining or improving product quality. Spray pyrolysis potentially offers advantages through simplified processing steps, reduced waste generation, and improved batch-to-batch consistency compared to conventional methods.
Environmental sustainability has emerged as another key market driver, with increasing preference for green manufacturing processes that minimize solvent use and energy consumption. Technologies that can operate with reduced environmental impact while maintaining performance are gaining competitive advantage in the current market landscape.
Recent market analyses indicate that pharmaceutical companies are increasingly seeking technologies that can produce particles with tailored characteristics to enhance drug performance. The growing prevalence of poorly water-soluble active pharmaceutical ingredients (APIs) presents a particular challenge, with approximately 70% of new chemical entities exhibiting low solubility. This has intensified the need for particle engineering approaches that can improve dissolution rates and bioavailability without compromising stability.
The rise of targeted and personalized medicine has further amplified demand for precise particle design capabilities. Delivery systems requiring specific release profiles, tissue targeting, or cellular uptake characteristics necessitate unprecedented control over particle properties at the micro and nano scales. Spray pyrolysis offers the potential to meet these requirements through its versatility in producing particles with customizable attributes.
Regulatory pressures are also driving market needs for more consistent and reproducible manufacturing processes. Quality by Design (QbD) initiatives from regulatory bodies worldwide emphasize the importance of understanding how manufacturing parameters influence final product characteristics. Technologies like spray pyrolysis that offer precise control and scalability align well with these regulatory expectations.
The global market for advanced drug delivery systems is projected to grow substantially, creating opportunities for innovative particle engineering technologies. Particular growth is anticipated in areas such as pulmonary drug delivery, where particle size and aerodynamic properties directly impact therapeutic efficacy. The inhalation drug delivery market segment specifically values technologies that can produce respirable particles with controlled characteristics.
Cost considerations remain significant, with pharmaceutical manufacturers seeking technologies that can reduce production costs while maintaining or improving product quality. Spray pyrolysis potentially offers advantages through simplified processing steps, reduced waste generation, and improved batch-to-batch consistency compared to conventional methods.
Environmental sustainability has emerged as another key market driver, with increasing preference for green manufacturing processes that minimize solvent use and energy consumption. Technologies that can operate with reduced environmental impact while maintaining performance are gaining competitive advantage in the current market landscape.
Current Spray Pyrolysis Technology Landscape and Barriers
Spray pyrolysis technology in pharmaceutical particle design has evolved significantly over the past two decades, transitioning from laboratory-scale experimentation to industrial applications. Currently, the global landscape shows varied adoption rates, with advanced pharmaceutical manufacturing hubs in North America, Europe, and parts of Asia leading implementation. The technology offers precise control over particle morphology, size distribution, and crystallinity—critical parameters for drug delivery systems and bioavailability enhancement.
Despite its advantages, spray pyrolysis faces several technical barriers that limit widespread adoption. The primary challenge remains scalability, as maintaining uniform particle characteristics during scale-up from laboratory to industrial production often results in quality inconsistencies. Temperature gradient control within large-scale reactors presents significant engineering difficulties, leading to batch-to-batch variability that is unacceptable in pharmaceutical manufacturing.
Energy efficiency represents another substantial barrier, with conventional spray pyrolysis systems requiring high thermal inputs that increase production costs and environmental impact. Current systems typically operate at energy efficiency rates of 40-60%, significantly lower than alternative particle engineering technologies. This inefficiency stems from heat loss during atomization and incomplete solvent evaporation processes.
Precursor solution formulation complexity creates additional challenges, particularly for heat-sensitive pharmaceutical compounds. Many active pharmaceutical ingredients (APIs) undergo degradation at the temperatures required for conventional spray pyrolysis (typically 150-300°C), limiting application scope. Current workarounds involve complex solvent systems and additives that introduce regulatory complications and stability concerns.
Equipment standardization remains underdeveloped, with most systems being custom-engineered for specific applications. This lack of standardization increases implementation costs and creates barriers to technology transfer between research and manufacturing environments. The absence of unified design principles also complicates regulatory approval pathways, as validation protocols vary significantly between equipment configurations.
Process control automation represents a significant gap in current technology. Real-time monitoring capabilities for critical process parameters such as droplet size, temperature distribution, and residence time remain limited. Most systems rely on indirect measurements and post-production quality testing rather than integrated process analytical technology (PAT) approaches recommended by regulatory authorities.
Regulatory uncertainty further complicates the landscape, as spray pyrolysis represents a relatively novel manufacturing approach for pharmaceuticals. Current good manufacturing practice (cGMP) guidelines specific to spray pyrolysis are still evolving, creating compliance challenges for early adopters. Documentation requirements and validation approaches vary significantly between regulatory jurisdictions, adding complexity to global implementation strategies.
Despite its advantages, spray pyrolysis faces several technical barriers that limit widespread adoption. The primary challenge remains scalability, as maintaining uniform particle characteristics during scale-up from laboratory to industrial production often results in quality inconsistencies. Temperature gradient control within large-scale reactors presents significant engineering difficulties, leading to batch-to-batch variability that is unacceptable in pharmaceutical manufacturing.
Energy efficiency represents another substantial barrier, with conventional spray pyrolysis systems requiring high thermal inputs that increase production costs and environmental impact. Current systems typically operate at energy efficiency rates of 40-60%, significantly lower than alternative particle engineering technologies. This inefficiency stems from heat loss during atomization and incomplete solvent evaporation processes.
Precursor solution formulation complexity creates additional challenges, particularly for heat-sensitive pharmaceutical compounds. Many active pharmaceutical ingredients (APIs) undergo degradation at the temperatures required for conventional spray pyrolysis (typically 150-300°C), limiting application scope. Current workarounds involve complex solvent systems and additives that introduce regulatory complications and stability concerns.
Equipment standardization remains underdeveloped, with most systems being custom-engineered for specific applications. This lack of standardization increases implementation costs and creates barriers to technology transfer between research and manufacturing environments. The absence of unified design principles also complicates regulatory approval pathways, as validation protocols vary significantly between equipment configurations.
Process control automation represents a significant gap in current technology. Real-time monitoring capabilities for critical process parameters such as droplet size, temperature distribution, and residence time remain limited. Most systems rely on indirect measurements and post-production quality testing rather than integrated process analytical technology (PAT) approaches recommended by regulatory authorities.
Regulatory uncertainty further complicates the landscape, as spray pyrolysis represents a relatively novel manufacturing approach for pharmaceuticals. Current good manufacturing practice (cGMP) guidelines specific to spray pyrolysis are still evolving, creating compliance challenges for early adopters. Documentation requirements and validation approaches vary significantly between regulatory jurisdictions, adding complexity to global implementation strategies.
Contemporary Spray Pyrolysis Approaches for Drug Particle Design
01 Precursor solution composition for spray pyrolysis
The composition of precursor solutions plays a crucial role in spray pyrolysis particle design. By carefully selecting and combining metal salts, organic compounds, and solvents, researchers can control the morphology, size distribution, and crystallinity of the resulting particles. Adjusting parameters such as concentration, pH, and viscosity of the precursor solution enables the tailoring of particle properties for specific applications.- Precursor solution composition for spray pyrolysis: The composition of precursor solutions plays a crucial role in spray pyrolysis particle design. By carefully selecting and combining metal salts, organic compounds, and solvents, researchers can control the morphology, size distribution, and crystallinity of the resulting particles. Adjusting parameters such as concentration, pH, and viscosity of the precursor solution enables the tailoring of particle properties for specific applications.
- Process parameters optimization for particle control: Various process parameters in spray pyrolysis significantly influence particle characteristics. These include atomization method, droplet size, reaction temperature, residence time, and gas flow rate. By optimizing these parameters, researchers can achieve precise control over particle size, density, porosity, and agglomeration behavior. Advanced monitoring and feedback systems allow for real-time adjustments to maintain consistent particle quality during production.
- Multi-component and core-shell particle synthesis: Spray pyrolysis enables the synthesis of complex multi-component and core-shell structured particles. By using multiple precursors or sequential deposition techniques, particles with layered structures or homogeneously distributed components can be produced. These advanced particle architectures offer enhanced properties such as improved catalytic activity, controlled release behavior, or unique optical and electronic characteristics for applications in energy storage, catalysis, and biomedicine.
- Nanoparticle design and size control methods: Specialized techniques in spray pyrolysis allow for the precise design and control of nanoparticle characteristics. Methods such as ultrasonic atomization, electrospray, and flame-assisted pyrolysis enable the production of nanoparticles with narrow size distributions and controlled morphologies. Additional strategies include the use of templating agents, surfactants, and post-processing treatments to further refine nanoparticle properties for advanced applications in electronics, sensors, and drug delivery systems.
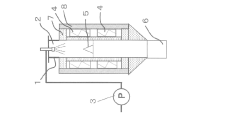
- Equipment design and scale-up considerations: The design of spray pyrolysis equipment significantly impacts particle characteristics and production efficiency. Innovations in reactor design, atomization systems, and collection methods enable improved control over particle properties and higher throughput. Considerations for industrial scale-up include continuous operation capabilities, energy efficiency, precursor delivery systems, and particle collection efficiency. Advanced modeling and simulation tools help optimize equipment design for specific particle production requirements.
02 Temperature control and thermal treatment in spray pyrolysis
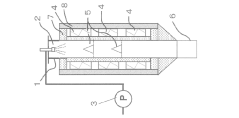
Temperature management during spray pyrolysis significantly impacts particle formation and characteristics. The process typically involves controlling the heating rate, maximum temperature, and cooling profile to achieve desired particle properties. Thermal treatment parameters affect particle crystallization, densification, and phase composition. Post-synthesis heat treatment can further modify particle structure, removing impurities and enhancing specific properties for applications in catalysis, electronics, and energy storage.Expand Specific Solutions03 Nozzle and atomization technology for particle size control
The design and operation of atomization systems are critical factors in controlling particle size and morphology in spray pyrolysis. Different nozzle types (ultrasonic, pressure, two-fluid) produce distinct droplet size distributions that directly translate to final particle characteristics. Adjusting parameters such as frequency, gas flow rate, and solution feed rate allows for precise control over the atomization process, enabling the production of particles with tailored size distributions ranging from nanometers to micrometers.Expand Specific Solutions04 Carrier gas composition and flow dynamics
The carrier gas used in spray pyrolysis significantly influences particle formation and properties. By selecting appropriate gases (air, nitrogen, argon, or reactive gases) and controlling flow rates, researchers can modify the reaction environment to achieve desired particle characteristics. Gas flow dynamics affect droplet transport, evaporation rates, and residence time in the reactor, which in turn determine particle size, morphology, and composition. Reactive carrier gases can also participate in the formation process, enabling in-situ surface modification of particles.Expand Specific Solutions05 Multi-component and core-shell particle synthesis
Advanced spray pyrolysis techniques enable the synthesis of complex multi-component and core-shell particles with enhanced functionalities. By using multiple precursors or sequential deposition approaches, researchers can create particles with controlled composition gradients or distinct core-shell structures. These sophisticated particle designs offer advantages in various applications, including improved catalytic activity, enhanced electrical properties, and controlled drug release. The process parameters can be optimized to achieve precise control over the spatial distribution of components within individual particles.Expand Specific Solutions
Leading Companies and Research Institutions in Spray Pyrolysis
Spray pyrolysis technology in pharmaceutical particle design is currently in a growth phase, with an expanding market driven by increasing demand for advanced drug delivery systems. The global market for this technology is estimated to reach significant scale as pharmaceutical companies seek innovative particle engineering solutions. Technologically, spray pyrolysis is advancing toward maturity, with key players demonstrating various levels of expertise. Companies like Novartis AG, Vertex Pharmaceuticals, and Novo Nordisk are leading commercial applications, while research institutions such as UNC Chapel Hill and Washington University in St. Louis contribute fundamental advancements. Pharmaceutical giants including Boehringer Ingelheim, Merck Patent GmbH, and GlaxoSmithKline are investing in this technology to enhance drug bioavailability and controlled release capabilities, indicating strong industry confidence in its future potential.
Novartis AG
Technical Solution: Novartis has developed an advanced spray pyrolysis platform for pharmaceutical particle engineering that enables precise control over particle morphology, size distribution, and crystallinity. Their technology utilizes a multi-nozzle system with controlled atomization parameters to produce uniform drug particles with enhanced bioavailability. The process incorporates real-time monitoring systems that adjust spray conditions based on feedback loops, ensuring consistent particle characteristics across batches. Novartis has particularly focused on developing spray pyrolysis methods for poorly water-soluble drugs, creating amorphous solid dispersions that significantly improve dissolution rates. Their system integrates specialized drying chambers with controlled temperature gradients that prevent premature crystallization while maintaining API stability. Recent innovations include the incorporation of excipients during the spray process to create pre-formulated particles ready for final dosage form development.
Strengths: Superior control over particle characteristics leading to enhanced bioavailability; scalable process suitable for commercial production; ability to handle thermally sensitive compounds. Weaknesses: Higher capital investment compared to conventional methods; complex process parameters requiring sophisticated control systems; potential challenges with certain solvent systems.
Merck Patent GmbH
Technical Solution: Merck has developed a sophisticated spray pyrolysis platform called "PrecisionSpray" that focuses on creating engineered pharmaceutical particles with enhanced stability and bioavailability profiles. Their technology employs a combination of electrohydrodynamic atomization and controlled thermal processing to achieve unprecedented control over particle architecture. The system utilizes specialized precursor formulations that incorporate stabilizing excipients directly into the spray solution, resulting in particles with integrated stability-enhancing features. Merck's approach includes a patented multi-zone thermal processing chamber that creates carefully controlled temperature gradients, allowing for precise manipulation of crystallization kinetics during particle formation. This enables the production of particles with tailored solid-state properties, including fully amorphous, partially crystalline, or fully crystalline structures depending on therapeutic requirements. The technology has been particularly successful in developing particles for sustained-release formulations, where specific dissolution profiles can be engineered through structural modifications during the spray pyrolysis process.
Strengths: Exceptional control over solid-state properties enabling customized release profiles; integrated excipients improve stability and functionality; highly reproducible process with minimal batch-to-batch variation. Weaknesses: Complex technology requiring specialized expertise; higher production costs compared to conventional methods; limited throughput for certain complex particle architectures.
Critical Patents and Innovations in Pharmaceutical Spray Pyrolysis
Spray pyrolysis method for in SITU production of graphene oxide based composites
PatentWO2012155196A1
Innovation
- A spray pyrolysis method is developed to produce graphene oxide-based nanocomposites in situ, involving the spraying of graphene oxide and precursors into a reaction chamber followed by heating, which allows for scalable, cost-effective, and highly homogeneous production of materials like GO-Mn2O3 and GO-Co3O4 composites with enhanced specific capacitance.
Fine particle manufacturing device by spray pyrolysis
PatentActiveJP2019122925A
Innovation
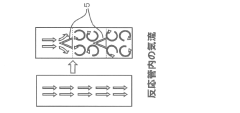
- A conical structure is installed inside the reaction tube with a hollow bottom and apex facing the spray nozzle, generating a venturi effect to increase mist retention time and promote turbulent airflow for uniform heating, using multiple temperature zones for drying, pyrolysis, and melting.
Regulatory Framework for Spray Pyrolysis in Pharmaceuticals
The regulatory landscape governing spray pyrolysis in pharmaceutical manufacturing is complex and multifaceted, requiring careful navigation by industry stakeholders. The FDA in the United States and the EMA in Europe have established specific guidelines for novel particle engineering technologies, including spray pyrolysis processes. These regulations primarily focus on ensuring product quality, safety, and consistency when implementing such advanced manufacturing techniques.
Quality by Design (QbD) principles have become increasingly important in the regulatory framework, with agencies requiring pharmaceutical companies to demonstrate thorough understanding of critical process parameters in spray pyrolysis that affect final product attributes. This includes detailed characterization of temperature profiles, precursor solution properties, and atomization parameters that influence particle morphology and drug release kinetics.
Current Good Manufacturing Practice (cGMP) compliance presents unique challenges for spray pyrolysis implementation, particularly regarding process validation and scale-up considerations. Regulatory bodies typically require extensive documentation of process controls, in-process testing protocols, and validation strategies specific to spray pyrolysis equipment and procedures.
Environmental regulations also impact spray pyrolysis adoption in pharmaceutical manufacturing, with emissions standards and solvent handling requirements varying significantly across different jurisdictions. Companies must address these considerations during technology transfer and commercial implementation phases to ensure full regulatory compliance.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has developed guidelines that indirectly affect spray pyrolysis applications, particularly ICH Q8, Q9, and Q10, which address pharmaceutical development, quality risk management, and pharmaceutical quality systems respectively. These guidelines provide a framework for implementing novel technologies like spray pyrolysis within a risk-based quality management approach.
Regulatory submissions for products manufactured using spray pyrolysis typically require enhanced characterization data compared to conventional formulations. This includes comprehensive particle size distribution analyses, surface morphology studies, and stability assessments under various conditions to demonstrate product robustness and consistency.
Recent regulatory trends indicate increasing acceptance of continuous manufacturing technologies, including spray pyrolysis, as part of broader initiatives to modernize pharmaceutical production. However, this acceptance comes with heightened expectations for real-time monitoring capabilities, process analytical technology implementation, and robust control strategies to ensure consistent product quality throughout the manufacturing process.
Quality by Design (QbD) principles have become increasingly important in the regulatory framework, with agencies requiring pharmaceutical companies to demonstrate thorough understanding of critical process parameters in spray pyrolysis that affect final product attributes. This includes detailed characterization of temperature profiles, precursor solution properties, and atomization parameters that influence particle morphology and drug release kinetics.
Current Good Manufacturing Practice (cGMP) compliance presents unique challenges for spray pyrolysis implementation, particularly regarding process validation and scale-up considerations. Regulatory bodies typically require extensive documentation of process controls, in-process testing protocols, and validation strategies specific to spray pyrolysis equipment and procedures.
Environmental regulations also impact spray pyrolysis adoption in pharmaceutical manufacturing, with emissions standards and solvent handling requirements varying significantly across different jurisdictions. Companies must address these considerations during technology transfer and commercial implementation phases to ensure full regulatory compliance.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has developed guidelines that indirectly affect spray pyrolysis applications, particularly ICH Q8, Q9, and Q10, which address pharmaceutical development, quality risk management, and pharmaceutical quality systems respectively. These guidelines provide a framework for implementing novel technologies like spray pyrolysis within a risk-based quality management approach.
Regulatory submissions for products manufactured using spray pyrolysis typically require enhanced characterization data compared to conventional formulations. This includes comprehensive particle size distribution analyses, surface morphology studies, and stability assessments under various conditions to demonstrate product robustness and consistency.
Recent regulatory trends indicate increasing acceptance of continuous manufacturing technologies, including spray pyrolysis, as part of broader initiatives to modernize pharmaceutical production. However, this acceptance comes with heightened expectations for real-time monitoring capabilities, process analytical technology implementation, and robust control strategies to ensure consistent product quality throughout the manufacturing process.
Scalability and Manufacturing Considerations
Scaling spray pyrolysis processes from laboratory to industrial production represents a significant challenge in pharmaceutical manufacturing. The transition requires careful consideration of equipment design, process parameters, and quality control systems to maintain consistent particle characteristics. Industrial spray pyrolysis equipment must be designed with pharmaceutical-grade materials that comply with Good Manufacturing Practice (GMP) regulations, featuring clean-in-place and sterilize-in-place capabilities essential for pharmaceutical production environments.
Process parameters that prove successful at laboratory scale often require substantial modification when implemented at production scale. Key variables including solution feed rate, atomization pressure, chamber temperature profiles, and residence time must be recalibrated to achieve comparable particle properties. Computational fluid dynamics modeling has emerged as a valuable tool for predicting how these parameters interact at larger scales, potentially reducing the number of experimental trials required during scale-up.
Continuous manufacturing approaches offer particular advantages for spray pyrolysis in pharmaceutical applications. These systems can provide more consistent product quality compared to batch processes by maintaining steady-state conditions throughout production. Real-time process analytical technology (PAT) integration enables continuous monitoring of critical quality attributes, allowing for automated adjustments to process parameters when deviations occur. This approach aligns with regulatory trends encouraging continuous manufacturing adoption in pharmaceutical production.
Economic considerations significantly impact the commercial viability of spray pyrolysis for pharmaceutical particle design. Capital investment for industrial-scale equipment is substantial, necessitating thorough cost-benefit analysis before implementation. Operating costs include energy consumption for maintaining high temperatures, specialized precursor materials, and maintenance of precision components. However, these costs may be offset by improved product performance, reduced downstream processing requirements, and potential intellectual property advantages.
Regulatory compliance presents additional manufacturing considerations. Validation protocols must demonstrate that scaled-up processes consistently produce particles meeting predetermined specifications. This includes establishing acceptable ranges for critical quality attributes such as particle size distribution, morphology, crystallinity, and purity. Documentation requirements are extensive, covering equipment qualification, process validation, and change control procedures. Early engagement with regulatory authorities can help identify potential compliance issues before significant resources are committed to scale-up efforts.
Environmental sustainability has become increasingly important in pharmaceutical manufacturing decisions. Spray pyrolysis processes should be evaluated for energy efficiency, solvent usage, and waste generation. Recovery systems for capturing and recycling solvents can significantly reduce environmental impact while potentially improving economic performance. Life cycle assessment methodologies provide a framework for comprehensive evaluation of environmental considerations throughout the product development and manufacturing process.
Process parameters that prove successful at laboratory scale often require substantial modification when implemented at production scale. Key variables including solution feed rate, atomization pressure, chamber temperature profiles, and residence time must be recalibrated to achieve comparable particle properties. Computational fluid dynamics modeling has emerged as a valuable tool for predicting how these parameters interact at larger scales, potentially reducing the number of experimental trials required during scale-up.
Continuous manufacturing approaches offer particular advantages for spray pyrolysis in pharmaceutical applications. These systems can provide more consistent product quality compared to batch processes by maintaining steady-state conditions throughout production. Real-time process analytical technology (PAT) integration enables continuous monitoring of critical quality attributes, allowing for automated adjustments to process parameters when deviations occur. This approach aligns with regulatory trends encouraging continuous manufacturing adoption in pharmaceutical production.
Economic considerations significantly impact the commercial viability of spray pyrolysis for pharmaceutical particle design. Capital investment for industrial-scale equipment is substantial, necessitating thorough cost-benefit analysis before implementation. Operating costs include energy consumption for maintaining high temperatures, specialized precursor materials, and maintenance of precision components. However, these costs may be offset by improved product performance, reduced downstream processing requirements, and potential intellectual property advantages.
Regulatory compliance presents additional manufacturing considerations. Validation protocols must demonstrate that scaled-up processes consistently produce particles meeting predetermined specifications. This includes establishing acceptable ranges for critical quality attributes such as particle size distribution, morphology, crystallinity, and purity. Documentation requirements are extensive, covering equipment qualification, process validation, and change control procedures. Early engagement with regulatory authorities can help identify potential compliance issues before significant resources are committed to scale-up efforts.
Environmental sustainability has become increasingly important in pharmaceutical manufacturing decisions. Spray pyrolysis processes should be evaluated for energy efficiency, solvent usage, and waste generation. Recovery systems for capturing and recycling solvents can significantly reduce environmental impact while potentially improving economic performance. Life cycle assessment methodologies provide a framework for comprehensive evaluation of environmental considerations throughout the product development and manufacturing process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!