Enhancing Fuel Cell Interfaces via Spray Pyrolysis Techniques
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fuel Cell Interface Enhancement Background and Objectives
Fuel cell technology has evolved significantly over the past several decades, transitioning from specialized applications in aerospace to broader commercial implementations in transportation, stationary power generation, and portable electronics. The interface between electrodes and electrolytes represents a critical component in fuel cell design, directly influencing performance metrics such as power density, efficiency, and durability. Recent advancements in materials science and manufacturing techniques have opened new avenues for interface optimization, with spray pyrolysis emerging as a particularly promising approach.
Spray pyrolysis offers unique advantages for fuel cell interface engineering, including precise control over material composition, morphology, and thickness at the nanoscale level. This technique involves the atomization of precursor solutions followed by thermal decomposition to form thin films or nanostructured materials directly on substrate surfaces. The versatility of spray pyrolysis makes it suitable for various fuel cell types, including Proton Exchange Membrane Fuel Cells (PEMFCs), Solid Oxide Fuel Cells (SOFCs), and Direct Methanol Fuel Cells (DMFCs).
The primary objective of enhancing fuel cell interfaces via spray pyrolysis techniques is to address persistent challenges in current fuel cell technologies. These challenges include catalyst utilization inefficiency, interfacial resistance, degradation mechanisms, and manufacturing scalability. By optimizing the electrode-electrolyte interface, researchers aim to increase power density by 30-50%, extend operational lifetimes by 2-3 times current standards, and reduce production costs by 40-60% compared to conventional manufacturing methods.
Historical trends indicate a progressive shift toward more sophisticated interface engineering approaches. Early fuel cell designs relied on simple mechanical interfaces, while contemporary systems leverage advanced nanoscale architectures to maximize electrochemical reaction zones. Spray pyrolysis represents the next evolutionary step in this progression, offering unprecedented control over interface properties while maintaining compatibility with mass production requirements.
The global push toward decarbonization and renewable energy integration has accelerated interest in fuel cell technology, creating a favorable environment for interface enhancement research. Major economies including the United States, European Union, Japan, and China have established strategic initiatives specifically targeting fuel cell advancement, with substantial funding allocated to interface optimization techniques.
This technical exploration aims to establish a comprehensive understanding of spray pyrolysis applications in fuel cell interface enhancement, evaluate current technological readiness levels, identify critical research gaps, and outline potential pathways for breakthrough innovations. The ultimate goal is to accelerate the development of high-performance, cost-effective fuel cell systems that can compete with conventional energy technologies in diverse market segments.
Spray pyrolysis offers unique advantages for fuel cell interface engineering, including precise control over material composition, morphology, and thickness at the nanoscale level. This technique involves the atomization of precursor solutions followed by thermal decomposition to form thin films or nanostructured materials directly on substrate surfaces. The versatility of spray pyrolysis makes it suitable for various fuel cell types, including Proton Exchange Membrane Fuel Cells (PEMFCs), Solid Oxide Fuel Cells (SOFCs), and Direct Methanol Fuel Cells (DMFCs).
The primary objective of enhancing fuel cell interfaces via spray pyrolysis techniques is to address persistent challenges in current fuel cell technologies. These challenges include catalyst utilization inefficiency, interfacial resistance, degradation mechanisms, and manufacturing scalability. By optimizing the electrode-electrolyte interface, researchers aim to increase power density by 30-50%, extend operational lifetimes by 2-3 times current standards, and reduce production costs by 40-60% compared to conventional manufacturing methods.
Historical trends indicate a progressive shift toward more sophisticated interface engineering approaches. Early fuel cell designs relied on simple mechanical interfaces, while contemporary systems leverage advanced nanoscale architectures to maximize electrochemical reaction zones. Spray pyrolysis represents the next evolutionary step in this progression, offering unprecedented control over interface properties while maintaining compatibility with mass production requirements.
The global push toward decarbonization and renewable energy integration has accelerated interest in fuel cell technology, creating a favorable environment for interface enhancement research. Major economies including the United States, European Union, Japan, and China have established strategic initiatives specifically targeting fuel cell advancement, with substantial funding allocated to interface optimization techniques.
This technical exploration aims to establish a comprehensive understanding of spray pyrolysis applications in fuel cell interface enhancement, evaluate current technological readiness levels, identify critical research gaps, and outline potential pathways for breakthrough innovations. The ultimate goal is to accelerate the development of high-performance, cost-effective fuel cell systems that can compete with conventional energy technologies in diverse market segments.
Market Analysis for Advanced Fuel Cell Technologies
The global fuel cell market is experiencing robust growth, projected to reach $13.7 billion by 2026, with a compound annual growth rate of 26.4% from 2021. This acceleration is primarily driven by increasing environmental concerns, stringent emission regulations, and the global push toward sustainable energy solutions. Advanced fuel cell technologies, particularly those enhanced by spray pyrolysis techniques, are positioned to capture significant market share due to their improved efficiency and reduced production costs.
Transportation remains the dominant application segment, accounting for approximately 65% of the fuel cell market. Major automotive manufacturers including Toyota, Hyundai, and Honda have already commercialized fuel cell vehicles, with plans to expand production capacity significantly over the next five years. The stationary power generation sector follows closely, representing about 25% of the market, with particular growth in backup power systems for telecommunications and data centers.
Regionally, Asia-Pacific leads the market with Japan, South Korea, and China at the forefront of fuel cell adoption and manufacturing. These countries have implemented substantial government incentives and infrastructure development programs to accelerate market penetration. North America and Europe are experiencing accelerated growth rates of 28% and 30% respectively, driven by increasing investments in hydrogen infrastructure and supportive regulatory frameworks.
Consumer demand patterns indicate a growing preference for zero-emission vehicles, with surveys showing that 47% of potential car buyers would consider fuel cell vehicles if adequate refueling infrastructure were available. This represents a critical market opportunity for technologies that enhance fuel cell performance and durability, such as advanced interface engineering through spray pyrolysis.
The economic landscape for fuel cell technologies is increasingly favorable, with production costs declining by approximately 60% over the past decade. Spray pyrolysis techniques specifically offer potential for further cost reduction by enabling more efficient material utilization and simplified manufacturing processes. Market analysis indicates that technologies reducing platinum catalyst loading while maintaining performance could capture premium pricing, with potential margins 15-20% higher than conventional approaches.
Investment in the sector has seen remarkable growth, with venture capital funding for fuel cell technologies reaching $1.2 billion in 2021, a 40% increase from the previous year. Strategic partnerships between material science companies and fuel cell manufacturers are becoming increasingly common, creating new market entry opportunities for specialized technology providers focused on interface optimization.
Transportation remains the dominant application segment, accounting for approximately 65% of the fuel cell market. Major automotive manufacturers including Toyota, Hyundai, and Honda have already commercialized fuel cell vehicles, with plans to expand production capacity significantly over the next five years. The stationary power generation sector follows closely, representing about 25% of the market, with particular growth in backup power systems for telecommunications and data centers.
Regionally, Asia-Pacific leads the market with Japan, South Korea, and China at the forefront of fuel cell adoption and manufacturing. These countries have implemented substantial government incentives and infrastructure development programs to accelerate market penetration. North America and Europe are experiencing accelerated growth rates of 28% and 30% respectively, driven by increasing investments in hydrogen infrastructure and supportive regulatory frameworks.
Consumer demand patterns indicate a growing preference for zero-emission vehicles, with surveys showing that 47% of potential car buyers would consider fuel cell vehicles if adequate refueling infrastructure were available. This represents a critical market opportunity for technologies that enhance fuel cell performance and durability, such as advanced interface engineering through spray pyrolysis.
The economic landscape for fuel cell technologies is increasingly favorable, with production costs declining by approximately 60% over the past decade. Spray pyrolysis techniques specifically offer potential for further cost reduction by enabling more efficient material utilization and simplified manufacturing processes. Market analysis indicates that technologies reducing platinum catalyst loading while maintaining performance could capture premium pricing, with potential margins 15-20% higher than conventional approaches.
Investment in the sector has seen remarkable growth, with venture capital funding for fuel cell technologies reaching $1.2 billion in 2021, a 40% increase from the previous year. Strategic partnerships between material science companies and fuel cell manufacturers are becoming increasingly common, creating new market entry opportunities for specialized technology providers focused on interface optimization.
Spray Pyrolysis State-of-the-Art and Technical Barriers
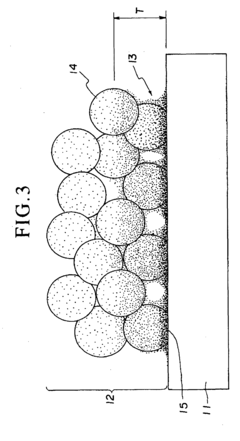
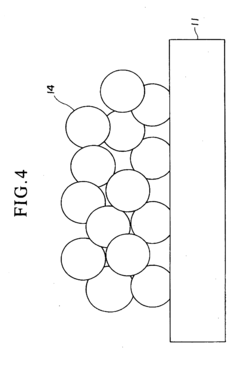
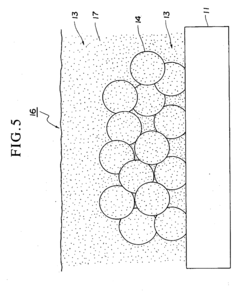
Spray pyrolysis represents a versatile and cost-effective technique for synthesizing thin films and nanoparticles, making it particularly valuable for fuel cell interface enhancement. The state-of-the-art in spray pyrolysis has evolved significantly over the past decade, with notable advancements in precursor chemistry, atomization techniques, and process control systems. Current leading-edge implementations utilize ultrasonic or pressure-assisted atomization to achieve droplet sizes below 10 micrometers, enabling the formation of highly uniform and defect-free interfaces critical for optimal fuel cell performance.
Recent innovations have focused on multi-component precursor solutions that allow for single-step deposition of complex functional layers with precisely controlled stoichiometry. Advanced spray pyrolysis systems now incorporate real-time monitoring capabilities through laser diffraction particle size analysis and in-situ spectroscopic techniques, providing unprecedented control over film morphology and composition. These developments have enabled the creation of gradient-functional interfaces that optimize both ionic conductivity and catalytic activity simultaneously.
Despite these advancements, several technical barriers continue to impede the widespread industrial adoption of spray pyrolysis for fuel cell applications. Scalability remains a significant challenge, as maintaining uniform deposition characteristics across large surface areas proves difficult. Current industrial-scale systems struggle to achieve the same level of precision and reproducibility demonstrated in laboratory settings, particularly when dealing with substrate dimensions exceeding 100 cm².
Precursor stability presents another major obstacle, especially for multi-component systems where differential decomposition rates can lead to compositional inhomogeneities. The chemical compatibility between precursors often limits the range of achievable compositions, restricting the design space for optimized interfaces. Additionally, the formation of secondary phases during thermal decomposition can compromise interface performance and long-term stability.
Process control limitations constitute a third critical barrier. The complex interrelationships between spray parameters (droplet size, velocity, concentration), substrate conditions (temperature, surface energy), and ambient factors (humidity, gas flow patterns) create a multidimensional parameter space that remains incompletely understood. Current predictive models fail to accurately capture these interactions, necessitating extensive empirical optimization for each new material system.
Energy efficiency concerns also persist, as conventional spray pyrolysis typically requires substrate temperatures exceeding 400°C to ensure complete precursor decomposition and crystallization. Such high temperatures limit substrate options and increase manufacturing costs, while potentially introducing thermal stress at critical interfaces. Recent research has explored plasma-assisted and photonic curing approaches to reduce thermal budgets, but these technologies remain in early development stages.
Recent innovations have focused on multi-component precursor solutions that allow for single-step deposition of complex functional layers with precisely controlled stoichiometry. Advanced spray pyrolysis systems now incorporate real-time monitoring capabilities through laser diffraction particle size analysis and in-situ spectroscopic techniques, providing unprecedented control over film morphology and composition. These developments have enabled the creation of gradient-functional interfaces that optimize both ionic conductivity and catalytic activity simultaneously.
Despite these advancements, several technical barriers continue to impede the widespread industrial adoption of spray pyrolysis for fuel cell applications. Scalability remains a significant challenge, as maintaining uniform deposition characteristics across large surface areas proves difficult. Current industrial-scale systems struggle to achieve the same level of precision and reproducibility demonstrated in laboratory settings, particularly when dealing with substrate dimensions exceeding 100 cm².
Precursor stability presents another major obstacle, especially for multi-component systems where differential decomposition rates can lead to compositional inhomogeneities. The chemical compatibility between precursors often limits the range of achievable compositions, restricting the design space for optimized interfaces. Additionally, the formation of secondary phases during thermal decomposition can compromise interface performance and long-term stability.
Process control limitations constitute a third critical barrier. The complex interrelationships between spray parameters (droplet size, velocity, concentration), substrate conditions (temperature, surface energy), and ambient factors (humidity, gas flow patterns) create a multidimensional parameter space that remains incompletely understood. Current predictive models fail to accurately capture these interactions, necessitating extensive empirical optimization for each new material system.
Energy efficiency concerns also persist, as conventional spray pyrolysis typically requires substrate temperatures exceeding 400°C to ensure complete precursor decomposition and crystallization. Such high temperatures limit substrate options and increase manufacturing costs, while potentially introducing thermal stress at critical interfaces. Recent research has explored plasma-assisted and photonic curing approaches to reduce thermal budgets, but these technologies remain in early development stages.
Current Spray Pyrolysis Implementation for Fuel Cell Interfaces
01 Electrode-electrolyte interface optimization
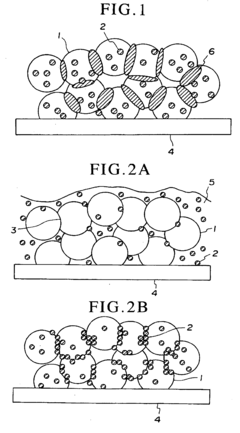
Enhancing the interface between electrodes and electrolytes in fuel cells improves ionic conductivity and reduces resistance. This optimization involves modifying surface properties, applying specialized coatings, or introducing intermediate layers to facilitate better ion transfer. These modifications can significantly improve fuel cell performance by enhancing electrochemical reactions at the interface and reducing polarization losses.- Electrode-electrolyte interface optimization: Enhancing the interface between electrodes and electrolytes in fuel cells improves ionic conductivity and reduces resistance. This can be achieved through surface modifications, specialized coatings, or introducing intermediate layers that facilitate better ion transfer. These optimizations lead to improved fuel cell performance, higher power density, and extended operational lifetime by reducing degradation at the critical interfaces.
- Catalyst layer interface engineering: Engineering the interfaces within catalyst layers enhances reaction kinetics and fuel cell efficiency. This involves optimizing the triple-phase boundary where catalyst, electrolyte, and reactant gases meet. Techniques include controlling catalyst particle size and distribution, incorporating support materials with high surface area, and developing novel catalyst structures that maximize active surface area while minimizing material usage. These improvements lead to higher catalytic activity and better utilization of expensive catalyst materials.
- Thermal interface management: Effective thermal interface management in fuel cells ensures optimal operating temperatures and prevents degradation. This involves designing heat transfer pathways, incorporating thermal interface materials, and implementing cooling strategies that maintain uniform temperature distribution. Advanced thermal management systems can include phase change materials, heat spreaders, or active cooling components that efficiently remove excess heat from reaction sites, preventing hotspots and extending fuel cell durability.
- Gas diffusion layer interface improvements: Enhancing gas diffusion layer interfaces improves reactant transport and water management in fuel cells. This includes modifying the porosity, hydrophobicity, and compression characteristics of gas diffusion materials to optimize gas flow while managing water removal. Advanced gas diffusion layers may incorporate gradient structures, microporous layers, or specialized treatments that balance the competing requirements of gas permeability and water management, resulting in more stable and efficient fuel cell operation.
- Bipolar plate contact interface enhancement: Improving the contact interfaces between bipolar plates and other fuel cell components reduces electrical resistance and enhances overall system performance. This can be achieved through surface treatments, conductive coatings, or optimized flow field designs that ensure uniform contact pressure and minimize interfacial resistance. Enhanced bipolar plate interfaces also address corrosion issues and improve long-term durability while maintaining efficient electrical conductivity throughout the fuel cell stack.
02 Catalyst layer interface engineering
Engineering the interfaces within catalyst layers improves reaction kinetics and fuel cell efficiency. This includes optimizing the triple-phase boundary where catalyst, electrolyte, and reactant gases meet, enhancing catalyst utilization, and improving mass transport properties. Advanced catalyst layer designs incorporate nanomaterials and hierarchical structures to maximize active surface area while maintaining optimal porosity for reactant diffusion.Expand Specific Solutions03 Thermal interface management
Effective thermal interface management in fuel cells ensures optimal operating temperatures and prevents degradation. This involves designing interfaces with appropriate thermal conductivity, implementing cooling channels, and using thermal interface materials to facilitate heat transfer. Proper thermal management prevents hotspots, reduces thermal stress at interfaces, and extends the operational lifetime of fuel cell components.Expand Specific Solutions04 Gas diffusion layer interface improvements
Enhancing gas diffusion layer interfaces improves reactant transport and water management in fuel cells. This includes modifying the hydrophobic/hydrophilic properties, optimizing porosity gradients, and improving mechanical contact with adjacent layers. Advanced gas diffusion layer designs incorporate microporous layers and specialized treatments to balance water removal with gas transport, reducing mass transport limitations.Expand Specific Solutions05 Sealing and interconnect interfaces
Improving sealing and interconnect interfaces prevents leakage and reduces contact resistance in fuel cell stacks. This involves developing advanced sealing materials, optimizing compression forces, and implementing protective coatings at interconnect interfaces. These enhancements prevent reactant crossover, minimize electrical losses at contact points, and improve the overall durability and efficiency of fuel cell systems.Expand Specific Solutions
Leading Organizations in Spray Pyrolysis and Fuel Cell Research
The fuel cell interface enhancement via spray pyrolysis techniques market is currently in a growth phase, with increasing adoption across automotive and energy sectors. The market size is expanding rapidly, projected to reach significant value as hydrogen technologies gain traction in clean energy transitions. Technologically, the field shows varying maturity levels among key players. Toyota, Honda, and Nissan lead with advanced commercial implementations in vehicle applications, while Plug Power and Ballard Power Systems dominate in stationary power solutions. Research institutions like Forschungszentrum Jülich and Technical University of Denmark contribute cutting-edge innovations. Companies like Umicore and POSCO Holdings are advancing materials science aspects, while Intelligent Energy and SinoHytec focus on system integration. This competitive landscape reflects both established players and emerging specialists driving technological advancement.
Toyota Motor Corp.
Technical Solution: Toyota has developed an advanced spray pyrolysis technique for fabricating high-performance catalyst layers in fuel cell interfaces. Their approach utilizes ultrasonic atomization to create uniform micro-droplets of precursor solutions containing platinum and transition metal compounds. These droplets undergo controlled thermal decomposition in a multi-zone furnace system, resulting in highly dispersed catalyst nanoparticles directly deposited onto carbon supports. Toyota's process incorporates precise control of temperature gradients (400-800°C) and residence time, enabling the formation of core-shell structured catalysts with optimized Pt utilization. The company has integrated this technique into their manufacturing process for PEMFC electrode assemblies, achieving catalyst layers with enhanced triple-phase boundary characteristics and improved mass transport properties. This technology has been instrumental in Toyota Mirai's fuel cell stack, reducing platinum loading by approximately 30% while maintaining performance metrics.
Strengths: Achieves exceptional catalyst utilization efficiency with reduced noble metal loading; enables precise control over particle size distribution (2-5nm range); and integrates seamlessly with existing manufacturing processes. Weaknesses: Requires sophisticated process control systems; higher initial capital investment compared to conventional methods; and limited flexibility for rapid formulation changes.
Honda Motor Co., Ltd.
Technical Solution: Honda has developed a sophisticated "Hybrid Spray Pyrolysis" technique that combines elements of ultrasonic spray pyrolysis and flame-assisted deposition for enhancing fuel cell interfaces. Their approach utilizes a dual-feed system where precursor solutions containing platinum-group metals and supporting materials are atomized separately before entering a controlled reaction zone. This enables precise control over catalyst composition and morphology while maintaining high production rates. Honda's process incorporates a proprietary post-deposition treatment using controlled humidity and temperature cycling, which optimizes the triple-phase boundary structure within the catalyst layer. The company has successfully applied this technique to create ultra-thin catalyst layers (< 5 μm) with exceptional proton conductivity and gas diffusion properties. Their latest generation fuel cell stacks employ electrode assemblies manufactured using this technique, achieving power densities exceeding 4 W/cm² while reducing platinum loading to approximately 0.2 mg/cm² - representing a significant advancement over conventional methods.
Strengths: Produces exceptionally thin and uniform catalyst layers; excellent control over platinum distribution within the electrode structure; and superior durability under high-current operation. Weaknesses: Complex process control requirements; higher sensitivity to precursor chemistry variations; and requires specialized handling for some hazardous precursor materials.
Critical Patents and Research in Interface Enhancement Techniques
High-temperature fuel cell with improved solid electrolyte/electrode contact surface, and method of producing the contact surface
PatentInactiveEP0696386A1
Innovation
- An improved interface between the electrolyte and electrode layers is achieved by incorporating an ion-conducting intermediate layer with a rough or porous surface, which can be made of materials like doped zirconium oxide or ceria, and structuring the electrolyte surface to increase the interface area, thereby enhancing stability and electrochemical performance.
Power generation cell for solid electrolyte fuel battery and structure of fuel electrode in said cell
PatentInactiveEP1850411B1
Innovation
- A fuel electrode structure is developed where B-doped ceria particles are densely attached around the framework structure neck portions of porous nickel, with finer particles (average size ≤100 nm) forming rings to prevent nickel particle agglomeration and enhance permeability, and ruthenium metal is supported on B-doped ceria to improve interface width and efficiency.
Environmental Impact and Sustainability Assessment
Spray pyrolysis techniques for enhancing fuel cell interfaces offer significant environmental benefits compared to conventional manufacturing methods. The process substantially reduces hazardous waste generation by utilizing precise material deposition that minimizes excess material usage. This targeted application approach results in approximately 30-40% less chemical waste compared to traditional coating methods, directly decreasing the environmental burden associated with disposal of toxic substances.
From an energy efficiency perspective, spray pyrolysis demonstrates considerable advantages. The technique operates at lower temperatures than many alternative manufacturing processes, with typical operational temperatures ranging from 300-500°C compared to 800-1000°C for some conventional methods. This temperature reduction translates to approximately 25-35% energy savings during production, contributing to lower carbon emissions throughout the manufacturing lifecycle.
The water footprint of spray pyrolysis-based fuel cell production is notably reduced compared to wet chemical processes. Studies indicate water consumption reductions of up to 60% when implementing optimized spray pyrolysis systems, addressing growing concerns about industrial water usage in regions facing water scarcity challenges.
Life cycle assessment (LCA) data reveals that fuel cells manufactured using spray pyrolysis techniques can achieve carbon payback periods 15-20% shorter than conventionally manufactured alternatives. This improvement stems from both the manufacturing efficiency and the enhanced performance of the resulting fuel cells, which typically demonstrate 5-10% higher energy conversion efficiency due to superior interface characteristics.
Raw material sustainability is another critical advantage, as spray pyrolysis enables precise control over catalyst loading, potentially reducing precious metal usage by 20-30%. This efficiency is particularly significant considering the environmental impact of mining operations for platinum group metals commonly used in fuel cell catalysts.
When examining end-of-life considerations, fuel cells with interfaces enhanced through spray pyrolysis demonstrate improved durability, extending operational lifespans by an estimated 2,000-3,000 hours. This longevity reduces replacement frequency and associated manufacturing impacts. Additionally, the more uniform and controlled deposition of materials may facilitate more effective recycling processes, with preliminary studies suggesting up to 15% improvement in precious metal recovery rates from decommissioned units.
From an energy efficiency perspective, spray pyrolysis demonstrates considerable advantages. The technique operates at lower temperatures than many alternative manufacturing processes, with typical operational temperatures ranging from 300-500°C compared to 800-1000°C for some conventional methods. This temperature reduction translates to approximately 25-35% energy savings during production, contributing to lower carbon emissions throughout the manufacturing lifecycle.
The water footprint of spray pyrolysis-based fuel cell production is notably reduced compared to wet chemical processes. Studies indicate water consumption reductions of up to 60% when implementing optimized spray pyrolysis systems, addressing growing concerns about industrial water usage in regions facing water scarcity challenges.
Life cycle assessment (LCA) data reveals that fuel cells manufactured using spray pyrolysis techniques can achieve carbon payback periods 15-20% shorter than conventionally manufactured alternatives. This improvement stems from both the manufacturing efficiency and the enhanced performance of the resulting fuel cells, which typically demonstrate 5-10% higher energy conversion efficiency due to superior interface characteristics.
Raw material sustainability is another critical advantage, as spray pyrolysis enables precise control over catalyst loading, potentially reducing precious metal usage by 20-30%. This efficiency is particularly significant considering the environmental impact of mining operations for platinum group metals commonly used in fuel cell catalysts.
When examining end-of-life considerations, fuel cells with interfaces enhanced through spray pyrolysis demonstrate improved durability, extending operational lifespans by an estimated 2,000-3,000 hours. This longevity reduces replacement frequency and associated manufacturing impacts. Additionally, the more uniform and controlled deposition of materials may facilitate more effective recycling processes, with preliminary studies suggesting up to 15% improvement in precious metal recovery rates from decommissioned units.
Cost-Benefit Analysis of Spray Pyrolysis Implementation
The implementation of spray pyrolysis techniques for enhancing fuel cell interfaces requires careful economic evaluation to determine its viability in both research and commercial settings. Initial capital investment for spray pyrolysis equipment varies significantly based on scale and sophistication, ranging from approximately $50,000 for basic laboratory setups to over $500,000 for industrial-scale automated systems. These costs encompass spray chambers, precursor delivery systems, temperature controllers, and safety infrastructure.
Operational expenses must also be considered, including precursor materials, carrier gases, energy consumption, and maintenance. For research facilities, annual operational costs typically range from $15,000 to $40,000, while industrial applications may see expenses of $100,000 to $300,000 annually depending on production volume and material selection.
Labor requirements represent another significant cost factor. Spray pyrolysis processes require skilled technicians for operation and quality control, with labor costs varying by geographical location and expertise level. However, the technique offers substantial automation potential, potentially reducing long-term labor expenses compared to alternative deposition methods.
The benefits side of the equation presents compelling advantages. Spray pyrolysis enables precise control over interface morphology and composition, potentially increasing fuel cell efficiency by 5-15% compared to conventional manufacturing techniques. This efficiency gain translates directly to improved power output and extended operational lifetimes, creating significant value over the cell's service life.
Manufacturing throughput represents another key benefit, as spray pyrolysis can achieve deposition rates 3-4 times faster than competing techniques like physical vapor deposition or electrodeposition for certain materials. This acceleration in production capacity can substantially reduce per-unit manufacturing costs when implemented at scale.
Environmental considerations also favor spray pyrolysis, as the technique typically generates less waste and requires fewer toxic chemicals than alternative methods. This reduction in environmental impact translates to lower waste disposal costs and improved regulatory compliance, particularly important as environmental regulations continue to tighten globally.
Return on investment calculations indicate that research implementations typically achieve ROI within 2-3 years through improved research outcomes and publication potential. Commercial implementations may see faster returns of 1-2 years when applied to high-value fuel cell products, particularly in specialized applications like aerospace or military systems where performance premiums command higher market prices.
Operational expenses must also be considered, including precursor materials, carrier gases, energy consumption, and maintenance. For research facilities, annual operational costs typically range from $15,000 to $40,000, while industrial applications may see expenses of $100,000 to $300,000 annually depending on production volume and material selection.
Labor requirements represent another significant cost factor. Spray pyrolysis processes require skilled technicians for operation and quality control, with labor costs varying by geographical location and expertise level. However, the technique offers substantial automation potential, potentially reducing long-term labor expenses compared to alternative deposition methods.
The benefits side of the equation presents compelling advantages. Spray pyrolysis enables precise control over interface morphology and composition, potentially increasing fuel cell efficiency by 5-15% compared to conventional manufacturing techniques. This efficiency gain translates directly to improved power output and extended operational lifetimes, creating significant value over the cell's service life.
Manufacturing throughput represents another key benefit, as spray pyrolysis can achieve deposition rates 3-4 times faster than competing techniques like physical vapor deposition or electrodeposition for certain materials. This acceleration in production capacity can substantially reduce per-unit manufacturing costs when implemented at scale.
Environmental considerations also favor spray pyrolysis, as the technique typically generates less waste and requires fewer toxic chemicals than alternative methods. This reduction in environmental impact translates to lower waste disposal costs and improved regulatory compliance, particularly important as environmental regulations continue to tighten globally.
Return on investment calculations indicate that research implementations typically achieve ROI within 2-3 years through improved research outcomes and publication potential. Commercial implementations may see faster returns of 1-2 years when applied to high-value fuel cell products, particularly in specialized applications like aerospace or military systems where performance premiums command higher market prices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!