Spray Pyrolysis Methodologies for High-Temperature Catalysts
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spray Pyrolysis Evolution and Research Objectives
Spray pyrolysis has evolved significantly since its inception in the mid-20th century, transforming from a simple laboratory technique to a sophisticated method for synthesizing high-performance catalytic materials. Initially developed for ceramic powder production, the technique gained prominence in the 1970s when researchers discovered its potential for creating fine particles with controlled morphology. By the 1990s, advancements in equipment design and process control enabled the synthesis of complex multi-component materials with precise compositional control.
The evolution of spray pyrolysis methodologies has been driven by increasing demands for catalysts capable of withstanding extreme operating conditions in industrial processes. Traditional wet chemistry methods often produce catalysts with limited thermal stability and active site accessibility. In contrast, spray pyrolysis offers a direct route to synthesize thermally stable catalysts with high surface area and well-dispersed active sites, addressing key limitations of conventional preparation techniques.
Recent technological breakthroughs have further expanded the capabilities of spray pyrolysis. The integration of ultrasonic atomization systems has enabled the production of sub-micron particles with narrow size distributions. Innovations in precursor chemistry have facilitated the synthesis of complex mixed-oxide catalysts with tailored surface properties. Additionally, the development of flame-assisted spray pyrolysis has opened new possibilities for creating highly crystalline materials with enhanced catalytic performance.
The primary research objectives in this field focus on optimizing spray pyrolysis parameters to enhance catalyst performance under high-temperature conditions. This includes investigating the relationship between precursor composition, atomization conditions, and the resulting catalyst structure. Understanding how these factors influence thermal stability, active site distribution, and resistance to sintering is crucial for developing next-generation catalysts for demanding applications such as methane reforming, automotive emission control, and chemical synthesis.
Another key research goal involves exploring novel precursor formulations that can yield catalysts with superior high-temperature characteristics. This includes the incorporation of rare earth elements as structural promoters, the development of core-shell architectures to prevent sintering, and the creation of self-regenerating catalyst systems that maintain activity during prolonged high-temperature operation.
The ultimate objective is to establish standardized spray pyrolysis protocols that enable the reproducible synthesis of high-performance catalysts at industrial scale. This requires addressing challenges related to process scalability, energy efficiency, and cost-effectiveness, while maintaining precise control over catalyst properties. Success in these endeavors would significantly advance capabilities in fields ranging from renewable energy production to environmental remediation and sustainable chemical manufacturing.
The evolution of spray pyrolysis methodologies has been driven by increasing demands for catalysts capable of withstanding extreme operating conditions in industrial processes. Traditional wet chemistry methods often produce catalysts with limited thermal stability and active site accessibility. In contrast, spray pyrolysis offers a direct route to synthesize thermally stable catalysts with high surface area and well-dispersed active sites, addressing key limitations of conventional preparation techniques.
Recent technological breakthroughs have further expanded the capabilities of spray pyrolysis. The integration of ultrasonic atomization systems has enabled the production of sub-micron particles with narrow size distributions. Innovations in precursor chemistry have facilitated the synthesis of complex mixed-oxide catalysts with tailored surface properties. Additionally, the development of flame-assisted spray pyrolysis has opened new possibilities for creating highly crystalline materials with enhanced catalytic performance.
The primary research objectives in this field focus on optimizing spray pyrolysis parameters to enhance catalyst performance under high-temperature conditions. This includes investigating the relationship between precursor composition, atomization conditions, and the resulting catalyst structure. Understanding how these factors influence thermal stability, active site distribution, and resistance to sintering is crucial for developing next-generation catalysts for demanding applications such as methane reforming, automotive emission control, and chemical synthesis.
Another key research goal involves exploring novel precursor formulations that can yield catalysts with superior high-temperature characteristics. This includes the incorporation of rare earth elements as structural promoters, the development of core-shell architectures to prevent sintering, and the creation of self-regenerating catalyst systems that maintain activity during prolonged high-temperature operation.
The ultimate objective is to establish standardized spray pyrolysis protocols that enable the reproducible synthesis of high-performance catalysts at industrial scale. This requires addressing challenges related to process scalability, energy efficiency, and cost-effectiveness, while maintaining precise control over catalyst properties. Success in these endeavors would significantly advance capabilities in fields ranging from renewable energy production to environmental remediation and sustainable chemical manufacturing.
Market Analysis for High-Temperature Catalyst Applications
The high-temperature catalyst market is experiencing robust growth driven by increasing industrial applications across multiple sectors. The global market for high-temperature catalysts was valued at approximately $5.2 billion in 2022 and is projected to reach $7.8 billion by 2028, representing a compound annual growth rate (CAGR) of 6.9%. This growth trajectory is primarily fueled by stringent environmental regulations, particularly in developed economies, necessitating advanced catalytic solutions for emission control.
The petroleum refining industry remains the largest consumer of high-temperature catalysts, accounting for roughly 38% of the total market share. These catalysts are essential for fluid catalytic cracking (FCC), hydrocracking, and reforming processes. The chemical synthesis sector follows closely, representing approximately 31% of market demand, with applications in ammonia production, methanol synthesis, and various petrochemical processes.
Environmental applications, particularly in automotive and industrial emission control systems, constitute the fastest-growing segment with a CAGR of 8.3%. This acceleration is directly linked to increasingly stringent emission standards worldwide, especially in Europe and North America. The energy sector, including clean coal technologies and syngas production, represents another significant growth area at 7.5% CAGR.
Geographically, Asia-Pacific dominates the market with 42% share, driven by rapid industrialization in China and India. North America and Europe follow with 27% and 23% respectively, with their markets primarily focused on specialty high-performance catalysts for advanced applications and emission control systems.
Spray pyrolysis methodologies for catalyst production are gaining particular traction in market segments requiring highly dispersed active phases and precise control over catalyst morphology. The market for spray pyrolysis-derived catalysts specifically is estimated at $780 million, with projected growth exceeding the overall market rate at 9.2% annually.
Key market drivers include the push toward carbon neutrality, which is accelerating demand for more efficient catalytic processes, and the hydrogen economy's emergence, creating new applications for high-temperature catalysts in reforming and water-gas shift reactions. Additionally, the circular economy trend is fostering interest in catalysts capable of converting waste materials into valuable chemicals.
Market challenges include price sensitivity, especially in developing regions, and raw material volatility, particularly for precious metal-based catalysts. The high cost of research and development for advanced spray pyrolysis techniques also presents a barrier to market entry for smaller players, contributing to ongoing market consolidation.
The petroleum refining industry remains the largest consumer of high-temperature catalysts, accounting for roughly 38% of the total market share. These catalysts are essential for fluid catalytic cracking (FCC), hydrocracking, and reforming processes. The chemical synthesis sector follows closely, representing approximately 31% of market demand, with applications in ammonia production, methanol synthesis, and various petrochemical processes.
Environmental applications, particularly in automotive and industrial emission control systems, constitute the fastest-growing segment with a CAGR of 8.3%. This acceleration is directly linked to increasingly stringent emission standards worldwide, especially in Europe and North America. The energy sector, including clean coal technologies and syngas production, represents another significant growth area at 7.5% CAGR.
Geographically, Asia-Pacific dominates the market with 42% share, driven by rapid industrialization in China and India. North America and Europe follow with 27% and 23% respectively, with their markets primarily focused on specialty high-performance catalysts for advanced applications and emission control systems.
Spray pyrolysis methodologies for catalyst production are gaining particular traction in market segments requiring highly dispersed active phases and precise control over catalyst morphology. The market for spray pyrolysis-derived catalysts specifically is estimated at $780 million, with projected growth exceeding the overall market rate at 9.2% annually.
Key market drivers include the push toward carbon neutrality, which is accelerating demand for more efficient catalytic processes, and the hydrogen economy's emergence, creating new applications for high-temperature catalysts in reforming and water-gas shift reactions. Additionally, the circular economy trend is fostering interest in catalysts capable of converting waste materials into valuable chemicals.
Market challenges include price sensitivity, especially in developing regions, and raw material volatility, particularly for precious metal-based catalysts. The high cost of research and development for advanced spray pyrolysis techniques also presents a barrier to market entry for smaller players, contributing to ongoing market consolidation.
Current Limitations and Technical Barriers in Spray Pyrolysis
Despite significant advancements in spray pyrolysis techniques for high-temperature catalyst synthesis, several critical limitations and technical barriers continue to challenge researchers and industry practitioners. One of the primary constraints is precise control over particle morphology and size distribution. The inherent rapid evaporation and precipitation dynamics during spray pyrolysis often lead to heterogeneous particle formation, resulting in catalysts with inconsistent active site distribution and variable performance characteristics.
Temperature gradient management presents another significant challenge. The temperature profile within droplets during the pyrolysis process is difficult to control uniformly, leading to compositional variations across the catalyst particles. This non-uniformity can significantly impact catalytic activity, selectivity, and long-term stability, particularly in high-temperature applications where thermal resilience is paramount.
Scalability remains a persistent barrier to widespread industrial adoption. While laboratory-scale spray pyrolysis demonstrates promising results, scaling production to industrial volumes introduces complications in maintaining consistent product quality. Larger reactors often experience flow pattern variations and temperature inconsistencies that are difficult to predict and control, resulting in batch-to-batch variability that is unacceptable for commercial catalyst production.
Precursor chemistry limitations further constrain the versatility of spray pyrolysis. Not all catalyst compositions can be effectively synthesized using this method due to solubility constraints, complex decomposition pathways, or undesired side reactions during the pyrolysis stage. This restricts the range of high-temperature catalysts that can be produced via spray pyrolysis.
Energy efficiency concerns also present significant challenges. The process requires substantial thermal input to achieve the necessary reaction conditions, making it energy-intensive compared to some alternative synthesis methods. This high energy demand impacts both the economic viability and environmental sustainability of spray pyrolysis for large-scale catalyst production.
Equipment design limitations contribute to technical barriers as well. Current atomization technologies struggle to consistently produce the ideal droplet size distribution needed for optimal catalyst formation. Additionally, reactor designs often fail to provide the perfect balance of residence time and temperature profile required for homogeneous catalyst development.
Material deposition on reactor walls represents another operational challenge, leading to reduced yield, potential contamination of subsequent batches, and increased maintenance requirements. This issue becomes particularly problematic during continuous operation, where system stability over extended periods is essential for industrial viability.
Temperature gradient management presents another significant challenge. The temperature profile within droplets during the pyrolysis process is difficult to control uniformly, leading to compositional variations across the catalyst particles. This non-uniformity can significantly impact catalytic activity, selectivity, and long-term stability, particularly in high-temperature applications where thermal resilience is paramount.
Scalability remains a persistent barrier to widespread industrial adoption. While laboratory-scale spray pyrolysis demonstrates promising results, scaling production to industrial volumes introduces complications in maintaining consistent product quality. Larger reactors often experience flow pattern variations and temperature inconsistencies that are difficult to predict and control, resulting in batch-to-batch variability that is unacceptable for commercial catalyst production.
Precursor chemistry limitations further constrain the versatility of spray pyrolysis. Not all catalyst compositions can be effectively synthesized using this method due to solubility constraints, complex decomposition pathways, or undesired side reactions during the pyrolysis stage. This restricts the range of high-temperature catalysts that can be produced via spray pyrolysis.
Energy efficiency concerns also present significant challenges. The process requires substantial thermal input to achieve the necessary reaction conditions, making it energy-intensive compared to some alternative synthesis methods. This high energy demand impacts both the economic viability and environmental sustainability of spray pyrolysis for large-scale catalyst production.
Equipment design limitations contribute to technical barriers as well. Current atomization technologies struggle to consistently produce the ideal droplet size distribution needed for optimal catalyst formation. Additionally, reactor designs often fail to provide the perfect balance of residence time and temperature profile required for homogeneous catalyst development.
Material deposition on reactor walls represents another operational challenge, leading to reduced yield, potential contamination of subsequent batches, and increased maintenance requirements. This issue becomes particularly problematic during continuous operation, where system stability over extended periods is essential for industrial viability.
Contemporary Spray Pyrolysis Methodologies Review
01 Ultrasonic spray pyrolysis techniques
Ultrasonic spray pyrolysis involves using ultrasonic waves to atomize precursor solutions into fine droplets before pyrolysis. This technique allows for better control over particle size distribution and morphology, resulting in more uniform nanoparticles. The ultrasonic atomization process creates smaller droplets compared to conventional spray methods, leading to improved material properties and performance in various applications including thin films and powder production.- Ultrasonic spray pyrolysis techniques: Ultrasonic spray pyrolysis involves using ultrasonic vibrations to atomize precursor solutions into fine droplets that are then pyrolyzed. This technique allows for precise control over particle size and morphology, resulting in high-quality thin films or nanoparticles. The ultrasonic atomization creates uniform droplets that lead to homogeneous material deposition, which is particularly valuable for creating advanced electronic and optical materials.
- Flame spray pyrolysis for nanomaterial synthesis: Flame spray pyrolysis is a method where precursor solutions are sprayed into a flame, causing rapid evaporation, decomposition, and particle formation. This technique is particularly effective for producing metal oxide nanoparticles with controlled size distribution and high purity. The high-temperature environment of the flame enables the formation of crystalline materials without the need for additional heat treatment, making it an energy-efficient process for large-scale nanomaterial production.
- Aerosol-assisted spray pyrolysis systems: Aerosol-assisted spray pyrolysis involves generating an aerosol from precursor solutions and directing it through a heated zone for decomposition and film formation. This methodology is particularly useful for creating thin films with controlled thickness and composition on various substrates. The aerosol can be generated using different methods such as pneumatic atomization or electrospray, allowing for versatility in material processing and application-specific optimization.
- Precursor formulations for spray pyrolysis: The composition and properties of precursor solutions significantly impact the quality of materials produced through spray pyrolysis. Optimized precursor formulations include considerations of solubility, viscosity, surface tension, and chemical stability. Various solvents, complexing agents, and additives can be incorporated to enhance the spray characteristics and subsequent decomposition behavior, leading to improved material properties such as crystallinity, density, and functional performance.
- Reactor design and process parameters for spray pyrolysis: The design of spray pyrolysis reactors and control of process parameters are crucial for achieving desired material properties. Key parameters include temperature profiles, residence time, carrier gas composition, and flow rates. Vertical tube furnaces, horizontal reactors, and custom-designed chambers with temperature gradient control are commonly employed. Advanced reactor designs incorporate features for precise droplet delivery, controlled atmosphere, and efficient collection of the synthesized materials, enabling scalable production of high-quality materials.
02 Flame spray pyrolysis for nanomaterial synthesis
Flame spray pyrolysis is a method where precursor solutions are sprayed directly into a flame, causing rapid evaporation, decomposition, and particle formation. This technique is particularly effective for producing metal oxide nanoparticles with high purity and crystallinity. The high-temperature environment of the flame enables the formation of complex oxide structures and allows for doping with various elements to tailor material properties for catalytic, electronic, and optical applications.Expand Specific Solutions03 Aerosol-assisted spray pyrolysis for thin film deposition
Aerosol-assisted spray pyrolysis involves generating an aerosol from precursor solutions and directing it onto heated substrates for thin film formation. This methodology enables the deposition of uniform thin films with controlled thickness and composition on various substrate materials. The technique is particularly valuable for producing functional coatings, semiconductor layers, and transparent conductive oxides for electronic devices, solar cells, and sensors.Expand Specific Solutions04 Reactor design and process parameters for spray pyrolysis
The design of spray pyrolysis reactors and optimization of process parameters significantly influence the properties of the resulting materials. Key parameters include temperature profiles, residence time, carrier gas flow rates, and precursor concentration. Vertical tube furnaces, horizontal reactors, and custom-designed chambers are commonly used configurations. Proper control of these parameters enables the synthesis of materials with desired morphology, crystallinity, and chemical composition for specific applications.Expand Specific Solutions05 Precursor solution engineering for enhanced material properties
The composition and properties of precursor solutions play a crucial role in spray pyrolysis outcomes. Engineering these solutions through the selection of appropriate solvents, stabilizers, and additives can control particle nucleation and growth mechanisms. Adjusting solution viscosity, surface tension, and concentration affects droplet formation and subsequent decomposition behavior. Advanced precursor formulations enable the synthesis of complex materials, including doped compounds, core-shell structures, and composite materials with enhanced functional properties.Expand Specific Solutions
Leading Research Institutions and Industrial Manufacturers
The spray pyrolysis methodology for high-temperature catalysts market is currently in a growth phase, with increasing demand driven by energy and chemical processing sectors. The global market size is expanding steadily, projected to reach significant value due to rising industrial applications requiring efficient catalytic processes. Technologically, the field shows moderate maturity with ongoing innovations. Leading players like China Petroleum & Chemical Corp. (Sinopec) and BASF are advancing commercial applications, while specialized firms such as Pyrochem Catalyst Co. offer proprietary technologies. Research institutions including Shanghai Petrochemical Research Institute and McGill University are driving fundamental innovations. Saudi Aramco, Shell, and PetroChina are investing in application-specific developments, particularly for petroleum refining and petrochemical processes, indicating strong industry commitment to this technology.
BASF Corp.
Technical Solution: BASF has developed advanced spray pyrolysis methodologies for high-temperature catalysts, focusing on their FlexCat™ technology platform. This approach utilizes ultrasonic spray pyrolysis to create highly dispersed metal oxide catalysts with controlled morphology and composition. Their process involves atomizing precursor solutions into micron-sized droplets that undergo rapid evaporation and decomposition in a high-temperature reactor (600-900°C), resulting in uniform spherical particles. BASF's methodology incorporates in-situ doping during the spray process, allowing precise control of catalyst properties. They've optimized the process parameters including precursor concentration, carrier gas flow rate, and residence time to achieve catalysts with superior thermal stability up to 1100°C. Recent innovations include the incorporation of rare earth elements as structural promoters to prevent sintering during high-temperature operations, particularly beneficial for automotive catalytic converters and petrochemical processes.
Strengths: Superior control over particle size distribution and morphology; excellent thermal stability; ability to create complex multi-component catalysts in a single step; scalable continuous production process. Weaknesses: Higher production costs compared to traditional precipitation methods; challenges in controlling porosity; energy-intensive process requiring precise temperature control.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has pioneered a proprietary spray pyrolysis methodology called Shell Catalytic Spray Process (SCSP) for high-temperature catalysts used in hydrocarbon conversion processes. Their approach utilizes a modified flame spray pyrolysis technique where metal-organic precursors are atomized into a high-temperature flame (1200-1500°C), creating nanoscale catalyst particles with exceptional thermal stability. Shell's innovation lies in their precursor formulation, which includes stabilizing agents that prevent agglomeration during the high-temperature synthesis. The process produces catalysts with controlled hierarchical porosity, enhancing mass transfer properties critical for reactions like methane reforming and Fischer-Tropsch synthesis. Shell has recently enhanced this technology by incorporating computational fluid dynamics to optimize spray patterns and residence times, resulting in more uniform catalyst properties. Their catalysts demonstrate remarkable resistance to coking and sintering at temperatures exceeding 900°C, making them particularly valuable for hydrogen production and gas-to-liquid technologies where severe operating conditions are common.
Strengths: Exceptional thermal stability at extreme temperatures; highly tunable surface properties; rapid one-step synthesis process; excellent resistance to catalyst poisoning. Weaknesses: High energy consumption during production; challenges in scaling up while maintaining quality consistency; limited control over secondary pore structure.
Critical Patents and Scientific Breakthroughs
Process for preparing catalyst composition for the synthesis of carbon nanotube with high yields using the spray pyrolysis method
PatentActiveUS9006132B2
Innovation
- A process involving dissolving multi-component metal precursors in de-ionized water, spraying the solution into a high-temperature reactor by gas atomization, and forming catalyst composition powder through pyrolysis at controlled temperatures and pressures, using Fe or Co as main catalysts with Al and optional co-catalysts like Ni, Cu, and Mg as support, to achieve high yields and low apparent density.
Method for producing metal oxides by means of spray pyrolysis
PatentActiveUS11434146B2
Innovation
- A process involving flame spray pyrolysis with a reaction space containing double-walled internals that introduce gas or vapor through slots to rotate the flame, increasing the residence time and adjusting the flame's geometry, which enhances the conversion and crystallinity of metal oxides by modifying the reaction conditions.
Materials Characterization and Performance Metrics
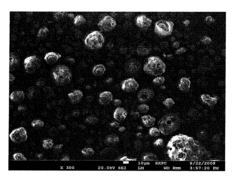
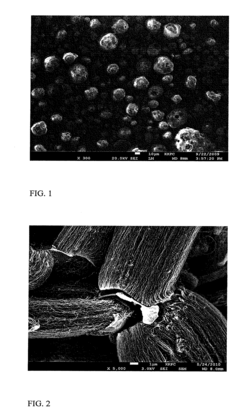
Characterization of catalysts produced through spray pyrolysis methodologies requires comprehensive analytical techniques to evaluate both structural properties and performance metrics. X-ray diffraction (XRD) serves as a primary tool for phase identification and crystallinity assessment, while scanning electron microscopy (SEM) and transmission electron microscopy (TEM) provide critical insights into morphology, particle size distribution, and surface features at micro and nano scales. These imaging techniques are particularly valuable for high-temperature catalysts where structural stability under thermal stress is paramount.
Surface area analysis using Brunauer-Emmett-Teller (BET) methodology represents another essential characterization approach, as catalytic activity correlates strongly with available surface area. For spray pyrolysis-derived catalysts, BET measurements typically reveal surface areas ranging from 20-150 m²/g depending on precursor composition and synthesis parameters. Temperature-programmed reduction (TPR) and temperature-programmed desorption (TPD) further elucidate redox properties and active site distribution.
Performance metrics for high-temperature catalysts must address both activity and durability under extreme conditions. Conversion efficiency, measured as percentage of reactant transformed per unit time, serves as the primary activity indicator. For industrial applications, catalysts must maintain at least 90% of initial conversion efficiency after extended operation at temperatures exceeding 800°C. Selectivity, quantified as the ratio of desired product to total products, represents another critical performance parameter, particularly for complex reactions where multiple pathways exist.
Thermal stability testing involves cyclic exposure to high temperatures (800-1200°C) followed by performance evaluation, with industry standards typically requiring less than 5% degradation after 100 cycles. Mechanical strength, measured through crush strength tests or attrition resistance evaluations, ensures catalyst integrity in industrial reactors. For spray pyrolysis catalysts, crush strength values exceeding 20 N/mm are generally considered acceptable for fixed-bed applications.
Deactivation kinetics provide insights into catalyst longevity, with time-on-stream studies revealing performance decay rates under various conditions. Advanced characterization techniques such as in-situ spectroscopy enable real-time monitoring of catalyst behavior during reaction conditions, bridging the gap between laboratory characterization and practical application. These comprehensive characterization approaches and standardized performance metrics facilitate meaningful comparison between different spray pyrolysis methodologies and guide optimization efforts for high-temperature catalytic applications.
Surface area analysis using Brunauer-Emmett-Teller (BET) methodology represents another essential characterization approach, as catalytic activity correlates strongly with available surface area. For spray pyrolysis-derived catalysts, BET measurements typically reveal surface areas ranging from 20-150 m²/g depending on precursor composition and synthesis parameters. Temperature-programmed reduction (TPR) and temperature-programmed desorption (TPD) further elucidate redox properties and active site distribution.
Performance metrics for high-temperature catalysts must address both activity and durability under extreme conditions. Conversion efficiency, measured as percentage of reactant transformed per unit time, serves as the primary activity indicator. For industrial applications, catalysts must maintain at least 90% of initial conversion efficiency after extended operation at temperatures exceeding 800°C. Selectivity, quantified as the ratio of desired product to total products, represents another critical performance parameter, particularly for complex reactions where multiple pathways exist.
Thermal stability testing involves cyclic exposure to high temperatures (800-1200°C) followed by performance evaluation, with industry standards typically requiring less than 5% degradation after 100 cycles. Mechanical strength, measured through crush strength tests or attrition resistance evaluations, ensures catalyst integrity in industrial reactors. For spray pyrolysis catalysts, crush strength values exceeding 20 N/mm are generally considered acceptable for fixed-bed applications.
Deactivation kinetics provide insights into catalyst longevity, with time-on-stream studies revealing performance decay rates under various conditions. Advanced characterization techniques such as in-situ spectroscopy enable real-time monitoring of catalyst behavior during reaction conditions, bridging the gap between laboratory characterization and practical application. These comprehensive characterization approaches and standardized performance metrics facilitate meaningful comparison between different spray pyrolysis methodologies and guide optimization efforts for high-temperature catalytic applications.
Environmental Impact and Sustainability Considerations
Spray pyrolysis methodologies for high-temperature catalyst production present significant environmental considerations that must be addressed for sustainable implementation. The process inherently consumes substantial energy due to the high temperatures required for pyrolysis reactions, typically ranging from 400°C to 800°C. This energy demand contributes to carbon emissions when fossil fuel sources are utilized, creating a notable environmental footprint that contradicts sustainability goals in green chemistry and manufacturing.
Water consumption represents another critical environmental factor, as spray pyrolysis techniques often require substantial quantities of water for solution preparation and cooling systems. In regions facing water scarcity, this dependency raises concerns about resource depletion and competition with other essential needs. Additionally, the process generates wastewater containing metal precursors and other chemicals that require proper treatment before discharge to prevent contamination of natural water bodies.
The precursor materials employed in spray pyrolysis frequently include metal salts and organic compounds that may pose environmental hazards. Some precursors contain toxic heavy metals or generate harmful byproducts during decomposition, necessitating careful handling and disposal protocols. Recent research has focused on developing greener precursor alternatives derived from sustainable sources, including bio-based materials and recovered metals from waste streams.
Airborne emissions constitute a significant environmental challenge in spray pyrolysis operations. The atomization process can release fine particulates and volatile organic compounds (VOCs) that contribute to air pollution if not properly contained. Modern systems increasingly incorporate advanced filtration technologies and closed-loop designs to minimize these emissions, though implementation varies across different production scales and regions.
Life cycle assessment (LCA) studies of spray pyrolysis catalysts reveal opportunities for sustainability improvements throughout the production chain. Researchers have demonstrated that optimizing process parameters can reduce energy consumption by 15-30% while maintaining catalyst performance. Furthermore, the development of ambient-temperature spray pyrolysis techniques represents a promising direction for dramatically reducing the energy requirements of this methodology.
The recyclability of spent catalysts presents both challenges and opportunities for sustainability. High-temperature catalysts produced via spray pyrolysis can often be regenerated through controlled thermal treatments, extending their useful lifespan. End-of-life recovery of precious metals from these catalysts is becoming increasingly economical as recovery technologies advance, creating closed material loops that reduce primary resource extraction demands.
Water consumption represents another critical environmental factor, as spray pyrolysis techniques often require substantial quantities of water for solution preparation and cooling systems. In regions facing water scarcity, this dependency raises concerns about resource depletion and competition with other essential needs. Additionally, the process generates wastewater containing metal precursors and other chemicals that require proper treatment before discharge to prevent contamination of natural water bodies.
The precursor materials employed in spray pyrolysis frequently include metal salts and organic compounds that may pose environmental hazards. Some precursors contain toxic heavy metals or generate harmful byproducts during decomposition, necessitating careful handling and disposal protocols. Recent research has focused on developing greener precursor alternatives derived from sustainable sources, including bio-based materials and recovered metals from waste streams.
Airborne emissions constitute a significant environmental challenge in spray pyrolysis operations. The atomization process can release fine particulates and volatile organic compounds (VOCs) that contribute to air pollution if not properly contained. Modern systems increasingly incorporate advanced filtration technologies and closed-loop designs to minimize these emissions, though implementation varies across different production scales and regions.
Life cycle assessment (LCA) studies of spray pyrolysis catalysts reveal opportunities for sustainability improvements throughout the production chain. Researchers have demonstrated that optimizing process parameters can reduce energy consumption by 15-30% while maintaining catalyst performance. Furthermore, the development of ambient-temperature spray pyrolysis techniques represents a promising direction for dramatically reducing the energy requirements of this methodology.
The recyclability of spent catalysts presents both challenges and opportunities for sustainability. High-temperature catalysts produced via spray pyrolysis can often be regenerated through controlled thermal treatments, extending their useful lifespan. End-of-life recovery of precious metals from these catalysts is becoming increasingly economical as recovery technologies advance, creating closed material loops that reduce primary resource extraction demands.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!