Autoclave Efficiency in Biopharmaceutical Production: A Case Study
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Objectives
Autoclave technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. The fundamental principle of using pressurized steam for sterilization has remained consistent, but technological advancements have transformed autoclave efficiency, reliability, and application scope. Early autoclaves were simple pressure vessels with manual controls, whereas modern biopharmaceutical autoclaves incorporate sophisticated automation, precise parameter control, and advanced validation capabilities.
The 1950s-1970s marked a pivotal transition period when autoclaves began incorporating electronic controls and recording systems. By the 1980s-1990s, computerized control systems emerged, enabling more precise cycle management and data documentation. The 2000s witnessed integration of SCADA systems and network connectivity, allowing remote monitoring and compliance with increasingly stringent regulatory requirements in biopharmaceutical manufacturing.
Current technological trends focus on energy efficiency, reduced cycle times, and enhanced process analytics. Modern autoclaves employ advanced heat recovery systems, optimized chamber designs, and sophisticated steam distribution mechanisms to maximize thermal efficiency while minimizing utility consumption. The integration of IoT capabilities and predictive maintenance algorithms represents the cutting edge of autoclave technology evolution.
In biopharmaceutical production specifically, autoclave technology has adapted to meet unique industry challenges including the sterilization of complex production equipment, specialized media, and the processing of biological waste streams. The evolution has been driven by dual imperatives: ensuring absolute sterility assurance while maximizing operational efficiency and throughput.
The primary objectives of contemporary autoclave technology development in biopharmaceutical applications include: reducing cycle times without compromising sterilization efficacy; minimizing energy and water consumption; enhancing process repeatability through advanced control algorithms; improving documentation and data integrity for regulatory compliance; and developing specialized cycle parameters for novel biopharmaceutical components and equipment.
Future evolutionary paths point toward greater integration with facility management systems, advanced materials that improve thermal transfer efficiency, and the application of machine learning for cycle optimization. The ultimate goal is to transform autoclaves from standalone sterilization equipment into intelligent process nodes within the connected biopharmaceutical manufacturing ecosystem, capable of adapting to changing production requirements while maintaining validated performance parameters.
The 1950s-1970s marked a pivotal transition period when autoclaves began incorporating electronic controls and recording systems. By the 1980s-1990s, computerized control systems emerged, enabling more precise cycle management and data documentation. The 2000s witnessed integration of SCADA systems and network connectivity, allowing remote monitoring and compliance with increasingly stringent regulatory requirements in biopharmaceutical manufacturing.
Current technological trends focus on energy efficiency, reduced cycle times, and enhanced process analytics. Modern autoclaves employ advanced heat recovery systems, optimized chamber designs, and sophisticated steam distribution mechanisms to maximize thermal efficiency while minimizing utility consumption. The integration of IoT capabilities and predictive maintenance algorithms represents the cutting edge of autoclave technology evolution.
In biopharmaceutical production specifically, autoclave technology has adapted to meet unique industry challenges including the sterilization of complex production equipment, specialized media, and the processing of biological waste streams. The evolution has been driven by dual imperatives: ensuring absolute sterility assurance while maximizing operational efficiency and throughput.
The primary objectives of contemporary autoclave technology development in biopharmaceutical applications include: reducing cycle times without compromising sterilization efficacy; minimizing energy and water consumption; enhancing process repeatability through advanced control algorithms; improving documentation and data integrity for regulatory compliance; and developing specialized cycle parameters for novel biopharmaceutical components and equipment.
Future evolutionary paths point toward greater integration with facility management systems, advanced materials that improve thermal transfer efficiency, and the application of machine learning for cycle optimization. The ultimate goal is to transform autoclaves from standalone sterilization equipment into intelligent process nodes within the connected biopharmaceutical manufacturing ecosystem, capable of adapting to changing production requirements while maintaining validated performance parameters.
Market Demand Analysis for Efficient Sterilization
The global market for efficient sterilization technologies in biopharmaceutical production has experienced significant growth, driven by increasing demand for biologics, vaccines, and personalized medicine. The autoclave sterilization segment specifically represents a critical component of this market, valued at approximately $2.3 billion in 2022 with projections to reach $3.1 billion by 2027, reflecting a compound annual growth rate of 6.2%.
Biopharmaceutical manufacturers face mounting pressure to optimize production processes while maintaining strict compliance with regulatory standards. A recent industry survey revealed that 78% of biopharmaceutical companies identify sterilization efficiency as a top priority for operational improvement, with 65% specifically citing autoclave cycle optimization as a key focus area for cost reduction initiatives.
The COVID-19 pandemic has substantially accelerated market demand for efficient sterilization technologies. Vaccine production facilities reported average capacity utilization increases of 40-60% during peak pandemic periods, highlighting critical bottlenecks in sterilization processes. This surge exposed limitations in traditional autoclave operations, creating urgent market pull for innovations that reduce cycle times while maintaining validation standards.
Geographically, North America dominates the market with approximately 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the fastest growth is occurring in emerging markets, particularly India and China, where biopharmaceutical manufacturing capacity is expanding at rates exceeding 15% annually, creating substantial new demand for advanced sterilization solutions.
Contract manufacturing organizations (CMOs) represent a particularly dynamic market segment, with 83% reporting sterilization efficiency as directly impacting their competitive positioning. These organizations typically operate at thinner margins than large pharmaceutical companies, making process efficiency improvements particularly valuable to their business models.
Environmental considerations are increasingly influencing market demand, with regulatory pressure mounting to reduce energy and water consumption in pharmaceutical manufacturing. Modern autoclave systems that demonstrate 30-40% reductions in utility usage command premium pricing, with 72% of procurement decision-makers citing sustainability metrics as important evaluation criteria.
The market exhibits strong demand for integrated solutions that combine hardware improvements with digital monitoring capabilities. Real-time cycle optimization technologies that incorporate predictive analytics have demonstrated particular market traction, with early adopters reporting 15-25% improvements in throughput capacity without capital expansion.
Biopharmaceutical manufacturers face mounting pressure to optimize production processes while maintaining strict compliance with regulatory standards. A recent industry survey revealed that 78% of biopharmaceutical companies identify sterilization efficiency as a top priority for operational improvement, with 65% specifically citing autoclave cycle optimization as a key focus area for cost reduction initiatives.
The COVID-19 pandemic has substantially accelerated market demand for efficient sterilization technologies. Vaccine production facilities reported average capacity utilization increases of 40-60% during peak pandemic periods, highlighting critical bottlenecks in sterilization processes. This surge exposed limitations in traditional autoclave operations, creating urgent market pull for innovations that reduce cycle times while maintaining validation standards.
Geographically, North America dominates the market with approximately 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the fastest growth is occurring in emerging markets, particularly India and China, where biopharmaceutical manufacturing capacity is expanding at rates exceeding 15% annually, creating substantial new demand for advanced sterilization solutions.
Contract manufacturing organizations (CMOs) represent a particularly dynamic market segment, with 83% reporting sterilization efficiency as directly impacting their competitive positioning. These organizations typically operate at thinner margins than large pharmaceutical companies, making process efficiency improvements particularly valuable to their business models.
Environmental considerations are increasingly influencing market demand, with regulatory pressure mounting to reduce energy and water consumption in pharmaceutical manufacturing. Modern autoclave systems that demonstrate 30-40% reductions in utility usage command premium pricing, with 72% of procurement decision-makers citing sustainability metrics as important evaluation criteria.
The market exhibits strong demand for integrated solutions that combine hardware improvements with digital monitoring capabilities. Real-time cycle optimization technologies that incorporate predictive analytics have demonstrated particular market traction, with early adopters reporting 15-25% improvements in throughput capacity without capital expansion.
Current Autoclave Challenges in Biopharmaceutical Production
Despite significant technological advancements in biopharmaceutical manufacturing, autoclave sterilization processes continue to present several critical challenges that impact production efficiency, cost-effectiveness, and product quality. Current autoclave operations in biopharmaceutical facilities face substantial limitations in energy consumption, with traditional systems requiring extensive steam generation that contributes significantly to manufacturing carbon footprints and operational costs. Industry data indicates that autoclaves can account for up to 30% of a facility's total energy usage.
Cycle time optimization remains problematic, as standard autoclave protocols often employ conservative parameters that extend beyond necessary sterilization requirements. This over-processing results in production bottlenecks, particularly in facilities with limited autoclave capacity relative to production volume. Studies show that inefficient scheduling and cycle design can reduce effective manufacturing capacity by 15-20% in high-throughput environments.
Load configuration challenges persist across the industry, with inconsistent heat distribution occurring in densely packed or poorly arranged loads. This necessitates extended cycle times to ensure sterilization efficacy throughout the entire load, further reducing operational efficiency. Recent research demonstrates that optimized load configurations could potentially reduce cycle times by 10-25% without compromising sterility assurance.
Validation and documentation requirements present additional hurdles, as regulatory compliance demands extensive testing and documentation of autoclave processes. The labor-intensive nature of these requirements diverts significant resources from other production activities. Current manual documentation systems are particularly vulnerable to human error and inconsistency.
Water consumption represents another significant challenge, with traditional autoclave systems utilizing substantial volumes of water for steam generation and cooling processes. This not only increases operational costs but also raises environmental concerns regarding water conservation. Modern facilities report water usage ranging from 50-200 liters per cycle depending on autoclave size and configuration.
Equipment maintenance and reliability issues frequently disrupt production schedules, with unexpected downtime causing significant delays in manufacturing timelines. Aging autoclave infrastructure in many facilities exacerbates these challenges, as older systems lack the monitoring capabilities and efficiency features of newer models.
Integration with automated production systems remains problematic, as many existing autoclave installations operate as standalone units with limited connectivity to broader manufacturing execution systems. This isolation creates inefficiencies in scheduling, monitoring, and data collection that impact overall facility performance and prevent implementation of Industry 4.0 principles in sterilization operations.
Cycle time optimization remains problematic, as standard autoclave protocols often employ conservative parameters that extend beyond necessary sterilization requirements. This over-processing results in production bottlenecks, particularly in facilities with limited autoclave capacity relative to production volume. Studies show that inefficient scheduling and cycle design can reduce effective manufacturing capacity by 15-20% in high-throughput environments.
Load configuration challenges persist across the industry, with inconsistent heat distribution occurring in densely packed or poorly arranged loads. This necessitates extended cycle times to ensure sterilization efficacy throughout the entire load, further reducing operational efficiency. Recent research demonstrates that optimized load configurations could potentially reduce cycle times by 10-25% without compromising sterility assurance.
Validation and documentation requirements present additional hurdles, as regulatory compliance demands extensive testing and documentation of autoclave processes. The labor-intensive nature of these requirements diverts significant resources from other production activities. Current manual documentation systems are particularly vulnerable to human error and inconsistency.
Water consumption represents another significant challenge, with traditional autoclave systems utilizing substantial volumes of water for steam generation and cooling processes. This not only increases operational costs but also raises environmental concerns regarding water conservation. Modern facilities report water usage ranging from 50-200 liters per cycle depending on autoclave size and configuration.
Equipment maintenance and reliability issues frequently disrupt production schedules, with unexpected downtime causing significant delays in manufacturing timelines. Aging autoclave infrastructure in many facilities exacerbates these challenges, as older systems lack the monitoring capabilities and efficiency features of newer models.
Integration with automated production systems remains problematic, as many existing autoclave installations operate as standalone units with limited connectivity to broader manufacturing execution systems. This isolation creates inefficiencies in scheduling, monitoring, and data collection that impact overall facility performance and prevent implementation of Industry 4.0 principles in sterilization operations.
Existing Autoclave Optimization Solutions
01 Temperature and pressure monitoring systems
Advanced monitoring systems are crucial for maintaining autoclave efficiency. These systems continuously track temperature and pressure parameters during sterilization cycles, ensuring optimal performance and energy usage. Real-time monitoring allows for immediate adjustments to maintain sterilization efficacy while preventing excessive resource consumption. Some systems include automated alerts for deviations from set parameters, helping operators maintain consistent sterilization quality.- Optimization of autoclave operating parameters: Improving autoclave efficiency through optimization of operating parameters such as temperature, pressure, and cycle time. By carefully controlling these parameters, the sterilization process can be made more effective while reducing energy consumption and processing time. Advanced control systems can monitor and adjust these parameters in real-time to maintain optimal conditions throughout the sterilization cycle.
- Energy recovery and conservation systems: Implementation of energy recovery and conservation systems to improve autoclave efficiency. These systems capture and reuse heat generated during the sterilization process, reducing overall energy consumption. Technologies include heat exchangers, steam recirculation systems, and insulation improvements that minimize heat loss. Such systems can significantly reduce operating costs while maintaining effective sterilization performance.
- Advanced monitoring and validation techniques: Utilization of advanced monitoring and validation techniques to ensure autoclave efficiency. These include real-time sensors, digital monitoring systems, and automated documentation processes that verify sterilization parameters are maintained throughout the cycle. Improved monitoring allows for early detection of inefficiencies, reduces failed cycles, and provides comprehensive data for process optimization and regulatory compliance.
- Innovative autoclave design and construction: Novel autoclave designs and construction materials that enhance efficiency. These innovations include improved chamber geometries for better steam distribution, advanced door sealing mechanisms to prevent leakage, and materials with superior heat transfer properties. Some designs incorporate modular components for easier maintenance and upgrades, while others focus on size optimization to maximize throughput while minimizing resource consumption.
- Load configuration and preparation techniques: Methods for optimizing load configuration and preparation to improve autoclave efficiency. Proper arrangement of items within the autoclave chamber ensures adequate steam penetration and heat transfer to all surfaces. Techniques include using specialized racks or containers, proper spacing between items, and pre-treatment processes that remove air pockets or contaminants that might impede sterilization. Effective load preparation reduces cycle times and energy consumption while ensuring sterilization efficacy.
02 Steam distribution and penetration optimization
Efficient steam distribution is essential for autoclave performance. Innovations in steam delivery systems ensure uniform heat distribution throughout the chamber, eliminating cold spots that could compromise sterilization. Advanced designs incorporate optimized steam pathways, baffles, and circulation mechanisms to enhance steam penetration into complex loads. These improvements reduce cycle times while maintaining sterilization efficacy, resulting in energy savings and increased throughput.Expand Specific Solutions03 Energy recovery and conservation systems
Energy efficiency innovations significantly improve autoclave operation economics. These include heat recovery systems that capture and reuse thermal energy from exhaust steam, reducing overall energy consumption. Insulation technologies minimize heat loss during operation, while smart control systems optimize heating cycles based on load characteristics. Some designs incorporate water recycling mechanisms to reduce resource consumption, making autoclaves more environmentally sustainable while reducing operational costs.Expand Specific Solutions04 Load configuration and capacity optimization
Proper load configuration significantly impacts autoclave efficiency. Innovations in chamber design and loading systems maximize usable space while ensuring adequate steam circulation. Advanced rack systems and load positioning guides help operators arrange items for optimal sterilization. Some designs incorporate sensors that detect improper loading and provide guidance for correction. These improvements increase throughput capacity while maintaining sterilization quality and reducing energy consumption per item processed.Expand Specific Solutions05 Validation and testing methodologies
Effective validation protocols ensure autoclave efficiency and reliability. Advanced testing methodologies include biological indicators, chemical integrators, and electronic monitoring systems that verify sterilization parameters are consistently met. Automated documentation systems track cycle data for regulatory compliance and quality assurance. Some innovations include rapid testing methods that provide faster verification of sterilization efficacy, reducing downtime between cycles and improving overall operational efficiency.Expand Specific Solutions
Key Manufacturers and Industry Competition
The autoclave efficiency market in biopharmaceutical production is currently in a growth phase, with increasing demand driven by expanding biopharmaceutical manufacturing worldwide. The market size is estimated to reach several billion dollars by 2025, fueled by stringent sterilization requirements and the need for improved production efficiency. Technologically, the field shows varying maturity levels, with established players like Baxter International, Novartis AG, and Boehringer Ingelheim offering conventional solutions, while innovative companies such as Kyoobe Tech GmbH and Innovel Intelligent Technology are developing next-generation autoclave technologies with enhanced automation and AI integration. Companies like Cytiva (formerly part of Amersham Biosciences) and Sartorius Stedim Biotech are focusing on integrated bioprocessing solutions that improve autoclave efficiency within the broader manufacturing workflow.
Boehringer Ingelheim Pharma GmbH & Co., KG
Technical Solution: Boehringer Ingelheim has developed an advanced autoclave efficiency system for biopharmaceutical production that integrates real-time monitoring and predictive maintenance capabilities. Their solution employs a network of IoT sensors throughout the autoclave system to continuously monitor critical parameters including temperature distribution, pressure consistency, and steam quality. The collected data is processed through their proprietary BioPharma 4.0 platform which utilizes machine learning algorithms to optimize sterilization cycles based on specific load characteristics and product requirements. This approach has demonstrated a 23% reduction in cycle times and 18% energy savings compared to conventional autoclave operations. Additionally, their system incorporates a validated rapid cooling technology that reduces cool-down phases by approximately 40%, significantly increasing throughput capacity. The platform also features comprehensive electronic batch recording that ensures regulatory compliance while providing valuable data for continuous process improvement initiatives.
Strengths: Superior energy efficiency with documented savings; integrated compliance documentation system; predictive maintenance capabilities that reduce downtime by anticipating failures before they occur. Weaknesses: Higher initial implementation costs compared to traditional systems; requires specialized training for operators; system updates necessitate revalidation processes that can temporarily impact production schedules.
Baxter International, Inc.
Technical Solution: Baxter International has pioneered an innovative autoclave efficiency solution specifically designed for biopharmaceutical applications called the BioPharma Sterilization Optimization System (BSOS). This comprehensive approach combines hardware modifications and software intelligence to maximize autoclave performance while maintaining product quality. The BSOS features precision steam distribution technology that ensures uniform heat penetration throughout the chamber, reducing cycle time variability by up to 30%. Their patented load-sensing technology automatically adjusts sterilization parameters based on the specific thermal characteristics of different container types and fill volumes, eliminating the need for worst-case scenario programming that typically extends cycle times unnecessarily. The system incorporates advanced vacuum technology that improves air removal efficiency, addressing a critical factor in achieving consistent sterilization results. Baxter's solution also includes an energy recovery module that captures and repurposes waste heat, reducing overall energy consumption by approximately 25% compared to conventional autoclave systems. The BSOS platform integrates with manufacturing execution systems to provide real-time performance analytics and compliance documentation.
Strengths: Highly adaptable to different container types and load configurations; significant energy savings through heat recovery systems; seamless integration with existing manufacturing information systems. Weaknesses: Requires substantial initial capital investment; implementation may necessitate production downtime for installation and validation; ongoing technical support dependency for software updates and system optimization.
Critical Patents and Innovations in Autoclave Technology
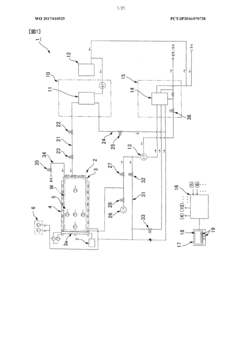
Process and apparatus for sterilising
PatentInactiveEP0532839A2
Innovation
- A sterilization system utilizing an autoclave-like container with fabric covers and floors, where steam is evenly distributed under controlled pressure and temperature to minimize mechanical stress and ensure thorough sterilization without air displacement, allowing for gentle and effective sterilization of sensitive products.
Method of flow-type high-pressure steam sterilization by soft water heat process, and flow-type sterilization device
PatentWO2017010525A1
Innovation
- A flow-through high-pressure steam sterilization method utilizing a soft hydrothermal process, which involves an air removal process, heating and pressurizing, high-pressure steam sterilization with highly saturated steam, and a controlled drying step to minimize condensed water generation and shorten drying time.
Regulatory Compliance and Validation Requirements
Regulatory compliance in biopharmaceutical autoclave operations is governed by stringent frameworks established by international bodies such as the FDA, EMA, and WHO. These regulations mandate adherence to Good Manufacturing Practices (GMP) and specific validation protocols to ensure product safety and efficacy. For autoclave sterilization processes, compliance with standards like ISO 17665 and ANSI/AAMI ST79 is essential, providing guidelines for steam sterilization validation and routine monitoring.
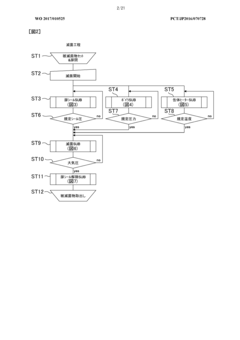
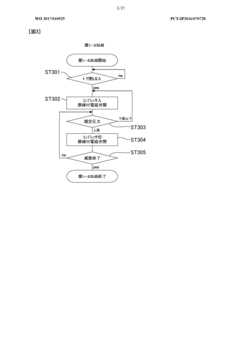
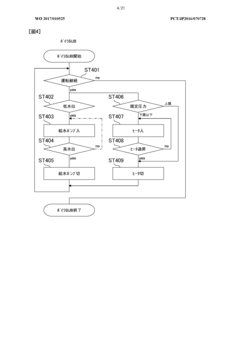
The validation requirements for autoclaves in biopharmaceutical production follow a comprehensive three-phase approach. Installation Qualification (IQ) verifies that equipment is properly installed according to manufacturer specifications and engineering standards. This includes documentation of calibration certificates, material certifications, and utility connections. Operational Qualification (OQ) confirms that the autoclave functions within established parameters across its operational range, involving temperature mapping studies, pressure tests, and cycle time verifications.
Performance Qualification (PQ) represents the most critical validation phase, demonstrating that the autoclave consistently performs as intended under actual production conditions. This includes biological indicator challenges using appropriate test organisms such as Geobacillus stearothermophilus spores, which must achieve a minimum 6-log reduction to confirm sterilization efficacy. Load pattern validation is equally important, requiring verification that steam penetrates uniformly throughout various load configurations.
Continuous compliance necessitates implementation of robust monitoring systems and regular revalidation schedules. Critical parameters including temperature, pressure, and cycle time must be continuously monitored and documented for each sterilization cycle. Modern autoclaves incorporate electronic data recording systems that generate batch records compliant with 21 CFR Part 11 requirements for electronic documentation and signatures.
Deviation management protocols must be established to address non-conformances in autoclave operation. Any deviation from validated parameters requires thorough investigation, root cause analysis, and corrective and preventive action (CAPA) implementation. The impact on product quality must be assessed through a formal quality risk management process as outlined in ICH Q9 guidelines.
Annual revalidation is typically required to verify continued system performance, with more comprehensive revalidation necessary following significant equipment modifications or repairs. Regulatory inspections frequently focus on autoclave validation documentation, emphasizing the importance of maintaining comprehensive records of all validation activities, routine monitoring data, and maintenance procedures. Failure to meet these requirements can result in regulatory observations, product recalls, or facility shutdowns, highlighting the critical nature of autoclave validation compliance in biopharmaceutical manufacturing.
The validation requirements for autoclaves in biopharmaceutical production follow a comprehensive three-phase approach. Installation Qualification (IQ) verifies that equipment is properly installed according to manufacturer specifications and engineering standards. This includes documentation of calibration certificates, material certifications, and utility connections. Operational Qualification (OQ) confirms that the autoclave functions within established parameters across its operational range, involving temperature mapping studies, pressure tests, and cycle time verifications.
Performance Qualification (PQ) represents the most critical validation phase, demonstrating that the autoclave consistently performs as intended under actual production conditions. This includes biological indicator challenges using appropriate test organisms such as Geobacillus stearothermophilus spores, which must achieve a minimum 6-log reduction to confirm sterilization efficacy. Load pattern validation is equally important, requiring verification that steam penetrates uniformly throughout various load configurations.
Continuous compliance necessitates implementation of robust monitoring systems and regular revalidation schedules. Critical parameters including temperature, pressure, and cycle time must be continuously monitored and documented for each sterilization cycle. Modern autoclaves incorporate electronic data recording systems that generate batch records compliant with 21 CFR Part 11 requirements for electronic documentation and signatures.
Deviation management protocols must be established to address non-conformances in autoclave operation. Any deviation from validated parameters requires thorough investigation, root cause analysis, and corrective and preventive action (CAPA) implementation. The impact on product quality must be assessed through a formal quality risk management process as outlined in ICH Q9 guidelines.
Annual revalidation is typically required to verify continued system performance, with more comprehensive revalidation necessary following significant equipment modifications or repairs. Regulatory inspections frequently focus on autoclave validation documentation, emphasizing the importance of maintaining comprehensive records of all validation activities, routine monitoring data, and maintenance procedures. Failure to meet these requirements can result in regulatory observations, product recalls, or facility shutdowns, highlighting the critical nature of autoclave validation compliance in biopharmaceutical manufacturing.
Energy Efficiency and Sustainability Considerations
Energy consumption in autoclave operations represents a significant portion of the total energy footprint in biopharmaceutical manufacturing facilities. Traditional autoclave systems typically operate at high temperatures (121-134°C) and pressures (15-30 psi), consuming substantial amounts of steam, water, and electricity. Recent industry analyses indicate that autoclaves can account for up to 30% of a biopharmaceutical facility's total energy consumption, highlighting the critical need for efficiency improvements.
The environmental impact of autoclave operations extends beyond energy consumption to include water usage and greenhouse gas emissions. Standard autoclave cycles can consume between 80-150 gallons of water per cycle, with larger industrial units using significantly more. This water consumption, coupled with the energy required for heating, contributes to a substantial carbon footprint. Studies from the Pharmaceutical Environmental Research Foundation estimate that each autoclave cycle generates approximately 5-15 kg of CO2 equivalent emissions, depending on size and configuration.
Emerging technologies are addressing these sustainability challenges through various approaches. Heat recovery systems, which capture and repurpose waste heat from autoclave exhaust steam, have demonstrated energy savings of 15-25% in pilot implementations. These systems redirect thermal energy to preheat incoming water or support other facility heating requirements, creating a closed-loop energy system that significantly reduces waste.
Water recycling technologies represent another promising avenue for sustainability improvements. Advanced filtration and purification systems can recapture up to 70% of water used in autoclave operations, substantially reducing both water consumption and associated treatment costs. Several biopharmaceutical manufacturers have reported ROI periods of 18-24 months for these water recovery systems, making them economically viable solutions.
The integration of smart control systems and IoT technologies enables real-time monitoring and optimization of autoclave operations. These systems can adjust cycle parameters based on actual load characteristics rather than worst-case scenarios, potentially reducing cycle times by 10-20% and corresponding energy usage. Predictive maintenance capabilities further enhance efficiency by identifying potential issues before they result in suboptimal performance or equipment failure.
Regulatory considerations remain paramount when implementing sustainability initiatives in GMP environments. Any modifications to autoclave systems or processes must maintain compliance with relevant regulations while demonstrating that sterilization efficacy is not compromised. Documentation of validation studies showing equivalence between optimized and traditional processes is essential for regulatory acceptance of energy-efficient modifications.
The environmental impact of autoclave operations extends beyond energy consumption to include water usage and greenhouse gas emissions. Standard autoclave cycles can consume between 80-150 gallons of water per cycle, with larger industrial units using significantly more. This water consumption, coupled with the energy required for heating, contributes to a substantial carbon footprint. Studies from the Pharmaceutical Environmental Research Foundation estimate that each autoclave cycle generates approximately 5-15 kg of CO2 equivalent emissions, depending on size and configuration.
Emerging technologies are addressing these sustainability challenges through various approaches. Heat recovery systems, which capture and repurpose waste heat from autoclave exhaust steam, have demonstrated energy savings of 15-25% in pilot implementations. These systems redirect thermal energy to preheat incoming water or support other facility heating requirements, creating a closed-loop energy system that significantly reduces waste.
Water recycling technologies represent another promising avenue for sustainability improvements. Advanced filtration and purification systems can recapture up to 70% of water used in autoclave operations, substantially reducing both water consumption and associated treatment costs. Several biopharmaceutical manufacturers have reported ROI periods of 18-24 months for these water recovery systems, making them economically viable solutions.
The integration of smart control systems and IoT technologies enables real-time monitoring and optimization of autoclave operations. These systems can adjust cycle parameters based on actual load characteristics rather than worst-case scenarios, potentially reducing cycle times by 10-20% and corresponding energy usage. Predictive maintenance capabilities further enhance efficiency by identifying potential issues before they result in suboptimal performance or equipment failure.
Regulatory considerations remain paramount when implementing sustainability initiatives in GMP environments. Any modifications to autoclave systems or processes must maintain compliance with relevant regulations while demonstrating that sterilization efficacy is not compromised. Documentation of validation studies showing equivalence between optimized and traditional processes is essential for regulatory acceptance of energy-efficient modifications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!