How to Safely Validate Autoclave Sterility for Mixed Loads
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Background and Objectives
Autoclave sterilization has been a cornerstone of microbial contamination control since its development in the 19th century. Originally conceived by Charles Chamberland in 1884, this technology has evolved from basic pressure cookers to sophisticated computer-controlled systems capable of precise temperature, pressure, and time management. The fundamental principle remains unchanged: using saturated steam under pressure to achieve thermal destruction of microorganisms through protein denaturation and coagulation.
The evolution of autoclave technology has been driven by increasing demands for efficiency, reliability, and safety across various industries. Modern autoclaves incorporate advanced features such as vacuum systems for air removal, rapid cooling mechanisms, and sophisticated validation protocols. These developments have expanded the application scope from basic medical instrument sterilization to complex pharmaceutical manufacturing, laboratory research, and waste management processes.
Mixed loads present a particular challenge in autoclave sterilization. These heterogeneous collections of items with varying thermal properties, densities, and configurations create complex heat transfer dynamics within the sterilization chamber. The variability in steam penetration rates across different materials can result in inconsistent sterilization outcomes, potentially compromising product safety and regulatory compliance.
Current industry standards, including ISO 17665 and AAMI ST79, provide general guidelines for sterilization validation but lack specific protocols for mixed load scenarios. This gap between standardized procedures and practical operational needs has created significant challenges for facilities that routinely process diverse materials simultaneously. The absence of clear validation methodologies increases operational risk and regulatory uncertainty.
The primary objective of autoclave sterility validation for mixed loads is to develop robust, scientifically sound protocols that ensure consistent microbial inactivation across all items regardless of their physical properties or positioning within the chamber. This requires comprehensive understanding of heat transfer dynamics, steam penetration patterns, and microbial inactivation kinetics under variable conditions.
Secondary objectives include optimizing cycle parameters to minimize processing time and utility consumption while maintaining sterilization efficacy, developing practical monitoring systems capable of detecting cold spots or inadequate steam penetration in real-time, and establishing clear documentation standards that satisfy regulatory requirements across different jurisdictions and industries.
The technological evolution in this field is trending toward more sophisticated modeling approaches, including computational fluid dynamics and thermal mapping, combined with advanced biological indicators and parametric release methodologies. These developments aim to transform autoclave validation from a largely empirical process to a more predictive, science-based approach capable of addressing the complexities of mixed load sterilization.
The evolution of autoclave technology has been driven by increasing demands for efficiency, reliability, and safety across various industries. Modern autoclaves incorporate advanced features such as vacuum systems for air removal, rapid cooling mechanisms, and sophisticated validation protocols. These developments have expanded the application scope from basic medical instrument sterilization to complex pharmaceutical manufacturing, laboratory research, and waste management processes.
Mixed loads present a particular challenge in autoclave sterilization. These heterogeneous collections of items with varying thermal properties, densities, and configurations create complex heat transfer dynamics within the sterilization chamber. The variability in steam penetration rates across different materials can result in inconsistent sterilization outcomes, potentially compromising product safety and regulatory compliance.
Current industry standards, including ISO 17665 and AAMI ST79, provide general guidelines for sterilization validation but lack specific protocols for mixed load scenarios. This gap between standardized procedures and practical operational needs has created significant challenges for facilities that routinely process diverse materials simultaneously. The absence of clear validation methodologies increases operational risk and regulatory uncertainty.
The primary objective of autoclave sterility validation for mixed loads is to develop robust, scientifically sound protocols that ensure consistent microbial inactivation across all items regardless of their physical properties or positioning within the chamber. This requires comprehensive understanding of heat transfer dynamics, steam penetration patterns, and microbial inactivation kinetics under variable conditions.
Secondary objectives include optimizing cycle parameters to minimize processing time and utility consumption while maintaining sterilization efficacy, developing practical monitoring systems capable of detecting cold spots or inadequate steam penetration in real-time, and establishing clear documentation standards that satisfy regulatory requirements across different jurisdictions and industries.
The technological evolution in this field is trending toward more sophisticated modeling approaches, including computational fluid dynamics and thermal mapping, combined with advanced biological indicators and parametric release methodologies. These developments aim to transform autoclave validation from a largely empirical process to a more predictive, science-based approach capable of addressing the complexities of mixed load sterilization.
Market Demand Analysis for Mixed Load Sterilization
The global market for autoclave sterilization has experienced significant growth, particularly in the healthcare sector where mixed load sterilization has become increasingly common. The demand for efficient and reliable mixed load sterilization solutions is driven by healthcare facilities seeking to optimize operational efficiency while maintaining strict sterility standards. According to recent market analyses, the global sterilization equipment market is projected to reach $20.9 billion by 2025, with autoclave systems representing a substantial segment of this market.
Healthcare facilities, including hospitals, clinics, and ambulatory surgical centers, constitute the largest market segment for mixed load sterilization solutions. These facilities process diverse medical instruments and materials daily, creating a persistent demand for validated sterilization methods that can accommodate heterogeneous loads. The COVID-19 pandemic has further accelerated this demand, as healthcare providers have had to increase their sterilization capacity while dealing with supply chain disruptions.
Pharmaceutical and biotechnology companies represent another significant market segment. These organizations require validated sterilization processes for various laboratory equipment, containers, and materials used in research and production. The growing emphasis on aseptic processing in pharmaceutical manufacturing has heightened the need for reliable mixed load sterilization validation protocols.
Regulatory pressures have substantially influenced market dynamics. Stringent standards from organizations such as the FDA, ISO, and various national health authorities have mandated comprehensive validation of sterilization processes. These regulations have created a market for specialized validation services and technologies that can demonstrate sterility assurance for mixed loads.
Cost considerations are driving innovation in this space. Healthcare facilities are increasingly seeking solutions that reduce the need for multiple sterilization cycles while ensuring sterility across different load compositions. The potential for operational cost savings through optimized mixed load processing represents a significant market driver.
Regional market analysis reveals varying adoption rates of advanced sterilization validation technologies. North America and Europe lead in implementing sophisticated validation protocols, while emerging economies in Asia-Pacific show the highest growth potential as healthcare infrastructure expands and quality standards improve.
The market also shows increasing demand for documentation and traceability solutions integrated with sterilization equipment. Digital systems that can track individual items within mixed loads throughout the sterilization process are gaining traction, particularly in settings where regulatory compliance requirements are stringent.
Healthcare facilities, including hospitals, clinics, and ambulatory surgical centers, constitute the largest market segment for mixed load sterilization solutions. These facilities process diverse medical instruments and materials daily, creating a persistent demand for validated sterilization methods that can accommodate heterogeneous loads. The COVID-19 pandemic has further accelerated this demand, as healthcare providers have had to increase their sterilization capacity while dealing with supply chain disruptions.
Pharmaceutical and biotechnology companies represent another significant market segment. These organizations require validated sterilization processes for various laboratory equipment, containers, and materials used in research and production. The growing emphasis on aseptic processing in pharmaceutical manufacturing has heightened the need for reliable mixed load sterilization validation protocols.
Regulatory pressures have substantially influenced market dynamics. Stringent standards from organizations such as the FDA, ISO, and various national health authorities have mandated comprehensive validation of sterilization processes. These regulations have created a market for specialized validation services and technologies that can demonstrate sterility assurance for mixed loads.
Cost considerations are driving innovation in this space. Healthcare facilities are increasingly seeking solutions that reduce the need for multiple sterilization cycles while ensuring sterility across different load compositions. The potential for operational cost savings through optimized mixed load processing represents a significant market driver.
Regional market analysis reveals varying adoption rates of advanced sterilization validation technologies. North America and Europe lead in implementing sophisticated validation protocols, while emerging economies in Asia-Pacific show the highest growth potential as healthcare infrastructure expands and quality standards improve.
The market also shows increasing demand for documentation and traceability solutions integrated with sterilization equipment. Digital systems that can track individual items within mixed loads throughout the sterilization process are gaining traction, particularly in settings where regulatory compliance requirements are stringent.
Current Challenges in Mixed Load Validation
The validation of mixed loads in autoclaves presents significant technical challenges that have persisted despite advancements in sterilization technology. Traditional validation protocols were primarily designed for homogeneous loads, creating substantial gaps when applied to the complex thermal dynamics of mixed items. The variability in material composition, density, size, and packaging configurations creates unpredictable heat penetration patterns that compromise validation reliability.
A critical challenge lies in identifying the true "cold spot" within mixed loads. Unlike uniform loads where cold spots can be predicted with reasonable accuracy, mixed loads create multiple potential cold spots that shift depending on load composition and arrangement. This variability makes consistent probe placement problematic and undermines the fundamental premise of validation protocols.
Load configuration inconsistency further exacerbates validation difficulties. Even minor variations in item positioning can significantly alter steam penetration and heat distribution. Healthcare facilities frequently process different combinations of items, making standardization nearly impossible without severely restricting operational flexibility.
The thermal mass differential between items presents another substantial hurdle. Dense surgical instruments may require significantly longer exposure times compared to lightweight textiles, creating a validation dilemma: insufficient cycles risk inadequate sterilization of dense items, while extended cycles may damage heat-sensitive components. This challenge is particularly pronounced in healthcare settings where efficiency demands often conflict with sterilization requirements.
Packaging diversity compounds these issues, as different wrapping materials and container systems create variable barriers to steam penetration. The interaction between different packaging types within the same load creates complex steam flow patterns that are difficult to model or predict consistently.
Regulatory compliance adds another layer of complexity, with standards bodies providing limited specific guidance for mixed load validation. The interpretive nature of existing standards leaves facilities uncertain about acceptable validation methodologies, particularly when dealing with novel item combinations or specialized medical devices.
Biological indicator positioning represents perhaps the most technically challenging aspect of mixed load validation. The traditional approach of placing indicators at predetermined "worst-case" locations becomes problematic when these locations constantly shift based on load composition. This fundamental uncertainty undermines confidence in sterilization efficacy and creates significant liability concerns for healthcare facilities.
A critical challenge lies in identifying the true "cold spot" within mixed loads. Unlike uniform loads where cold spots can be predicted with reasonable accuracy, mixed loads create multiple potential cold spots that shift depending on load composition and arrangement. This variability makes consistent probe placement problematic and undermines the fundamental premise of validation protocols.
Load configuration inconsistency further exacerbates validation difficulties. Even minor variations in item positioning can significantly alter steam penetration and heat distribution. Healthcare facilities frequently process different combinations of items, making standardization nearly impossible without severely restricting operational flexibility.
The thermal mass differential between items presents another substantial hurdle. Dense surgical instruments may require significantly longer exposure times compared to lightweight textiles, creating a validation dilemma: insufficient cycles risk inadequate sterilization of dense items, while extended cycles may damage heat-sensitive components. This challenge is particularly pronounced in healthcare settings where efficiency demands often conflict with sterilization requirements.
Packaging diversity compounds these issues, as different wrapping materials and container systems create variable barriers to steam penetration. The interaction between different packaging types within the same load creates complex steam flow patterns that are difficult to model or predict consistently.
Regulatory compliance adds another layer of complexity, with standards bodies providing limited specific guidance for mixed load validation. The interpretive nature of existing standards leaves facilities uncertain about acceptable validation methodologies, particularly when dealing with novel item combinations or specialized medical devices.
Biological indicator positioning represents perhaps the most technically challenging aspect of mixed load validation. The traditional approach of placing indicators at predetermined "worst-case" locations becomes problematic when these locations constantly shift based on load composition. This fundamental uncertainty undermines confidence in sterilization efficacy and creates significant liability concerns for healthcare facilities.
Current Validation Protocols for Mixed Loads
01 Validation methods for autoclave sterilization
Various methods are employed to validate the effectiveness of autoclave sterilization processes. These include biological indicators containing bacterial spores, chemical indicators that change color when exposed to specific temperature and pressure conditions, and physical monitoring systems that track critical parameters throughout the sterilization cycle. These validation methods ensure that sterilization processes meet required safety standards and effectively eliminate microorganisms.- Validation methods for autoclave sterilization: Various methods are used to validate the effectiveness of autoclave sterilization processes. These include biological indicators containing bacterial spores, chemical indicators that change color when exposed to specific temperature and pressure conditions, and physical monitoring systems that track critical parameters throughout the sterilization cycle. These validation methods ensure that the autoclave is functioning properly and achieving sterility, which is essential for safety in medical and laboratory settings.
- Safety features in autoclave design: Modern autoclaves incorporate various safety features to protect operators and ensure effective sterilization. These include pressure relief valves, door interlock mechanisms that prevent opening during operation, temperature monitoring systems, and automatic shut-off features in case of malfunction. Advanced autoclaves also include alarm systems that alert users to potential safety issues or sterilization failures, reducing the risk of accidents and ensuring validation requirements are met.
- Automated monitoring systems for sterility validation: Automated systems for monitoring and documenting autoclave sterilization cycles have been developed to enhance safety and validation processes. These systems continuously track critical parameters such as temperature, pressure, and time, providing real-time data and generating detailed reports for regulatory compliance. Some advanced systems include remote monitoring capabilities, allowing for immediate detection of deviations from validated sterilization parameters and ensuring consistent sterility assurance levels.
- Specialized validation protocols for different materials: Different materials and items require specialized validation protocols to ensure effective sterilization in autoclaves. Porous materials, dense loads, hollow instruments, and heat-sensitive items each present unique challenges for sterilization validation. Specific protocols have been developed that account for these differences, including appropriate placement of biological indicators, extended exposure times, and modified temperature settings. These specialized protocols ensure safety by confirming that all items, regardless of their composition or configuration, achieve proper sterility.
- Documentation and regulatory compliance for autoclave validation: Comprehensive documentation systems are essential for autoclave sterility validation and regulatory compliance. These systems include standardized procedures for recording sterilization parameters, validation test results, maintenance records, and operator training. Regular calibration and qualification of autoclaves, including installation qualification, operational qualification, and performance qualification, are documented to demonstrate ongoing compliance with safety standards. This documentation provides evidence of proper sterilization processes and is critical for meeting regulatory requirements in healthcare, pharmaceutical, and laboratory settings.
02 Safety features in autoclave design
Modern autoclaves incorporate various safety features to protect operators and ensure effective sterilization. These include pressure relief valves, door interlocking mechanisms that prevent opening during operation, temperature monitoring systems, and automatic shut-off capabilities in case of process deviations. Advanced designs also include redundant safety systems and alarms to alert users of potential hazards or sterilization failures.Expand Specific Solutions03 Monitoring systems for autoclave processes
Sophisticated monitoring systems are essential for ensuring autoclave sterility validation and safety. These systems continuously track critical parameters such as temperature, pressure, and time throughout the sterilization cycle. Real-time monitoring allows for immediate detection of deviations from validated parameters, while data logging capabilities provide documentation for regulatory compliance and quality assurance purposes. Some advanced systems include remote monitoring capabilities and integration with facility management systems.Expand Specific Solutions04 Regulatory compliance and documentation requirements
Autoclave sterility validation must adhere to strict regulatory standards and documentation requirements. This includes establishing validated sterilization protocols, maintaining detailed records of each sterilization cycle, performing regular calibration of monitoring equipment, and conducting periodic revalidation studies. Documentation must demonstrate consistent achievement of sterility assurance levels and may include parametric release criteria, where products are released based on meeting critical process parameters rather than end-product testing.Expand Specific Solutions05 Innovations in autoclave validation technology
Recent innovations have enhanced the reliability and efficiency of autoclave sterility validation. These include the development of rapid readout biological indicators that provide results in hours rather than days, wireless sensor technologies for improved process monitoring, and advanced algorithms for analyzing sterilization cycle data. Other innovations include integrated validation systems that combine multiple monitoring approaches and automated documentation systems that reduce human error and improve compliance with regulatory requirements.Expand Specific Solutions
Key Industry Players and Equipment Manufacturers
The autoclave sterility validation for mixed loads market is in a growth phase, driven by increasing regulatory scrutiny and healthcare facility demands for efficient sterilization processes. The global market is estimated at approximately $2.5 billion, with projected annual growth of 6-8%. Technologically, the field is maturing with key innovations in validation methodologies and monitoring systems. Leading players include Olympus Medical Systems, which dominates with advanced endoscope sterilization solutions, and Shinva Medical Instrument Co., offering comprehensive pharmaceutical-grade validation systems. Rapid Micro Biosystems has disrupted the space with automated microbial detection technologies, while established players like Advanced Sterilization Products and Turbett Surgical focus on specialized mixed-load validation protocols. The industry is seeing increased integration of IoT and real-time monitoring capabilities to enhance validation reliability.
Olympus Corp.
Technical Solution: Olympus Corporation has developed an integrated validation system for mixed load autoclave sterility that focuses particularly on complex medical devices. Their approach centers on the ETD (Endoscope Thermal Distribution) methodology, which maps heat distribution patterns within complex instruments when combined with other items in mixed loads. For validation of mixed loads containing endoscopes and surgical instruments, Olympus employs multi-point temperature monitoring with sensors placed at identified cold spots within lumens, joints, and other challenging areas. Their validation protocol incorporates specialized biological indicators designed to be placed within instrument channels and other hard-to-reach areas that represent the greatest challenge to sterilization. Olympus has also developed load configuration guidelines that specify optimal positioning of different device types within mixed loads to ensure adequate steam penetration. Their validation system includes comprehensive documentation tools that track the specific devices included in each validated load configuration, ensuring that future loads remain within validated parameters. Additionally, they've implemented parametric monitoring systems that continuously verify that critical sterilization parameters remain within validated ranges during routine operation.
Strengths: Their specialized expertise with complex medical devices provides exceptional insight into challenging mixed load configurations. The ETD methodology effectively identifies cold spots in complex instruments. Weaknesses: Their approach is heavily focused on medical devices and may be less applicable to other industries. The system requires significant technical expertise to implement properly.
Thermal Compliance Ltd.
Technical Solution: Thermal Compliance Ltd. has developed a comprehensive validation approach for mixed load autoclave sterility that centers on thermal mapping technology. Their system employs multiple temperature sensors strategically placed throughout the autoclave chamber and within test loads to create detailed thermal profiles. This data-driven approach allows for precise identification of cold spots and heat distribution patterns specific to different load configurations. Their proprietary software analyzes thermal penetration data to establish minimum sterilization parameters for various load combinations, ensuring that the most challenging items receive adequate sterilization. The company's validation protocol includes biological indicator testing with resistant bacterial spores (typically Geobacillus stearothermophilus) placed at identified cold spots to verify sterility achievement. Additionally, they've implemented parametric release methodologies that combine physical measurements with statistical process control to validate sterilization cycles without relying solely on biological indicators.
Strengths: Their thermal mapping technology provides exceptional precision in identifying cold spots specific to mixed loads, reducing validation failures. The data-driven approach enables optimization of cycle parameters for efficiency while maintaining safety margins. Weaknesses: The system requires significant initial investment in sensor technology and software. The complexity of the validation process necessitates specialized training for operators.
Critical Technologies for Sterility Assurance
Heatable form for sterilization of bulk material in a flexible container
PatentWO2007124906A1
Innovation
- A mold with integrated heating elements is used to sterilize containers within a filling and capping machine, allowing for rapid and controlled temperature attainment, eliminating the need for an autoclave and enabling direct documentation of sterilization temperature for each container.
Autoclave operation to accommodate fluid sterilization cycles
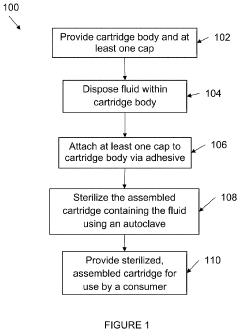
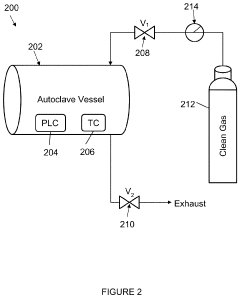
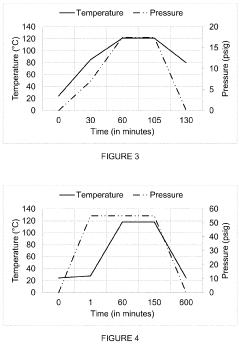
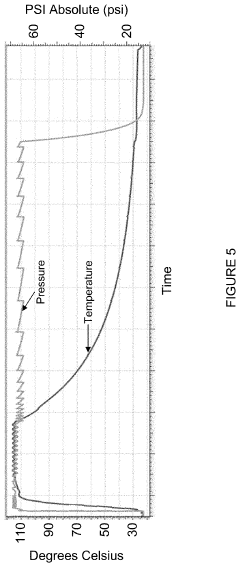
PatentInactiveUS20200179548A1
Innovation
- The autoclave system pre-pressurizes to an initial pressure before heating, maintaining pressure within ±10% of the initial level during temperature increase, and then cools, using a programmable logic controller and temperature controller to manage pressure and temperature profiles.
Regulatory Compliance and Standards
Autoclave sterilization processes for mixed loads must adhere to stringent regulatory frameworks established by international and national authorities. The FDA's Quality System Regulation (21 CFR Part 820) mandates comprehensive validation protocols for medical device manufacturers, requiring documented evidence that sterilization processes consistently achieve predetermined specifications. Similarly, the European Medical Device Regulation (EU MDR 2017/745) emphasizes rigorous validation procedures with particular attention to mixed load configurations.
ISO 17665-1:2006 serves as the cornerstone standard for moist heat sterilization validation, outlining specific requirements for development, validation, and routine control. This standard explicitly addresses the challenges of mixed loads, requiring validation studies that account for the worst-case scenarios within load compositions. Complementary to this, ISO/TS 17665-2:2009 provides practical guidance for applying the principles established in the primary standard.
For healthcare facilities, compliance with standards such as ANSI/AAMI ST79 is essential, as it provides comprehensive guidelines for steam sterilization in healthcare facilities, including specific protocols for mixed load validation. The standard emphasizes the critical importance of load configuration documentation and consistent placement patterns to ensure reproducible sterilization outcomes.
Regulatory bodies increasingly require risk-based approaches to validation, as outlined in ISO 14971 for medical device risk management. This necessitates thorough analysis of potential failure modes in mixed load configurations, with particular attention to cold spots, air entrapment, and moisture penetration barriers that may compromise sterilization efficacy.
Validation documentation must demonstrate compliance with these standards through three sequential phases: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). For mixed loads, PQ protocols must incorporate challenging configurations that represent the most difficult-to-sterilize combinations encountered in routine operations.
Recent regulatory trends indicate increasing scrutiny of mixed load validation, with authorities requiring more comprehensive data on thermal mapping, biological indicator positioning, and load composition variability. Manufacturers and healthcare facilities must maintain awareness of evolving standards, as organizations like AAMI, ISO, and regulatory agencies regularly update requirements based on emerging scientific evidence and technological advancements in sterilization monitoring and validation methodologies.
ISO 17665-1:2006 serves as the cornerstone standard for moist heat sterilization validation, outlining specific requirements for development, validation, and routine control. This standard explicitly addresses the challenges of mixed loads, requiring validation studies that account for the worst-case scenarios within load compositions. Complementary to this, ISO/TS 17665-2:2009 provides practical guidance for applying the principles established in the primary standard.
For healthcare facilities, compliance with standards such as ANSI/AAMI ST79 is essential, as it provides comprehensive guidelines for steam sterilization in healthcare facilities, including specific protocols for mixed load validation. The standard emphasizes the critical importance of load configuration documentation and consistent placement patterns to ensure reproducible sterilization outcomes.
Regulatory bodies increasingly require risk-based approaches to validation, as outlined in ISO 14971 for medical device risk management. This necessitates thorough analysis of potential failure modes in mixed load configurations, with particular attention to cold spots, air entrapment, and moisture penetration barriers that may compromise sterilization efficacy.
Validation documentation must demonstrate compliance with these standards through three sequential phases: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). For mixed loads, PQ protocols must incorporate challenging configurations that represent the most difficult-to-sterilize combinations encountered in routine operations.
Recent regulatory trends indicate increasing scrutiny of mixed load validation, with authorities requiring more comprehensive data on thermal mapping, biological indicator positioning, and load composition variability. Manufacturers and healthcare facilities must maintain awareness of evolving standards, as organizations like AAMI, ISO, and regulatory agencies regularly update requirements based on emerging scientific evidence and technological advancements in sterilization monitoring and validation methodologies.
Risk Assessment and Quality Control Measures
Risk assessment for mixed load autoclave validation requires a systematic approach to identify and mitigate potential failure points. The primary risks include uneven heat distribution, steam penetration barriers, load configuration issues, and biological indicator placement challenges. Each risk factor must be quantified using a standardized risk priority number (RPN) calculation that considers severity, occurrence probability, and detection difficulty. This methodology enables facilities to prioritize control measures for the most critical risks.
Quality control measures should be implemented at multiple levels, beginning with pre-validation protocols that establish clear acceptance criteria for different load combinations. These protocols must specify minimum spacing requirements between items, maximum load densities, and orientation guidelines for various materials. Documentation systems should track load compositions across validation cycles to ensure comprehensive coverage of all potential combinations.
Process challenge devices (PCDs) represent a critical quality control element, designed to simulate worst-case scenarios within mixed loads. These devices should be strategically positioned at identified cold spots and within the most challenging items to verify sterilization efficacy. The PCD selection must account for the specific characteristics of the mixed load components, including dense instruments, porous materials, and lumened devices.
Parametric monitoring systems provide real-time quality control through continuous measurement of critical parameters including temperature, pressure, and time. Modern validation approaches incorporate multiple independent sensing systems with redundant probes positioned throughout the chamber and within representative load items. These systems should feature automated alert mechanisms that trigger when parameters deviate from predetermined specifications.
Microbiological verification remains the gold standard for quality control in autoclave validation. A comprehensive biological indicator program should utilize appropriate bacterial spores (typically Geobacillus stearothermophilus) placed at multiple locations within mixed loads. The program must include positive controls to verify indicator viability and negative controls to confirm test validity. Statistical analysis of biological indicator results across multiple validation cycles provides confidence in sterilization consistency.
Post-cycle quality control measures include visual inspection protocols, chemical indicator verification, and physical parameter review. These measures should be integrated into a comprehensive quality management system that includes regular audits, staff competency assessments, and validation requalification schedules. The frequency of revalidation should be risk-based, with more frequent verification for highly variable load compositions or when introducing new item types.
Quality control measures should be implemented at multiple levels, beginning with pre-validation protocols that establish clear acceptance criteria for different load combinations. These protocols must specify minimum spacing requirements between items, maximum load densities, and orientation guidelines for various materials. Documentation systems should track load compositions across validation cycles to ensure comprehensive coverage of all potential combinations.
Process challenge devices (PCDs) represent a critical quality control element, designed to simulate worst-case scenarios within mixed loads. These devices should be strategically positioned at identified cold spots and within the most challenging items to verify sterilization efficacy. The PCD selection must account for the specific characteristics of the mixed load components, including dense instruments, porous materials, and lumened devices.
Parametric monitoring systems provide real-time quality control through continuous measurement of critical parameters including temperature, pressure, and time. Modern validation approaches incorporate multiple independent sensing systems with redundant probes positioned throughout the chamber and within representative load items. These systems should feature automated alert mechanisms that trigger when parameters deviate from predetermined specifications.
Microbiological verification remains the gold standard for quality control in autoclave validation. A comprehensive biological indicator program should utilize appropriate bacterial spores (typically Geobacillus stearothermophilus) placed at multiple locations within mixed loads. The program must include positive controls to verify indicator viability and negative controls to confirm test validity. Statistical analysis of biological indicator results across multiple validation cycles provides confidence in sterilization consistency.
Post-cycle quality control measures include visual inspection protocols, chemical indicator verification, and physical parameter review. These measures should be integrated into a comprehensive quality management system that includes regular audits, staff competency assessments, and validation requalification schedules. The frequency of revalidation should be risk-based, with more frequent verification for highly variable load compositions or when introducing new item types.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!