Autoclave Condensate Management for Enhanced Sterility
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Condensate Technology Background and Objectives
Autoclave sterilization has been a cornerstone technology in medical, pharmaceutical, and laboratory settings for over a century, with its origins dating back to Charles Chamberland's invention of the first pressure steam sterilizer in 1879. This technology leverages the powerful sterilizing properties of saturated steam under pressure to eliminate microorganisms, including resistant bacterial spores, through protein denaturation and coagulation. The evolution of autoclave technology has been marked by continuous improvements in design, automation, and efficiency, yet condensate management remains a critical challenge that directly impacts sterilization efficacy.
Condensate forms naturally during the autoclave process as steam contacts cooler surfaces and transitions from gaseous to liquid state. Historically, condensate was viewed primarily as a waste product to be drained. However, contemporary understanding recognizes condensate as a critical factor in achieving and maintaining sterility. Improperly managed condensate can harbor microorganisms, create wet loads that compromise sterility, and potentially reintroduce contaminants to sterilized materials during the exhaust phase.
The technological trajectory in this field has shifted from simple gravity displacement systems to more sophisticated vacuum-assisted and steam-flush pressure-pulse designs that enhance steam penetration and condensate removal. Recent innovations have focused on integrating advanced sensors, automated control systems, and specialized drainage configurations to optimize the condensate management process throughout the sterilization cycle.
The primary objective of modern autoclave condensate management technology is to ensure complete sterility assurance while maximizing operational efficiency. This encompasses several specific goals: preventing condensate-related recontamination of sterilized items, reducing cycle times through efficient moisture removal, minimizing water and energy consumption, and extending equipment lifespan by preventing corrosion and mechanical failures associated with improper condensate handling.
Industry standards and regulatory requirements have become increasingly stringent, with organizations such as the Association for the Advancement of Medical Instrumentation (AAMI) and the International Organization for Standardization (ISO) establishing specific parameters for condensate management in sterilization processes. These standards drive technological innovation and adoption across healthcare facilities, pharmaceutical manufacturing, and research laboratories worldwide.
The future trajectory of autoclave condensate management technology aims to achieve perfect sterility assurance through real-time monitoring systems, predictive analytics for condensate formation, and intelligent drainage systems that adapt to varying load conditions. Additionally, sustainability considerations are driving research into condensate recovery and reuse systems that can significantly reduce water consumption while maintaining or enhancing sterilization efficacy.
Condensate forms naturally during the autoclave process as steam contacts cooler surfaces and transitions from gaseous to liquid state. Historically, condensate was viewed primarily as a waste product to be drained. However, contemporary understanding recognizes condensate as a critical factor in achieving and maintaining sterility. Improperly managed condensate can harbor microorganisms, create wet loads that compromise sterility, and potentially reintroduce contaminants to sterilized materials during the exhaust phase.
The technological trajectory in this field has shifted from simple gravity displacement systems to more sophisticated vacuum-assisted and steam-flush pressure-pulse designs that enhance steam penetration and condensate removal. Recent innovations have focused on integrating advanced sensors, automated control systems, and specialized drainage configurations to optimize the condensate management process throughout the sterilization cycle.
The primary objective of modern autoclave condensate management technology is to ensure complete sterility assurance while maximizing operational efficiency. This encompasses several specific goals: preventing condensate-related recontamination of sterilized items, reducing cycle times through efficient moisture removal, minimizing water and energy consumption, and extending equipment lifespan by preventing corrosion and mechanical failures associated with improper condensate handling.
Industry standards and regulatory requirements have become increasingly stringent, with organizations such as the Association for the Advancement of Medical Instrumentation (AAMI) and the International Organization for Standardization (ISO) establishing specific parameters for condensate management in sterilization processes. These standards drive technological innovation and adoption across healthcare facilities, pharmaceutical manufacturing, and research laboratories worldwide.
The future trajectory of autoclave condensate management technology aims to achieve perfect sterility assurance through real-time monitoring systems, predictive analytics for condensate formation, and intelligent drainage systems that adapt to varying load conditions. Additionally, sustainability considerations are driving research into condensate recovery and reuse systems that can significantly reduce water consumption while maintaining or enhancing sterilization efficacy.
Market Analysis for Sterile Processing Solutions
The global sterile processing solutions market is experiencing robust growth, driven by increasing healthcare-associated infections and stringent regulatory requirements for infection control. Currently valued at approximately 7.5 billion USD, the market is projected to reach 12.3 billion USD by 2028, representing a compound annual growth rate of 8.7%. This growth trajectory is particularly evident in regions with expanding healthcare infrastructure, such as Asia-Pacific and Latin America.
Hospital-acquired infections remain a significant concern worldwide, with an estimated 1.7 million patients affected annually in the United States alone. This has intensified the demand for advanced sterile processing solutions, including improved autoclave condensate management systems that minimize contamination risks. Healthcare facilities are increasingly recognizing that effective condensate management is not merely a technical requirement but a critical component of patient safety protocols.
The market segmentation reveals distinct categories within sterile processing solutions. Equipment segments, including sterilizers, washers, and disinfectors, account for approximately 45% of the market share. Consumables and accessories represent 30%, while services, including maintenance and validation, comprise the remaining 25%. Autoclave condensate management systems specifically fall within a specialized subsegment that has shown accelerated growth of 10.2% annually, outpacing the broader market.
Demand patterns vary significantly across different healthcare settings. Large hospitals and academic medical centers typically invest in comprehensive, integrated sterile processing systems with advanced condensate management capabilities. Meanwhile, ambulatory surgical centers and smaller clinics often seek more compact, cost-effective solutions that still maintain high sterility standards. This market stratification has prompted manufacturers to develop tiered product offerings to address diverse customer needs.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 22%. However, the highest growth rates are observed in emerging economies where healthcare infrastructure development is accelerating. Countries like China, India, and Brazil are experiencing annual market expansion exceeding 12%, creating significant opportunities for sterile processing solution providers.
Customer purchasing behaviors have evolved toward value-based procurement models, with increasing emphasis on total cost of ownership rather than initial acquisition costs. Healthcare facilities are prioritizing solutions that demonstrate measurable improvements in infection control outcomes, operational efficiency, and regulatory compliance. This shift has elevated the importance of condensate management systems that can provide documented sterility assurance while optimizing resource utilization.
Hospital-acquired infections remain a significant concern worldwide, with an estimated 1.7 million patients affected annually in the United States alone. This has intensified the demand for advanced sterile processing solutions, including improved autoclave condensate management systems that minimize contamination risks. Healthcare facilities are increasingly recognizing that effective condensate management is not merely a technical requirement but a critical component of patient safety protocols.
The market segmentation reveals distinct categories within sterile processing solutions. Equipment segments, including sterilizers, washers, and disinfectors, account for approximately 45% of the market share. Consumables and accessories represent 30%, while services, including maintenance and validation, comprise the remaining 25%. Autoclave condensate management systems specifically fall within a specialized subsegment that has shown accelerated growth of 10.2% annually, outpacing the broader market.
Demand patterns vary significantly across different healthcare settings. Large hospitals and academic medical centers typically invest in comprehensive, integrated sterile processing systems with advanced condensate management capabilities. Meanwhile, ambulatory surgical centers and smaller clinics often seek more compact, cost-effective solutions that still maintain high sterility standards. This market stratification has prompted manufacturers to develop tiered product offerings to address diverse customer needs.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 22%. However, the highest growth rates are observed in emerging economies where healthcare infrastructure development is accelerating. Countries like China, India, and Brazil are experiencing annual market expansion exceeding 12%, creating significant opportunities for sterile processing solution providers.
Customer purchasing behaviors have evolved toward value-based procurement models, with increasing emphasis on total cost of ownership rather than initial acquisition costs. Healthcare facilities are prioritizing solutions that demonstrate measurable improvements in infection control outcomes, operational efficiency, and regulatory compliance. This shift has elevated the importance of condensate management systems that can provide documented sterility assurance while optimizing resource utilization.
Current Challenges in Condensate Management Systems
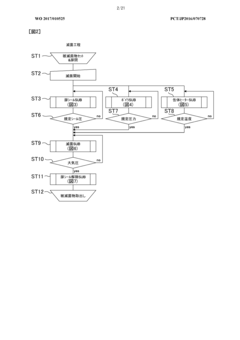
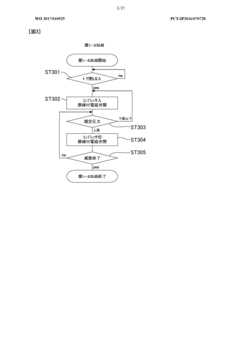
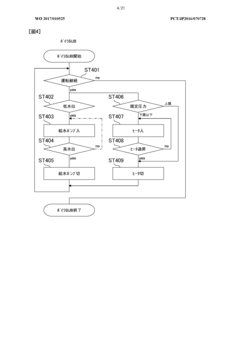
Despite significant advancements in autoclave technology, condensate management remains one of the most challenging aspects of sterilization processes. Current condensate management systems face several critical limitations that compromise sterility assurance levels and operational efficiency. The primary challenge lies in the inconsistent removal of condensate during sterilization cycles, leading to wet packs and potential recontamination of sterilized items. This issue is particularly pronounced in healthcare settings where even minor sterility breaches can have severe consequences for patient safety.
Conventional gravity displacement autoclaves struggle with air pockets and inadequate steam penetration, resulting in condensate accumulation in complex medical instruments with narrow lumens or multiple chambers. The physical properties of condensate, including surface tension and viscosity, make complete removal technically difficult without specialized systems. Many existing condensate management solutions rely on outdated drainage designs that fail to address the dynamic nature of condensate formation during different sterilization phases.
Material compatibility presents another significant challenge. Aggressive condensate removal methods can accelerate corrosion of sensitive instruments, particularly those containing dissimilar metals. The chemical composition of condensate often includes dissolved minerals and process chemicals that can leave residues on sterilized items, potentially causing functional issues or patient reactions. Current filtration and treatment systems for autoclave condensate frequently underperform in removing these contaminants before discharge.
Energy efficiency concerns further complicate condensate management. Many existing systems waste significant thermal energy contained in hot condensate, failing to implement effective heat recovery mechanisms. This inefficiency contributes to higher operational costs and environmental impact. Additionally, the integration of condensate management with broader facility water and steam systems remains problematic, with interface issues causing backflow risks and cross-contamination concerns.
Monitoring and validation of condensate management effectiveness represent another critical gap. Current systems typically lack real-time sensors capable of detecting condensate accumulation in critical areas during the sterilization process. This monitoring deficiency makes it difficult to validate cycle effectiveness and ensure consistent sterility outcomes. Documentation and traceability of condensate-related parameters are often inadequate for regulatory compliance, particularly in pharmaceutical and medical device manufacturing environments.
Regulatory requirements for condensate handling continue to evolve, with increasingly stringent standards for both sterility assurance and environmental discharge. Many existing systems struggle to meet these dual demands, particularly in facilities with space constraints or outdated infrastructure. The cost of implementing comprehensive condensate management solutions remains prohibitively high for many smaller healthcare facilities, creating disparities in sterilization quality across different settings.
Conventional gravity displacement autoclaves struggle with air pockets and inadequate steam penetration, resulting in condensate accumulation in complex medical instruments with narrow lumens or multiple chambers. The physical properties of condensate, including surface tension and viscosity, make complete removal technically difficult without specialized systems. Many existing condensate management solutions rely on outdated drainage designs that fail to address the dynamic nature of condensate formation during different sterilization phases.
Material compatibility presents another significant challenge. Aggressive condensate removal methods can accelerate corrosion of sensitive instruments, particularly those containing dissimilar metals. The chemical composition of condensate often includes dissolved minerals and process chemicals that can leave residues on sterilized items, potentially causing functional issues or patient reactions. Current filtration and treatment systems for autoclave condensate frequently underperform in removing these contaminants before discharge.
Energy efficiency concerns further complicate condensate management. Many existing systems waste significant thermal energy contained in hot condensate, failing to implement effective heat recovery mechanisms. This inefficiency contributes to higher operational costs and environmental impact. Additionally, the integration of condensate management with broader facility water and steam systems remains problematic, with interface issues causing backflow risks and cross-contamination concerns.
Monitoring and validation of condensate management effectiveness represent another critical gap. Current systems typically lack real-time sensors capable of detecting condensate accumulation in critical areas during the sterilization process. This monitoring deficiency makes it difficult to validate cycle effectiveness and ensure consistent sterility outcomes. Documentation and traceability of condensate-related parameters are often inadequate for regulatory compliance, particularly in pharmaceutical and medical device manufacturing environments.
Regulatory requirements for condensate handling continue to evolve, with increasingly stringent standards for both sterility assurance and environmental discharge. Many existing systems struggle to meet these dual demands, particularly in facilities with space constraints or outdated infrastructure. The cost of implementing comprehensive condensate management solutions remains prohibitively high for many smaller healthcare facilities, creating disparities in sterilization quality across different settings.
Contemporary Condensate Management Solutions
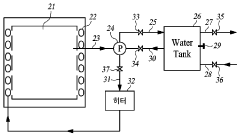
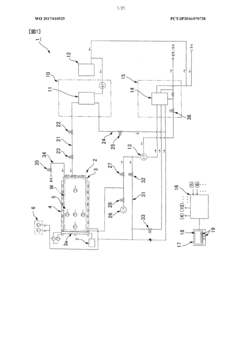
01 Condensate collection and sterilization systems
Systems designed for collecting and sterilizing condensate from autoclaves to maintain sterility. These systems include specialized containers, filters, and treatment mechanisms that prevent contamination of the sterilized items by ensuring that condensate is properly collected and treated. The systems often incorporate temperature and pressure monitoring to verify that sterilization conditions are maintained throughout the condensate management process.- Condensate collection and sterilization systems: Systems designed for collecting and sterilizing condensate from autoclaves to maintain sterility. These systems typically include collection chambers, filters, and sterilization mechanisms to prevent contamination. The condensate is collected and treated to ensure that no viable microorganisms are present before disposal or reuse, maintaining the sterile environment of the autoclave and surrounding areas.
- Temperature and pressure management in condensate handling: Methods and systems for managing temperature and pressure during condensate formation and removal in autoclaves. These approaches focus on controlling the thermal conditions to prevent contamination risks associated with condensate formation. By maintaining appropriate temperature gradients and pressure levels, these systems help ensure that condensate does not compromise sterility during the sterilization process.
- Filtration and barrier technologies for condensate: Advanced filtration and barrier technologies specifically designed to maintain sterility when handling autoclave condensate. These include specialized membrane filters, HEPA filtration systems, and physical barriers that prevent microbial contamination. The technologies ensure that condensate is properly filtered before being released from the sterilization system, preventing potential cross-contamination.
- Automated condensate management systems: Automated systems for monitoring and managing condensate in autoclave operations. These systems incorporate sensors, controllers, and automated valves to detect, collect, and process condensate without manual intervention. The automation helps maintain consistent sterility by reducing human error and providing real-time monitoring of condensate parameters to ensure compliance with sterilization standards.
- Condensate treatment for reuse or disposal: Methods for treating autoclave condensate to enable safe reuse or disposal. These approaches include chemical treatments, heat treatments, and other processes that ensure condensate is free from viable microorganisms. The treatments address both sterility concerns and environmental considerations, allowing for the condensate to be either recycled within the system or safely disposed of without contamination risks.
02 Condensate drainage and removal techniques
Methods and devices for efficiently draining and removing condensate from autoclaves during the sterilization cycle. These techniques include gravity-based drainage systems, vacuum-assisted removal, and specialized valve configurations that ensure complete evacuation of condensate. Proper drainage prevents water accumulation that could compromise sterility by allowing microbial growth or recontamination of sterilized items.Expand Specific Solutions03 Condensate treatment for sterility assurance
Processes for treating autoclave condensate to ensure it does not compromise sterility. These treatments include thermal processing, chemical disinfection, filtration systems, and UV irradiation to eliminate potential microbial contaminants. The treated condensate can either be safely disposed of or, in some systems, recycled for reuse, maintaining the sterile environment throughout the autoclave operation.Expand Specific Solutions04 Monitoring and validation of condensate sterility
Systems and methods for continuous monitoring and validation of condensate sterility in autoclave operations. These include sensors, indicators, and testing protocols that verify the absence of microbial contamination in condensate. Real-time monitoring allows for immediate detection of potential sterility breaches, while validation processes document compliance with sterilization standards and regulatory requirements.Expand Specific Solutions05 Energy-efficient condensate management systems
Innovative approaches to managing autoclave condensate while optimizing energy efficiency. These systems recover heat from condensate for reuse in preheating or other processes, reducing overall energy consumption. Advanced designs incorporate heat exchangers, insulation techniques, and automated control systems that maintain sterility requirements while minimizing resource usage and environmental impact.Expand Specific Solutions
Leading Manufacturers and Industry Landscape
The autoclave condensate management market is currently in a growth phase, driven by increasing focus on sterility assurance in healthcare and pharmaceutical sectors. The global market size is estimated to reach $1.2 billion by 2025, with a CAGR of 6.8%. Technologically, the field shows varying maturity levels across different applications. Leading players like Olympus Corp. and Ethicon (Johnson & Johnson) demonstrate advanced solutions with integrated monitoring systems, while companies such as Shinva Medical Instrument and MEDIVATORS are rapidly innovating in condensate recycling technologies. Regional players like LTE Scientific and SciCan are specializing in niche applications, particularly for laboratory settings. The competitive landscape is characterized by increasing consolidation as larger medical device manufacturers acquire specialized sterilization technology providers to enhance their infection control portfolios.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva has developed an advanced autoclave condensate management system that incorporates a multi-stage filtration process combined with real-time monitoring technology. Their solution features a closed-loop condensate collection system that prevents environmental contamination while maximizing water recovery. The system employs specialized heat exchangers that rapidly cool steam condensate before it enters the filtration stages, which include mechanical filtration, activated carbon treatment, and final membrane filtration to remove potential biological contaminants. Shinva's approach integrates IoT sensors throughout the system to continuously monitor condensate quality parameters including temperature, pH, conductivity, and microbial load, ensuring sterility is maintained throughout the process. The system automatically adjusts treatment protocols based on detected contamination levels and provides comprehensive data logging for regulatory compliance.
Strengths: Comprehensive real-time monitoring capabilities allow for immediate detection of sterility breaches; high water recovery rate (up to 85%) reduces operational costs; fully automated operation minimizes human intervention and contamination risk. Weaknesses: Higher initial capital investment compared to conventional systems; requires specialized maintenance personnel; system complexity can lead to longer troubleshooting times when issues arise.
Barriquand SAS
Technical Solution: Barriquand has developed the ThermoSteril Condensate Management System, leveraging their expertise in heat exchange technology to address autoclave condensate sterility challenges. Their approach centers on a plate heat exchanger design that enables rapid cooling of condensate while maintaining physical separation between potentially contaminated fluids and the cooling medium. The system incorporates a proprietary three-stage treatment process: first, a cyclonic separator removes particulates and steam droplets; second, a specialized thermochemical treatment chamber introduces precisely controlled amounts of biocidal agents activated by residual heat in the condensate; and third, a final cooling stage reduces temperatures to levels safe for discharge or recycling. Barriquand's system features adaptive control technology that continuously monitors condensate flow rates and temperatures, automatically adjusting treatment parameters to ensure consistent sterility outcomes regardless of autoclave operation patterns. The ThermoSteril system includes an integrated clean-in-place (CIP) capability that periodically sanitizes all wetted surfaces using a combination of high-temperature circulation and chemical disinfection, preventing biofilm formation that could compromise system performance.
Strengths: Exceptional thermal efficiency through advanced plate heat exchanger design; adaptive control system ensures consistent treatment regardless of variable condensate conditions; integrated CIP functionality reduces maintenance requirements. Weaknesses: Reliance on chemical treatments increases operational costs and requires careful handling; system requires specialized technical expertise for optimal configuration; larger physical footprint compared to simpler systems.
Key Technical Innovations in Sterility Assurance
High-pressure steam sterilizer
PatentActiveKR1020140086324A
Innovation
- The apparatus incorporates an improved packing mechanism with an expandable sealing member and a cooling water recovery system that reduces water consumption by cooling high-temperature steam and water before discharge.
Method of flow-type high-pressure steam sterilization by soft water heat process, and flow-type sterilization device
PatentWO2017010525A1
Innovation
- A flow-through high-pressure steam sterilization method utilizing a soft hydrothermal process, which involves an air removal process, heating and pressurizing, high-pressure steam sterilization with highly saturated steam, and a controlled drying step to minimize condensed water generation and shorten drying time.
Regulatory Compliance and Standards
Autoclave sterilization processes are governed by stringent regulatory frameworks designed to ensure patient safety and product integrity. The FDA's Quality System Regulation (21 CFR Part 820) establishes comprehensive requirements for medical device manufacturers, with specific provisions addressing sterilization validation and process controls. Similarly, the European Medical Device Regulation (EU MDR 2017/745) mandates strict adherence to sterilization protocols, particularly emphasizing the management of critical process parameters including condensate handling.
ISO 17665-1:2006 serves as the international standard specifically addressing moist heat sterilization processes, providing detailed guidelines for validation and routine control. This standard explicitly addresses condensate management as a critical factor affecting sterilization efficacy. Complementary to this, ISO 13485:2016 outlines quality management system requirements for medical device manufacturers, incorporating sterilization process validation as a fundamental component.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed ST79, a comprehensive guide to steam sterilization and sterility assurance in healthcare facilities. This document provides specific recommendations for condensate removal systems and monitoring protocols to prevent recontamination risks.
Regulatory bodies increasingly emphasize a risk-based approach to sterilization validation. The ICH Q9 Quality Risk Management framework has been adapted for sterilization processes, requiring manufacturers to identify, assess, and mitigate risks associated with condensate management. This includes potential cross-contamination pathways and microbial growth opportunities in improperly managed condensate systems.
Compliance verification typically involves regular audits and inspections by regulatory authorities. Documentation requirements are extensive, including validation protocols, routine monitoring records, and deviation management procedures specific to condensate handling systems. Many jurisdictions mandate real-time monitoring and recording of critical parameters, with condensate flow rates and quality metrics increasingly included among these parameters.
Recent regulatory trends indicate growing scrutiny of water quality standards applicable to autoclave systems. USP <1231> Water for Pharmaceutical Purposes provides guidelines for water quality in sterilization processes, while the European Pharmacopoeia establishes specific standards for water purity in medical applications. These standards directly impact condensate management strategies, as recycled condensate must meet increasingly stringent quality requirements.
Emerging regulations are beginning to address environmental considerations in autoclave operations. The EU's Industrial Emissions Directive and similar regulations worldwide are imposing stricter controls on wastewater discharge, including autoclave condensate. This regulatory evolution is driving innovation in condensate recovery and treatment technologies to ensure both sterility assurance and environmental compliance.
ISO 17665-1:2006 serves as the international standard specifically addressing moist heat sterilization processes, providing detailed guidelines for validation and routine control. This standard explicitly addresses condensate management as a critical factor affecting sterilization efficacy. Complementary to this, ISO 13485:2016 outlines quality management system requirements for medical device manufacturers, incorporating sterilization process validation as a fundamental component.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed ST79, a comprehensive guide to steam sterilization and sterility assurance in healthcare facilities. This document provides specific recommendations for condensate removal systems and monitoring protocols to prevent recontamination risks.
Regulatory bodies increasingly emphasize a risk-based approach to sterilization validation. The ICH Q9 Quality Risk Management framework has been adapted for sterilization processes, requiring manufacturers to identify, assess, and mitigate risks associated with condensate management. This includes potential cross-contamination pathways and microbial growth opportunities in improperly managed condensate systems.
Compliance verification typically involves regular audits and inspections by regulatory authorities. Documentation requirements are extensive, including validation protocols, routine monitoring records, and deviation management procedures specific to condensate handling systems. Many jurisdictions mandate real-time monitoring and recording of critical parameters, with condensate flow rates and quality metrics increasingly included among these parameters.
Recent regulatory trends indicate growing scrutiny of water quality standards applicable to autoclave systems. USP <1231> Water for Pharmaceutical Purposes provides guidelines for water quality in sterilization processes, while the European Pharmacopoeia establishes specific standards for water purity in medical applications. These standards directly impact condensate management strategies, as recycled condensate must meet increasingly stringent quality requirements.
Emerging regulations are beginning to address environmental considerations in autoclave operations. The EU's Industrial Emissions Directive and similar regulations worldwide are imposing stricter controls on wastewater discharge, including autoclave condensate. This regulatory evolution is driving innovation in condensate recovery and treatment technologies to ensure both sterility assurance and environmental compliance.
Environmental Impact and Sustainability Considerations
Autoclave condensate management systems have significant environmental implications that must be considered in their design and operation. Traditional condensate handling methods often involve direct discharge of potentially contaminated water into municipal sewage systems without adequate treatment. This practice raises serious environmental concerns as autoclave condensate may contain trace amounts of biological materials, chemicals, and heat that can disrupt aquatic ecosystems and strain municipal water treatment facilities.
Water conservation represents a critical sustainability consideration in modern autoclave operations. Advanced condensate management systems now incorporate water recycling technologies that can reduce freshwater consumption by up to 60-80% compared to conventional systems. These closed-loop systems capture, treat, and reuse condensate water for subsequent autoclave cycles, significantly reducing the facility's water footprint while simultaneously decreasing wastewater discharge volumes.
Energy recovery from autoclave condensate presents another important sustainability opportunity. The high-temperature condensate contains substantial thermal energy that can be harvested through heat exchangers and redirected to preheat incoming water or support other facility heating requirements. Implementation of such energy recovery systems has demonstrated potential energy savings of 15-30% in autoclave operations, contributing to reduced carbon emissions and operational costs.
Chemical usage in condensate treatment processes requires careful environmental consideration. Modern systems increasingly employ biodegradable treatment chemicals and advanced filtration methods that minimize the introduction of harmful substances into wastewater streams. Some cutting-edge facilities have successfully implemented chemical-free treatment approaches using UV sterilization and mechanical filtration, further reducing environmental impact.
Regulatory compliance frameworks worldwide are evolving to address the environmental aspects of autoclave condensate management. Organizations such as the Environmental Protection Agency (EPA) in the United States and the European Environmental Agency have established increasingly stringent guidelines for temperature, biological oxygen demand, and chemical content of discharged water from sterilization processes. Forward-thinking facilities are adopting management systems that not only meet current requirements but anticipate future regulatory developments.
Life cycle assessment of condensate management systems reveals that initial investment in environmentally responsible technologies typically yields positive returns through reduced resource consumption, lower compliance costs, and enhanced corporate sustainability profiles. The most effective systems balance sterility assurance with environmental protection, recognizing that these objectives can be complementary rather than competing priorities in modern healthcare and industrial settings.
Water conservation represents a critical sustainability consideration in modern autoclave operations. Advanced condensate management systems now incorporate water recycling technologies that can reduce freshwater consumption by up to 60-80% compared to conventional systems. These closed-loop systems capture, treat, and reuse condensate water for subsequent autoclave cycles, significantly reducing the facility's water footprint while simultaneously decreasing wastewater discharge volumes.
Energy recovery from autoclave condensate presents another important sustainability opportunity. The high-temperature condensate contains substantial thermal energy that can be harvested through heat exchangers and redirected to preheat incoming water or support other facility heating requirements. Implementation of such energy recovery systems has demonstrated potential energy savings of 15-30% in autoclave operations, contributing to reduced carbon emissions and operational costs.
Chemical usage in condensate treatment processes requires careful environmental consideration. Modern systems increasingly employ biodegradable treatment chemicals and advanced filtration methods that minimize the introduction of harmful substances into wastewater streams. Some cutting-edge facilities have successfully implemented chemical-free treatment approaches using UV sterilization and mechanical filtration, further reducing environmental impact.
Regulatory compliance frameworks worldwide are evolving to address the environmental aspects of autoclave condensate management. Organizations such as the Environmental Protection Agency (EPA) in the United States and the European Environmental Agency have established increasingly stringent guidelines for temperature, biological oxygen demand, and chemical content of discharged water from sterilization processes. Forward-thinking facilities are adopting management systems that not only meet current requirements but anticipate future regulatory developments.
Life cycle assessment of condensate management systems reveals that initial investment in environmentally responsible technologies typically yields positive returns through reduced resource consumption, lower compliance costs, and enhanced corporate sustainability profiles. The most effective systems balance sterility assurance with environmental protection, recognizing that these objectives can be complementary rather than competing priorities in modern healthcare and industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!