How to Utilize Autoclave Data for Predictive Sterilization Quality
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Technology Background and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This technology leverages the principle of using saturated steam under pressure to eliminate microorganisms through protein denaturation and coagulation. Over the decades, autoclave systems have progressed from simple pressure vessels to sophisticated computerized equipment with precise control mechanisms and data collection capabilities.
The evolution of autoclave technology has been marked by several key advancements, including the introduction of vacuum systems for air removal, microprocessor controls for cycle management, and integrated sensors for real-time monitoring of critical parameters such as temperature, pressure, and time. Recent developments have focused on enhancing energy efficiency, reducing cycle times, and implementing comprehensive data logging systems that capture detailed information about each sterilization cycle.
Current autoclave systems generate vast amounts of process data, including temperature profiles, pressure curves, F0 values (measure of lethality), and cycle phase timing. Historically, this data has primarily been used for compliance documentation and basic quality assurance rather than predictive analytics. The untapped potential lies in leveraging this rich dataset to move from reactive to proactive quality management approaches.
The primary objective of utilizing autoclave data for predictive sterilization quality is to develop robust predictive models that can anticipate sterilization failures before they occur, optimize cycle parameters for different load configurations, and ensure consistent sterilization efficacy while maximizing operational efficiency. This approach aims to transform traditional sterilization processes into data-driven operations that continuously improve through machine learning and statistical analysis.
Additional technical goals include establishing correlations between process parameters and sterilization outcomes, identifying early warning indicators of potential failures, developing algorithms for real-time process adjustments, and creating standardized methodologies for data interpretation across different autoclave models and manufacturers. These advancements would significantly enhance quality assurance in critical industries such as healthcare, pharmaceuticals, and food processing.
The technological trajectory points toward integrated sterilization ecosystems where autoclave data interfaces with broader quality management systems, supply chain logistics, and regulatory compliance frameworks. Future developments will likely focus on implementing edge computing capabilities for real-time analytics, developing industry-specific predictive models, and establishing interoperability standards for sterilization data exchange across different platforms and equipment types.
The evolution of autoclave technology has been marked by several key advancements, including the introduction of vacuum systems for air removal, microprocessor controls for cycle management, and integrated sensors for real-time monitoring of critical parameters such as temperature, pressure, and time. Recent developments have focused on enhancing energy efficiency, reducing cycle times, and implementing comprehensive data logging systems that capture detailed information about each sterilization cycle.
Current autoclave systems generate vast amounts of process data, including temperature profiles, pressure curves, F0 values (measure of lethality), and cycle phase timing. Historically, this data has primarily been used for compliance documentation and basic quality assurance rather than predictive analytics. The untapped potential lies in leveraging this rich dataset to move from reactive to proactive quality management approaches.
The primary objective of utilizing autoclave data for predictive sterilization quality is to develop robust predictive models that can anticipate sterilization failures before they occur, optimize cycle parameters for different load configurations, and ensure consistent sterilization efficacy while maximizing operational efficiency. This approach aims to transform traditional sterilization processes into data-driven operations that continuously improve through machine learning and statistical analysis.
Additional technical goals include establishing correlations between process parameters and sterilization outcomes, identifying early warning indicators of potential failures, developing algorithms for real-time process adjustments, and creating standardized methodologies for data interpretation across different autoclave models and manufacturers. These advancements would significantly enhance quality assurance in critical industries such as healthcare, pharmaceuticals, and food processing.
The technological trajectory points toward integrated sterilization ecosystems where autoclave data interfaces with broader quality management systems, supply chain logistics, and regulatory compliance frameworks. Future developments will likely focus on implementing edge computing capabilities for real-time analytics, developing industry-specific predictive models, and establishing interoperability standards for sterilization data exchange across different platforms and equipment types.
Market Demand Analysis for Predictive Sterilization Solutions
The global market for predictive sterilization solutions is experiencing significant growth, driven by increasing demands for quality assurance in healthcare, pharmaceutical manufacturing, food processing, and other industries where sterilization is critical. The market size for sterilization equipment and services was valued at approximately $7.5 billion in 2022 and is projected to reach $12.3 billion by 2028, with predictive analytics solutions representing the fastest-growing segment at a CAGR of 11.2%.
Healthcare facilities represent the largest market segment, accounting for nearly 40% of the demand. Hospitals and surgical centers are increasingly seeking solutions that can predict sterilization failures before they occur, as healthcare-associated infections (HAIs) cost the U.S. healthcare system alone over $28 billion annually. The ability to predict and prevent sterilization failures represents a significant value proposition for these institutions.
Pharmaceutical manufacturing constitutes the second-largest market segment, where sterilization quality directly impacts product safety and regulatory compliance. With regulatory bodies worldwide tightening requirements for sterilization validation, manufacturers are actively seeking predictive solutions that can provide real-time quality assurance and documentation. The FDA reported a 23% increase in sterilization-related recalls between 2018 and 2022, highlighting the critical need for improved predictive capabilities.
Medical device manufacturers represent another significant market segment, particularly as devices become more complex and sensitive to sterilization processes. These manufacturers require solutions that can predict how different sterilization parameters might affect product integrity and functionality across various materials and designs.
From a geographical perspective, North America currently leads the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to show the highest growth rate over the next five years due to expanding healthcare infrastructure and increasing adoption of advanced manufacturing practices in countries like China and India.
Customer pain points driving market demand include regulatory compliance challenges, cost pressures from failed batches, increasing complexity of items requiring sterilization, and the need for comprehensive documentation. Survey data indicates that 78% of potential customers prioritize predictive capabilities that can reduce sterilization failures, while 65% seek solutions offering real-time monitoring and alerts.
The market shows strong receptivity to data-driven solutions, with 82% of surveyed facilities expressing interest in autoclave systems with predictive analytics capabilities. However, price sensitivity remains a concern, particularly among smaller facilities, with 56% indicating that cost is a significant barrier to adoption of advanced predictive systems.
Healthcare facilities represent the largest market segment, accounting for nearly 40% of the demand. Hospitals and surgical centers are increasingly seeking solutions that can predict sterilization failures before they occur, as healthcare-associated infections (HAIs) cost the U.S. healthcare system alone over $28 billion annually. The ability to predict and prevent sterilization failures represents a significant value proposition for these institutions.
Pharmaceutical manufacturing constitutes the second-largest market segment, where sterilization quality directly impacts product safety and regulatory compliance. With regulatory bodies worldwide tightening requirements for sterilization validation, manufacturers are actively seeking predictive solutions that can provide real-time quality assurance and documentation. The FDA reported a 23% increase in sterilization-related recalls between 2018 and 2022, highlighting the critical need for improved predictive capabilities.
Medical device manufacturers represent another significant market segment, particularly as devices become more complex and sensitive to sterilization processes. These manufacturers require solutions that can predict how different sterilization parameters might affect product integrity and functionality across various materials and designs.
From a geographical perspective, North America currently leads the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to show the highest growth rate over the next five years due to expanding healthcare infrastructure and increasing adoption of advanced manufacturing practices in countries like China and India.
Customer pain points driving market demand include regulatory compliance challenges, cost pressures from failed batches, increasing complexity of items requiring sterilization, and the need for comprehensive documentation. Survey data indicates that 78% of potential customers prioritize predictive capabilities that can reduce sterilization failures, while 65% seek solutions offering real-time monitoring and alerts.
The market shows strong receptivity to data-driven solutions, with 82% of surveyed facilities expressing interest in autoclave systems with predictive analytics capabilities. However, price sensitivity remains a concern, particularly among smaller facilities, with 56% indicating that cost is a significant barrier to adoption of advanced predictive systems.
Current State and Challenges in Autoclave Data Utilization
The current state of autoclave data utilization in sterilization processes reveals a significant gap between data collection capabilities and analytical implementation. Modern autoclaves are equipped with sophisticated sensors that monitor critical parameters such as temperature, pressure, time, and steam quality throughout the sterilization cycle. However, many facilities still operate in a reactive rather than predictive mode, primarily using this data for basic compliance documentation rather than advanced quality prediction.
Global regulatory bodies, including the FDA and EMA, have established stringent requirements for sterilization validation, yet the industry's approach to data analysis remains largely retrospective. Most facilities analyze autoclave data only after process deviations occur, missing opportunities for early intervention. This reactive approach results in increased batch rejections, production delays, and higher operational costs.
Technical challenges in autoclave data utilization include data fragmentation across multiple systems, inconsistent data formats, and the absence of standardized analytical frameworks. Many facilities struggle with legacy equipment that lacks modern connectivity features, creating data silos that impede comprehensive analysis. The volume and velocity of data generated during sterilization cycles—often capturing measurements every second—present significant computational challenges for real-time processing.
Integration difficulties between autoclave systems and enterprise quality management systems (EQMS) further complicate data utilization efforts. Without seamless integration, valuable correlations between sterilization parameters and final product quality remain undiscovered. Additionally, many organizations lack the specialized expertise required to implement advanced statistical process control (SPC) and machine learning algorithms for predictive analytics.
The geographical distribution of technological advancement in this field shows notable disparities. European pharmaceutical manufacturers, particularly in Germany and Switzerland, lead in implementing data-driven autoclave monitoring systems. North American facilities demonstrate strong regulatory compliance but slower adoption of predictive technologies. Asian manufacturing hubs are rapidly modernizing their capabilities, with significant investments in IoT-enabled sterilization equipment.
Cybersecurity concerns represent another significant challenge, as connected autoclave systems potentially create new vulnerabilities in production environments. The sensitive nature of pharmaceutical and medical device manufacturing requires robust data protection measures that sometimes conflict with the accessibility needed for advanced analytics.
Despite these challenges, pioneering organizations have demonstrated that effective utilization of autoclave data can reduce sterilization cycle failures by up to 40% and decrease validation times by 25-30%, highlighting the substantial potential benefits of overcoming current limitations.
Global regulatory bodies, including the FDA and EMA, have established stringent requirements for sterilization validation, yet the industry's approach to data analysis remains largely retrospective. Most facilities analyze autoclave data only after process deviations occur, missing opportunities for early intervention. This reactive approach results in increased batch rejections, production delays, and higher operational costs.
Technical challenges in autoclave data utilization include data fragmentation across multiple systems, inconsistent data formats, and the absence of standardized analytical frameworks. Many facilities struggle with legacy equipment that lacks modern connectivity features, creating data silos that impede comprehensive analysis. The volume and velocity of data generated during sterilization cycles—often capturing measurements every second—present significant computational challenges for real-time processing.
Integration difficulties between autoclave systems and enterprise quality management systems (EQMS) further complicate data utilization efforts. Without seamless integration, valuable correlations between sterilization parameters and final product quality remain undiscovered. Additionally, many organizations lack the specialized expertise required to implement advanced statistical process control (SPC) and machine learning algorithms for predictive analytics.
The geographical distribution of technological advancement in this field shows notable disparities. European pharmaceutical manufacturers, particularly in Germany and Switzerland, lead in implementing data-driven autoclave monitoring systems. North American facilities demonstrate strong regulatory compliance but slower adoption of predictive technologies. Asian manufacturing hubs are rapidly modernizing their capabilities, with significant investments in IoT-enabled sterilization equipment.
Cybersecurity concerns represent another significant challenge, as connected autoclave systems potentially create new vulnerabilities in production environments. The sensitive nature of pharmaceutical and medical device manufacturing requires robust data protection measures that sometimes conflict with the accessibility needed for advanced analytics.
Despite these challenges, pioneering organizations have demonstrated that effective utilization of autoclave data can reduce sterilization cycle failures by up to 40% and decrease validation times by 25-30%, highlighting the substantial potential benefits of overcoming current limitations.
Current Data-Driven Approaches for Sterilization Quality Prediction
01 Monitoring and validation of sterilization parameters
Autoclave sterilization quality depends on monitoring critical parameters such as temperature, pressure, and time. Advanced monitoring systems collect and analyze real-time data to ensure sterilization cycles meet required standards. These systems can detect deviations from optimal conditions and provide alerts when parameters fall outside acceptable ranges, ensuring effective microbial inactivation and maintaining sterilization quality.- Monitoring and validation of sterilization parameters: Autoclave sterilization quality depends on monitoring critical parameters such as temperature, pressure, and time. Advanced monitoring systems collect real-time data during the sterilization cycle to ensure that all parameters meet predetermined specifications. These systems can detect deviations from the standard sterilization protocol and alert operators to potential issues, ensuring consistent sterilization quality and compliance with regulatory standards.
- Biological indicators for sterilization verification: Biological indicators containing resistant microorganisms are used to verify the effectiveness of autoclave sterilization processes. These indicators provide direct evidence that sterilization conditions were sufficient to kill highly resistant bacterial spores, offering a higher level of assurance than physical or chemical indicators alone. The growth or non-growth of these microorganisms after the sterilization cycle serves as a definitive test of sterilization efficacy.
- Data logging and documentation systems: Automated data logging systems record and store sterilization cycle information for quality assurance and regulatory compliance. These systems capture critical parameters throughout the sterilization process, creating permanent records that can be reviewed for quality control purposes or during audits. Advanced systems may include features such as electronic signatures, data encryption, and cloud storage to ensure data integrity and accessibility.
- Process optimization through data analysis: Analysis of autoclave sterilization data enables process optimization and quality improvement. By examining patterns and trends in sterilization parameters across multiple cycles, operators can identify opportunities to enhance efficiency, consistency, and reliability. This data-driven approach helps in developing more effective sterilization protocols, reducing cycle times, and minimizing resource consumption while maintaining or improving sterilization quality.
- Equipment design and validation for sterilization quality: The design and validation of autoclave equipment significantly impacts sterilization quality. Modern autoclaves incorporate features such as pre-vacuum systems, steam quality monitoring, and uniform heat distribution to ensure effective sterilization. Equipment validation protocols include installation qualification, operational qualification, and performance qualification to verify that the autoclave consistently delivers the required sterilization conditions across all load configurations.
02 Biological and chemical indicators for sterilization verification
Biological and chemical indicators are used to verify the effectiveness of autoclave sterilization processes. Biological indicators contain resistant bacterial spores that are exposed to the sterilization cycle, while chemical indicators change color or physical state when exposed to specific sterilization conditions. These indicators provide visual confirmation that sterilization parameters were achieved throughout the load, enhancing quality assurance in sterilization processes.Expand Specific Solutions03 Data logging and documentation systems
Automated data logging systems record and store sterilization cycle information, creating comprehensive documentation for quality control and regulatory compliance. These systems capture parameters throughout the entire sterilization process, generate reports, and maintain searchable records. Digital documentation improves traceability, facilitates audit processes, and helps identify trends or issues that might affect sterilization quality over time.Expand Specific Solutions04 Load configuration and steam penetration optimization
Proper load configuration is critical for ensuring steam penetration and achieving uniform sterilization throughout the autoclave chamber. Techniques include appropriate spacing between items, correct positioning of containers, and consideration of material density. Optimized load configurations prevent the formation of air pockets that could shield microorganisms from steam exposure, thereby enhancing sterilization quality and consistency across all items in the load.Expand Specific Solutions05 Automated quality control and process improvement
Advanced autoclave systems incorporate automated quality control features that analyze sterilization data to identify process improvements. These systems use algorithms to detect patterns, predict potential failures, and recommend optimization strategies. By continuously monitoring performance metrics and comparing them against established standards, automated quality control systems help maintain consistent sterilization quality while reducing human error and resource consumption.Expand Specific Solutions
Key Industry Players in Autoclave and Sterilization Analytics
The sterilization quality prediction market is in a growth phase, with increasing adoption of data-driven approaches to enhance autoclave performance and ensure compliance with stringent medical standards. The global market size for sterilization monitoring and quality control systems is expanding rapidly due to heightened focus on infection control in healthcare settings. Technologically, the field is advancing from basic monitoring to predictive analytics, with companies at varying maturity levels. Industry leaders like Steris and Getinge dominate the market, while specialized players such as Nakanishi, Truking Technology, and W&H Sterilization offer innovative solutions for specific applications. Midmark and Eschmann Holdings are developing integrated data management platforms, while technology companies like Solstice Medical are introducing RFID-based tracking systems to complement traditional sterilization monitoring approaches.
Truking Technology Ltd.
Technical Solution: Truking Technology has developed an integrated sterilization data management platform called "SterilSense" that utilizes industrial IoT sensors and advanced analytics for predictive quality control in pharmaceutical and medical device sterilization processes. Their system employs a network of high-precision sensors that capture over 30 distinct parameters throughout the sterilization cycle, including multi-point temperature mapping, pressure gradients, steam quality metrics, and air removal efficiency. The platform implements a hybrid analytics approach combining physics-based models with machine learning algorithms to establish process capability indices (Cpk) for each critical parameter, enabling real-time prediction of sterilization effectiveness. Their solution features a digital twin modeling capability that simulates the thermal penetration characteristics of different load configurations, allowing operators to optimize cycle parameters before physical processing. The system's predictive quality module analyzes historical performance patterns to identify subtle deviations that may indicate developing equipment issues or process drift, generating proactive alerts before sterility assurance levels are compromised.
Strengths: Comprehensive parameter monitoring beyond standard requirements; sophisticated digital twin modeling capabilities; strong integration with pharmaceutical manufacturing execution systems. Weaknesses: Higher complexity requiring specialized technical expertise; significant computational resources needed for real-time digital twin simulations; primarily optimized for pharmaceutical rather than hospital environments.
W&H Sterilization SRL
Technical Solution: W&H Sterilization has developed an advanced autoclave monitoring system called "Lisa Remote Monitoring" that utilizes real-time data collection and analysis for predictive sterilization quality. Their solution employs multiple sensors throughout the sterilization chamber to continuously monitor critical parameters including temperature, pressure, humidity, and cycle time. The system implements machine learning algorithms that analyze historical performance data against current operation metrics to predict potential failures before they occur. Their proprietary Adaptive Sterilization Technology adjusts cycle parameters in real-time based on load characteristics and historical performance data, optimizing both energy efficiency and sterilization efficacy. The platform includes comprehensive data logging capabilities that maintain detailed records of all sterilization cycles, enabling traceability and compliance with regulatory standards such as EN 13060 and EN 285. Their cloud-based analytics dashboard provides visualization of sterilization trends and predictive maintenance alerts, allowing healthcare facilities to schedule maintenance before critical failures occur.
Strengths: Industry-leading predictive analytics capabilities specifically designed for dental and medical sterilization equipment; seamless integration with existing healthcare IT infrastructure; comprehensive regulatory compliance features. Weaknesses: Higher initial implementation cost compared to basic monitoring systems; requires consistent internet connectivity for full functionality; more complex user interface requiring additional staff training.
Core Innovations in Autoclave Data Analytics and Modeling
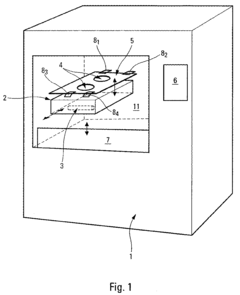
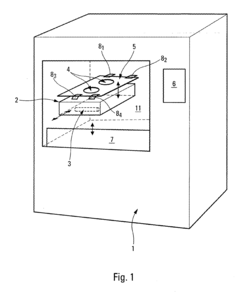
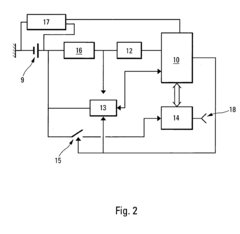
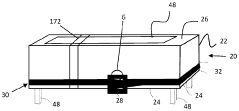
Process and device for controlling the sterilization of articles within an autoclave
PatentInactiveEP1820519A1
Innovation
- An on-board, autonomous device within the container measures sterilization parameters during the cycle and transmits results via electromagnetic waves or magnetic induction to an external reader without opening the container, allowing immediate verification of sterilization compliance before or after removal from the autoclave.
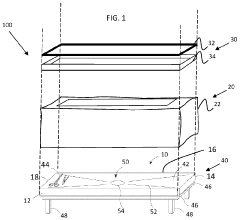
Sterilization packaging system and method
PatentWO2024020239A1
Innovation
- A sterilization packaging system comprising a base with a support surface and a wrap that seals around the base and object, using a compressible layer and sealing device to form a sealed volume, allowing for efficient steam penetration and reducing the need for additional draping, while being designed for easy expansion and removal post-sterilization.
Regulatory Compliance and Validation Requirements
Regulatory compliance represents a critical framework governing autoclave sterilization processes across healthcare, pharmaceutical, and food processing industries. The FDA's 21 CFR Part 11 establishes requirements for electronic records and signatures, mandating that autoclave data collection systems maintain data integrity, security, and traceability. Similarly, the EU Medical Device Regulation (MDR) and ISO 13485:2016 specify stringent documentation requirements for sterilization processes, emphasizing the need for comprehensive data management systems.
Validation requirements for autoclave sterilization follow a structured approach outlined in international standards such as ISO 17665 for moist heat sterilization. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) phases. Each validation stage generates substantial data that must be systematically collected, analyzed, and archived to demonstrate ongoing compliance. Modern predictive quality systems must incorporate these validation frameworks while maintaining regulatory alignment.
The concept of Process Analytical Technology (PAT), introduced by regulatory bodies, encourages real-time monitoring and analysis of critical process parameters. For autoclave sterilization, this translates to continuous monitoring of temperature, pressure, time, and load configuration data. Predictive models must be validated according to GAMP 5 (Good Automated Manufacturing Practice) guidelines, which provide a risk-based approach to computerized system validation in regulated environments.
Data integrity requirements present particular challenges for predictive sterilization quality systems. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be incorporated into any data collection and analysis framework. Predictive models utilizing autoclave data must demonstrate how these principles are maintained throughout the data lifecycle.
Regulatory bodies increasingly recognize the value of advanced analytics while maintaining cautious oversight. The FDA's framework for AI/ML-based Software as a Medical Device (SaMD) provides guidance that can be adapted for predictive sterilization quality systems. This includes expectations for algorithm transparency, continuous learning protocols, and change control procedures that ensure ongoing compliance as predictive models evolve with additional data inputs.
International harmonization efforts, such as those by the International Medical Device Regulators Forum (IMDRF), are establishing consistent approaches to novel technologies including predictive analytics. Organizations implementing predictive sterilization quality systems must monitor these evolving regulatory landscapes to ensure their data utilization strategies remain compliant across global markets.
Validation requirements for autoclave sterilization follow a structured approach outlined in international standards such as ISO 17665 for moist heat sterilization. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) phases. Each validation stage generates substantial data that must be systematically collected, analyzed, and archived to demonstrate ongoing compliance. Modern predictive quality systems must incorporate these validation frameworks while maintaining regulatory alignment.
The concept of Process Analytical Technology (PAT), introduced by regulatory bodies, encourages real-time monitoring and analysis of critical process parameters. For autoclave sterilization, this translates to continuous monitoring of temperature, pressure, time, and load configuration data. Predictive models must be validated according to GAMP 5 (Good Automated Manufacturing Practice) guidelines, which provide a risk-based approach to computerized system validation in regulated environments.
Data integrity requirements present particular challenges for predictive sterilization quality systems. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be incorporated into any data collection and analysis framework. Predictive models utilizing autoclave data must demonstrate how these principles are maintained throughout the data lifecycle.
Regulatory bodies increasingly recognize the value of advanced analytics while maintaining cautious oversight. The FDA's framework for AI/ML-based Software as a Medical Device (SaMD) provides guidance that can be adapted for predictive sterilization quality systems. This includes expectations for algorithm transparency, continuous learning protocols, and change control procedures that ensure ongoing compliance as predictive models evolve with additional data inputs.
International harmonization efforts, such as those by the International Medical Device Regulators Forum (IMDRF), are establishing consistent approaches to novel technologies including predictive analytics. Organizations implementing predictive sterilization quality systems must monitor these evolving regulatory landscapes to ensure their data utilization strategies remain compliant across global markets.
Implementation Challenges and ROI Assessment
Implementing predictive sterilization quality systems based on autoclave data presents several significant challenges that organizations must address. The integration of data collection systems with existing autoclave equipment often requires substantial hardware modifications, particularly for older sterilization units that lack built-in sensors or digital interfaces. This retrofitting process can be costly and may necessitate temporary production shutdowns, impacting operational continuity. Additionally, the development of reliable data pipelines capable of handling the high-volume, high-frequency data generated during sterilization cycles demands specialized expertise in both sterilization processes and data engineering.
Data quality issues represent another major implementation hurdle. Sensor calibration drift, measurement inconsistencies, and environmental interference can compromise the integrity of collected data, potentially leading to inaccurate predictions. Organizations must establish rigorous validation protocols to ensure data reliability before building predictive models upon this foundation. Furthermore, the regulatory compliance landscape for sterilization processes is complex, with stringent requirements from bodies such as FDA and EMA that must be satisfied when implementing new quality prediction systems.
From a return on investment perspective, predictive sterilization quality systems offer compelling financial benefits despite these implementation challenges. Direct cost savings emerge from reduced batch rejections, with organizations reporting 15-30% fewer failed sterilization cycles after implementation. The prevention of even a single batch rejection can save pharmaceutical manufacturers hundreds of thousands of dollars in lost product value. Operational efficiency improvements, including optimized cycle times and reduced energy consumption, typically yield 5-12% reductions in sterilization-related operational costs.
The ROI timeline for these systems generally shows break-even points between 12-24 months, depending on implementation scale and existing infrastructure. Organizations with higher sterilization volumes or those producing high-value products tend to realize returns more quickly. Beyond direct financial returns, these systems deliver significant risk mitigation value by reducing the probability of sterility failures reaching later production stages or, most critically, the market. Such preventive capabilities, while harder to quantify precisely, represent substantial value in terms of brand protection and regulatory compliance.
Human resource optimization represents another ROI dimension, as predictive systems reduce the manual quality assurance burden, allowing specialized personnel to focus on higher-value activities rather than routine monitoring and troubleshooting. This reallocation of human capital often yields productivity improvements across sterilization-dependent operations.
Data quality issues represent another major implementation hurdle. Sensor calibration drift, measurement inconsistencies, and environmental interference can compromise the integrity of collected data, potentially leading to inaccurate predictions. Organizations must establish rigorous validation protocols to ensure data reliability before building predictive models upon this foundation. Furthermore, the regulatory compliance landscape for sterilization processes is complex, with stringent requirements from bodies such as FDA and EMA that must be satisfied when implementing new quality prediction systems.
From a return on investment perspective, predictive sterilization quality systems offer compelling financial benefits despite these implementation challenges. Direct cost savings emerge from reduced batch rejections, with organizations reporting 15-30% fewer failed sterilization cycles after implementation. The prevention of even a single batch rejection can save pharmaceutical manufacturers hundreds of thousands of dollars in lost product value. Operational efficiency improvements, including optimized cycle times and reduced energy consumption, typically yield 5-12% reductions in sterilization-related operational costs.
The ROI timeline for these systems generally shows break-even points between 12-24 months, depending on implementation scale and existing infrastructure. Organizations with higher sterilization volumes or those producing high-value products tend to realize returns more quickly. Beyond direct financial returns, these systems deliver significant risk mitigation value by reducing the probability of sterility failures reaching later production stages or, most critically, the market. Such preventive capabilities, while harder to quantify precisely, represent substantial value in terms of brand protection and regulatory compliance.
Human resource optimization represents another ROI dimension, as predictive systems reduce the manual quality assurance burden, allowing specialized personnel to focus on higher-value activities rather than routine monitoring and troubleshooting. This reallocation of human capital often yields productivity improvements across sterilization-dependent operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!