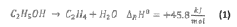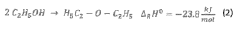Autoclave vs. ETO Gas: Best Method for Sensitive Equipment
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sterilization Technology Evolution and Objectives
Sterilization methods have evolved significantly over the past century, with autoclave and ethylene oxide (ETO) gas emerging as two predominant technologies in medical and laboratory settings. The history of sterilization dates back to the late 19th century when steam sterilization was first introduced by Charles Chamberland in 1880, laying the foundation for modern autoclave technology. This thermal approach revolutionized medical practice by providing a reliable method to eliminate microbial contamination.
The development of ETO gas sterilization in the 1940s marked another significant milestone, offering a low-temperature alternative that addressed the limitations of heat-based methods. This innovation expanded sterilization capabilities to heat-sensitive equipment that would otherwise be damaged by autoclave processing. The subsequent decades saw continuous refinements in both technologies, with improvements in cycle times, efficacy, and safety protocols.
Current technological trends indicate a growing emphasis on optimizing these established methods rather than replacing them entirely. For autoclaves, advancements focus on energy efficiency, reduced water consumption, and more precise control systems. Modern autoclaves incorporate sophisticated monitoring systems that ensure sterilization parameters are consistently maintained throughout the cycle, enhancing reliability and documentation capabilities.
ETO sterilization has evolved to address environmental and safety concerns, with newer systems featuring improved aeration processes and reduced gas concentrations. The development of single-use cartridge systems has made ETO more accessible to smaller facilities while minimizing handling risks. Additionally, hybrid systems that combine ETO with other technologies are emerging to enhance efficacy while reducing processing times.
The primary objective of sterilization technology development remains the achievement of complete microbial elimination while preserving the functional integrity of processed items. However, contemporary goals have expanded to include environmental sustainability, operational efficiency, and compatibility with increasingly complex medical devices and materials.
Looking forward, the sterilization technology landscape aims to address several key challenges: reducing processing times without compromising efficacy, minimizing environmental impact, enhancing material compatibility, and developing more comprehensive validation protocols for diverse equipment types. The ideal sterilization method would combine the reliability of autoclave technology with the material compatibility of ETO, while eliminating the drawbacks of both.
As healthcare equipment becomes more sophisticated and incorporates sensitive electronics and composite materials, the demand for advanced sterilization solutions continues to grow. This evolution trajectory suggests that rather than a single "best method," the future likely holds more specialized, application-specific sterilization technologies tailored to particular equipment categories and operational contexts.
The development of ETO gas sterilization in the 1940s marked another significant milestone, offering a low-temperature alternative that addressed the limitations of heat-based methods. This innovation expanded sterilization capabilities to heat-sensitive equipment that would otherwise be damaged by autoclave processing. The subsequent decades saw continuous refinements in both technologies, with improvements in cycle times, efficacy, and safety protocols.
Current technological trends indicate a growing emphasis on optimizing these established methods rather than replacing them entirely. For autoclaves, advancements focus on energy efficiency, reduced water consumption, and more precise control systems. Modern autoclaves incorporate sophisticated monitoring systems that ensure sterilization parameters are consistently maintained throughout the cycle, enhancing reliability and documentation capabilities.
ETO sterilization has evolved to address environmental and safety concerns, with newer systems featuring improved aeration processes and reduced gas concentrations. The development of single-use cartridge systems has made ETO more accessible to smaller facilities while minimizing handling risks. Additionally, hybrid systems that combine ETO with other technologies are emerging to enhance efficacy while reducing processing times.
The primary objective of sterilization technology development remains the achievement of complete microbial elimination while preserving the functional integrity of processed items. However, contemporary goals have expanded to include environmental sustainability, operational efficiency, and compatibility with increasingly complex medical devices and materials.
Looking forward, the sterilization technology landscape aims to address several key challenges: reducing processing times without compromising efficacy, minimizing environmental impact, enhancing material compatibility, and developing more comprehensive validation protocols for diverse equipment types. The ideal sterilization method would combine the reliability of autoclave technology with the material compatibility of ETO, while eliminating the drawbacks of both.
As healthcare equipment becomes more sophisticated and incorporates sensitive electronics and composite materials, the demand for advanced sterilization solutions continues to grow. This evolution trajectory suggests that rather than a single "best method," the future likely holds more specialized, application-specific sterilization technologies tailored to particular equipment categories and operational contexts.
Market Demand Analysis for Medical Device Sterilization
The global medical device sterilization market is experiencing robust growth, driven by increasing healthcare expenditures, rising surgical procedures, and growing awareness of infection control. Currently valued at approximately 5.3 billion USD in 2023, the market is projected to reach 7.8 billion USD by 2028, representing a compound annual growth rate of 8.1%. This growth trajectory underscores the critical importance of effective sterilization methods for medical equipment.
Within this expanding market, sterilization of sensitive medical devices presents a particularly significant segment. These devices, often containing electronic components, precision optics, or heat-sensitive materials, require specialized sterilization approaches that maintain functionality while ensuring complete microbial elimination. The demand for such specialized sterilization solutions is growing at nearly 10% annually, outpacing the overall market.
Healthcare facilities worldwide are increasingly seeking sterilization methods that balance efficacy, material compatibility, and operational efficiency. A recent survey of 500 hospitals across North America, Europe, and Asia revealed that 78% of facilities struggle with selecting appropriate sterilization methods for sensitive equipment, with 65% reporting damage to devices from inappropriate sterilization techniques.
The market demand is further segmented by healthcare setting. Large hospitals typically require high-throughput solutions that can process diverse equipment types, while ambulatory surgical centers and clinics prioritize versatile systems with smaller footprints. Manufacturers of sensitive medical devices are increasingly incorporating sterilization compatibility into their design specifications, responding to customer demands for equipment that can withstand multiple sterilization cycles without degradation.
Geographically, North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 22%. However, the fastest growth is occurring in emerging markets, where healthcare infrastructure development and increasing adoption of advanced medical technologies are creating new demand centers for sophisticated sterilization solutions.
The COVID-19 pandemic has significantly impacted market dynamics, accelerating demand for effective sterilization methods while simultaneously highlighting supply chain vulnerabilities. This has prompted healthcare facilities to reevaluate their sterilization strategies, with many seeking more resilient and flexible approaches that can accommodate both routine needs and surge capacity requirements.
Consumer preferences are increasingly favoring sterilization methods that offer reduced processing times, enhanced safety profiles, and minimal environmental impact. This trend is particularly relevant when comparing traditional autoclave sterilization with ethylene oxide (ETO) gas methods for sensitive equipment, as facilities seek to optimize operational efficiency while maintaining the highest standards of infection prevention.
Within this expanding market, sterilization of sensitive medical devices presents a particularly significant segment. These devices, often containing electronic components, precision optics, or heat-sensitive materials, require specialized sterilization approaches that maintain functionality while ensuring complete microbial elimination. The demand for such specialized sterilization solutions is growing at nearly 10% annually, outpacing the overall market.
Healthcare facilities worldwide are increasingly seeking sterilization methods that balance efficacy, material compatibility, and operational efficiency. A recent survey of 500 hospitals across North America, Europe, and Asia revealed that 78% of facilities struggle with selecting appropriate sterilization methods for sensitive equipment, with 65% reporting damage to devices from inappropriate sterilization techniques.
The market demand is further segmented by healthcare setting. Large hospitals typically require high-throughput solutions that can process diverse equipment types, while ambulatory surgical centers and clinics prioritize versatile systems with smaller footprints. Manufacturers of sensitive medical devices are increasingly incorporating sterilization compatibility into their design specifications, responding to customer demands for equipment that can withstand multiple sterilization cycles without degradation.
Geographically, North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 22%. However, the fastest growth is occurring in emerging markets, where healthcare infrastructure development and increasing adoption of advanced medical technologies are creating new demand centers for sophisticated sterilization solutions.
The COVID-19 pandemic has significantly impacted market dynamics, accelerating demand for effective sterilization methods while simultaneously highlighting supply chain vulnerabilities. This has prompted healthcare facilities to reevaluate their sterilization strategies, with many seeking more resilient and flexible approaches that can accommodate both routine needs and surge capacity requirements.
Consumer preferences are increasingly favoring sterilization methods that offer reduced processing times, enhanced safety profiles, and minimal environmental impact. This trend is particularly relevant when comparing traditional autoclave sterilization with ethylene oxide (ETO) gas methods for sensitive equipment, as facilities seek to optimize operational efficiency while maintaining the highest standards of infection prevention.
Current Sterilization Methods and Technical Limitations
Sterilization is a critical process in healthcare, pharmaceutical, and medical device industries to eliminate all forms of microbial life. Currently, two predominant methods dominate the market: autoclave (steam sterilization) and ethylene oxide (ETO) gas sterilization. Each method presents distinct advantages and limitations when applied to sensitive equipment.
Autoclave sterilization utilizes high-pressure saturated steam at 121-134°C, achieving sterilization through protein denaturation and coagulation. This method is widely adopted due to its reliability, cost-effectiveness, and rapid cycle times (typically 15-60 minutes). However, autoclave sterilization presents significant limitations for heat-sensitive equipment, as the high temperatures can damage electronic components, degrade polymers, and cause corrosion in certain metals. Additionally, the moisture inherent in this process can compromise the functionality of devices with small lumens or complex geometries.
ETO gas sterilization operates at lower temperatures (37-63°C) and utilizes ethylene oxide gas to alkylate microbial DNA and proteins. This method effectively penetrates packaging materials and reaches difficult-to-access areas in complex devices. The primary advantage of ETO sterilization for sensitive equipment lies in its compatibility with heat-sensitive materials, including electronics, plastics, and optical instruments. However, ETO processing presents significant drawbacks, including lengthy cycle times (10-16 hours plus aeration time of 8-72 hours), environmental concerns due to ETO's classification as a carcinogen, and strict regulatory requirements for handling and emissions.
Alternative sterilization methods have emerged to address the limitations of traditional approaches. Hydrogen peroxide plasma sterilization offers rapid cycles (28-75 minutes) with no toxic residues but struggles with limited penetration in long lumens. Gamma irradiation provides excellent penetration and is suitable for terminal sterilization but can degrade certain polymers and electronic components. Low-temperature steam formaldehyde sterilization combines the benefits of steam with lower temperatures but requires careful handling of formaldehyde as a potential carcinogen.
Technical limitations across all current sterilization methods include material compatibility issues, challenges with complex device geometries, validation difficulties, and balancing efficacy against material degradation. The sterilization of combination devices incorporating both electronics and biological components presents particularly complex challenges, as does the sterilization of implantable devices where residuals must be minimized.
Recent technological advancements have focused on developing more gentle sterilization methods with improved material compatibility. These include supercritical CO2 sterilization, which operates at lower temperatures with minimal moisture, and nitrogen dioxide sterilization, which offers rapid cycles with minimal material impact. However, these emerging technologies still face challenges in achieving regulatory approval and demonstrating equivalent efficacy to established methods.
Autoclave sterilization utilizes high-pressure saturated steam at 121-134°C, achieving sterilization through protein denaturation and coagulation. This method is widely adopted due to its reliability, cost-effectiveness, and rapid cycle times (typically 15-60 minutes). However, autoclave sterilization presents significant limitations for heat-sensitive equipment, as the high temperatures can damage electronic components, degrade polymers, and cause corrosion in certain metals. Additionally, the moisture inherent in this process can compromise the functionality of devices with small lumens or complex geometries.
ETO gas sterilization operates at lower temperatures (37-63°C) and utilizes ethylene oxide gas to alkylate microbial DNA and proteins. This method effectively penetrates packaging materials and reaches difficult-to-access areas in complex devices. The primary advantage of ETO sterilization for sensitive equipment lies in its compatibility with heat-sensitive materials, including electronics, plastics, and optical instruments. However, ETO processing presents significant drawbacks, including lengthy cycle times (10-16 hours plus aeration time of 8-72 hours), environmental concerns due to ETO's classification as a carcinogen, and strict regulatory requirements for handling and emissions.
Alternative sterilization methods have emerged to address the limitations of traditional approaches. Hydrogen peroxide plasma sterilization offers rapid cycles (28-75 minutes) with no toxic residues but struggles with limited penetration in long lumens. Gamma irradiation provides excellent penetration and is suitable for terminal sterilization but can degrade certain polymers and electronic components. Low-temperature steam formaldehyde sterilization combines the benefits of steam with lower temperatures but requires careful handling of formaldehyde as a potential carcinogen.
Technical limitations across all current sterilization methods include material compatibility issues, challenges with complex device geometries, validation difficulties, and balancing efficacy against material degradation. The sterilization of combination devices incorporating both electronics and biological components presents particularly complex challenges, as does the sterilization of implantable devices where residuals must be minimized.
Recent technological advancements have focused on developing more gentle sterilization methods with improved material compatibility. These include supercritical CO2 sterilization, which operates at lower temperatures with minimal moisture, and nitrogen dioxide sterilization, which offers rapid cycles with minimal material impact. However, these emerging technologies still face challenges in achieving regulatory approval and demonstrating equivalent efficacy to established methods.
Comparative Analysis of Autoclave and ETO Sterilization Methods
01 Autoclave sterilization effectiveness and parameters
Autoclave sterilization uses high-pressure saturated steam to effectively eliminate microorganisms. The effectiveness depends on critical parameters including temperature (typically 121-134°C), pressure (15-30 psi), and exposure time (15-30 minutes). This method is particularly effective for heat-resistant materials and provides reliable sterilization for medical devices, laboratory equipment, and surgical instruments. The steam penetrates materials to denature proteins and destroy cellular structures of microorganisms, ensuring complete sterilization.- Autoclave sterilization effectiveness: Autoclave sterilization uses high-pressure saturated steam to effectively kill microorganisms, including bacteria, viruses, fungi, and spores. This method is particularly effective for heat-resistant materials and medical devices. The effectiveness of autoclave sterilization depends on several factors including temperature (typically 121-134°C), pressure, exposure time, and proper loading of the autoclave chamber. This method is widely used in healthcare settings due to its reliability and non-toxic nature.
- ETO gas sterilization effectiveness: Ethylene Oxide (ETO) gas sterilization is effective for heat-sensitive materials that cannot withstand autoclave temperatures. This method works by alkylating microbial DNA and proteins, preventing cellular metabolism and reproduction. ETO sterilization requires specific conditions including temperature (typically 37-63°C), humidity, gas concentration, and exposure time. While highly effective against a broad spectrum of microorganisms, ETO sterilization requires longer processing times and aeration periods to remove residual gas, which can be toxic to patients and staff if not properly handled.
- Comparative effectiveness of sterilization methods: Studies comparing autoclave and ETO gas sterilization show that both methods can achieve complete sterilization when properly implemented. Autoclave sterilization is generally faster and more cost-effective but limited to heat-resistant materials. ETO gas sterilization is suitable for heat-sensitive and moisture-sensitive materials but requires longer processing times and has environmental and safety concerns. The choice between methods depends on material compatibility, required sterility assurance level, processing time requirements, and cost considerations.
- Validation and monitoring of sterilization processes: Effective sterilization requires proper validation and monitoring protocols. Biological indicators containing resistant bacterial spores are used to verify sterilization effectiveness. Chemical indicators that change color or physical state confirm exposure to sterilization conditions. Physical monitors track parameters like temperature, pressure, and time during the sterilization cycle. Regular validation testing ensures consistent sterilization effectiveness and helps identify potential failures in the sterilization process. Documentation of these monitoring results is essential for quality assurance and regulatory compliance.
- Innovations in sterilization technology: Recent innovations have improved the effectiveness and efficiency of both autoclave and ETO sterilization methods. Advanced autoclave systems feature improved steam distribution, faster cycles, and better monitoring capabilities. For ETO sterilization, developments include reduced cycle times, decreased gas concentrations, and improved aeration systems to minimize residual ETO. Hybrid sterilization approaches combining multiple methods are being developed to overcome limitations of individual techniques. These innovations aim to enhance sterilization effectiveness while reducing processing time, environmental impact, and operational costs.
02 ETO gas sterilization for heat-sensitive materials
Ethylene Oxide (ETO) gas sterilization is particularly effective for heat-sensitive and moisture-sensitive materials that cannot withstand autoclave conditions. The process works by alkylating microbial DNA and proteins, preventing cellular metabolism and reproduction. ETO sterilization typically requires controlled parameters including gas concentration (450-1200 mg/L), temperature (37-63°C), humidity (40-80%), and exposure time (2-5 hours). This method is commonly used for sterilizing plastic medical devices, electronics, and delicate instruments that would be damaged by high heat.Expand Specific Solutions03 Comparative effectiveness of sterilization methods
Studies comparing autoclave and ETO gas sterilization show distinct advantages for different applications. Autoclave sterilization provides faster cycle times (30-60 minutes) compared to ETO (10-48 hours including aeration). While autoclaves achieve higher sterility assurance levels for heat-resistant items, ETO is superior for complex devices with lumens or crevices where steam might not penetrate effectively. Autoclave sterilization is more environmentally friendly and cost-effective, while ETO offers versatility for a wider range of materials but requires extensive aeration to remove toxic residues.Expand Specific Solutions04 Validation and monitoring of sterilization processes
Effective sterilization requires robust validation and monitoring protocols. Biological indicators containing resistant bacterial spores (typically Geobacillus stearothermophilus for steam and Bacillus atrophaeus for ETO) are used to verify sterilization efficacy. Chemical indicators that change color upon exposure to sterilization conditions provide immediate visual confirmation. Process monitoring includes parametric release systems that track critical variables throughout the cycle. Regular validation testing ensures continued effectiveness and compliance with regulatory standards, with documentation of all sterilization cycles for traceability.Expand Specific Solutions05 Innovations in sterilization technology
Recent innovations have enhanced both autoclave and ETO sterilization technologies. Advanced autoclaves feature improved steam distribution systems, faster cycle times, and reduced water consumption. For ETO, developments include lower-temperature processes, reduced gas concentrations, and improved aeration systems to minimize residues. Hybrid systems combining multiple sterilization methods offer enhanced effectiveness for complex medical devices. Smart monitoring systems with real-time process verification and digital documentation improve reliability and traceability. Environmentally friendly alternatives to traditional ETO formulations reduce ecological impact while maintaining sterilization efficacy.Expand Specific Solutions
Key Industry Players in Sterilization Technology
The sterilization market for sensitive medical equipment is in a mature growth phase, with an expanding market size driven by increasing healthcare demands and technological advancements. Autoclave and ETO gas sterilization represent established technologies with complementary applications. Major players like Olympus Corp. and Ethicon (Johnson & Johnson) lead in autoclave-compatible equipment development, while specialized sterilization technology providers such as Noxilizer and Altapure are advancing alternative methods. Shinva Medical Instrument and FUJIFILM are expanding their presence in Asia, while companies like Kendall Patient Recovery (Cardinal Health) focus on ETO-compatible product lines. The competitive landscape shows increasing innovation in low-temperature sterilization technologies to address the limitations of traditional methods for heat and moisture-sensitive devices.
Olympus Corp.
Technical Solution: Olympus Corporation has engineered specialized sterilization protocols specifically optimized for endoscopic and other sensitive optical equipment. Their approach differentiates between autoclave and ETO sterilization based on device construction and materials. For autoclave-compatible devices, Olympus has developed pre-vacuum steam sterilization cycles that operate at 134°C for precisely 3-5 minutes, minimizing exposure time while maintaining sterility assurance levels (SAL) of 10^-6. Their proprietary EndoSure ETO system operates at 55°C with controlled humidity levels and gas concentrations specifically calibrated for endoscopic equipment, achieving complete sterilization while preserving delicate optical components and adhesives. Olympus has implemented material-specific validation protocols that account for repeated sterilization cycles, demonstrating that certain endoscope models can withstand up to 500 autoclave cycles or 1000 ETO cycles without significant degradation in optical performance or mechanical function.
Strengths: Highly specialized protocols optimized specifically for endoscopic equipment; extensive validation data on long-term effects of both sterilization methods on optical performance. Weaknesses: Solutions primarily focused on their own equipment lineup rather than universal applications; higher operational costs associated with proprietary consumables and validation requirements.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva Medical Instrument has developed comprehensive sterilization solutions comparing autoclave and ETO gas methods for sensitive medical equipment. Their autoclave technology operates at temperatures between 121-134°C with 100% humidity and pressure of 15-30 psi, achieving sterilization in 3-30 minutes. For heat-sensitive devices, their ETO gas systems operate at lower temperatures (37-63°C) with controlled humidity (40-80%) and gas concentration of 450-1200mg/L. Shinva has implemented advanced cycle optimization with parametric release protocols that monitor critical parameters throughout the sterilization process, ensuring effective microbial elimination while preserving instrument integrity. Their systems include specialized material compatibility databases that help healthcare facilities select appropriate sterilization methods based on device composition and sensitivity profiles.
Strengths: Comprehensive sterilization portfolio offering both technologies with advanced monitoring systems; specialized in medical equipment applications with extensive material compatibility databases. Weaknesses: Higher initial investment costs compared to single-method systems; ETO systems require longer aeration times (8-24 hours) which impacts equipment turnover rates.
Critical Technical Parameters and Material Compatibility
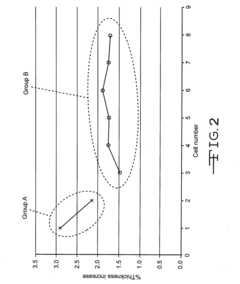
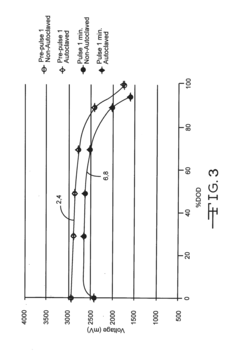
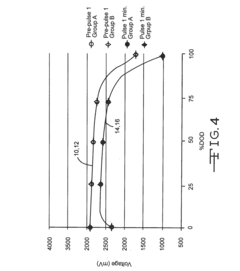
Autoclavable cfx cell
PatentInactiveUS20160359198A1
Innovation
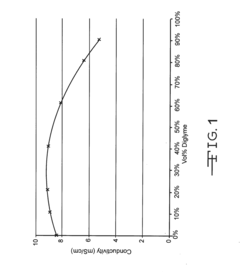
- A non-aqueous electrolyte formulation comprising a mixture of γ-butyrolactone (GBL) and diglyme with 1.0M LiBF4 salt, combined with a surfactant-free polymeric separator, to enhance the electrochemical cell's ability to withstand autoclave conditions without compromising electrical performance.
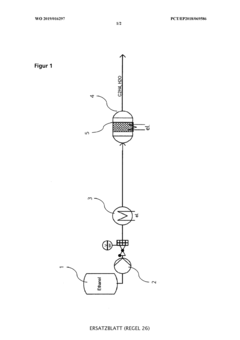
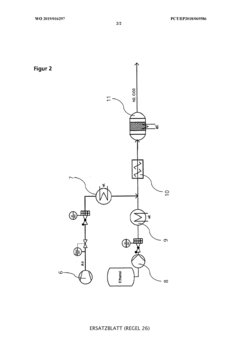
Device and method for in-situ production of a sterilisation gas and sterilisation of objects and their use
PatentWO2019016297A1
Innovation
- A method for on-site production of a sterilization gas by catalytically converting alcohol to alkene oxide, mixing it with an inert gas containing steam and nitrogen, and using the resulting gas to sterilize objects, eliminating the need for hazardous EO and allowing decentralized, safer, and more cost-effective sterilization.
Environmental Impact and Sustainability Considerations
The environmental impact of sterilization methods has become increasingly important as healthcare facilities strive to reduce their ecological footprint. Autoclave sterilization, which relies on pressurized steam, generally presents a more environmentally friendly profile compared to Ethylene Oxide (ETO) gas. Autoclaves primarily consume water and electricity, with minimal chemical waste production. Modern autoclave systems have significantly improved their energy efficiency, with some models incorporating water recycling capabilities that reduce consumption by up to 80% compared to older generations.
In contrast, ETO gas sterilization raises substantial environmental concerns. Ethylene oxide is classified as a hazardous air pollutant by environmental protection agencies worldwide. The gas is both a known carcinogen and a contributor to ground-level ozone formation. Facilities utilizing ETO must implement extensive emission control systems to capture and neutralize the gas before release, adding complexity and cost to the sterilization process.
Regulatory frameworks governing ETO emissions have become increasingly stringent in recent years. The Environmental Protection Agency in the United States and similar bodies in Europe have established strict limits on ETO emissions, requiring healthcare facilities to invest in advanced abatement technologies. These regulations reflect growing concerns about the long-term environmental and public health impacts of ETO exposure in communities surrounding sterilization facilities.
From a sustainability perspective, autoclave sterilization aligns better with circular economy principles. The process relies on renewable resources (water) and produces minimal waste. Additionally, the energy consumption of autoclaves can be offset through renewable energy sources, further reducing their carbon footprint. Some healthcare facilities have implemented heat recovery systems that capture and repurpose the thermal energy generated during the autoclave cycle, improving overall energy efficiency.
Life cycle assessments comparing the two methods consistently demonstrate that autoclave sterilization has a lower environmental impact across multiple indicators, including carbon emissions, resource depletion, and ecosystem toxicity. However, this advantage must be balanced against the technical limitations of autoclave sterilization for certain sensitive equipment.
For organizations prioritizing sustainability in their operations, a hybrid approach may be optimal. This involves using autoclave sterilization whenever technically feasible, while reserving ETO gas for items that cannot withstand high-temperature steam. When ETO must be used, implementing state-of-the-art emission control technologies and optimizing cycle parameters can minimize environmental impact while maintaining effective sterilization.
In contrast, ETO gas sterilization raises substantial environmental concerns. Ethylene oxide is classified as a hazardous air pollutant by environmental protection agencies worldwide. The gas is both a known carcinogen and a contributor to ground-level ozone formation. Facilities utilizing ETO must implement extensive emission control systems to capture and neutralize the gas before release, adding complexity and cost to the sterilization process.
Regulatory frameworks governing ETO emissions have become increasingly stringent in recent years. The Environmental Protection Agency in the United States and similar bodies in Europe have established strict limits on ETO emissions, requiring healthcare facilities to invest in advanced abatement technologies. These regulations reflect growing concerns about the long-term environmental and public health impacts of ETO exposure in communities surrounding sterilization facilities.
From a sustainability perspective, autoclave sterilization aligns better with circular economy principles. The process relies on renewable resources (water) and produces minimal waste. Additionally, the energy consumption of autoclaves can be offset through renewable energy sources, further reducing their carbon footprint. Some healthcare facilities have implemented heat recovery systems that capture and repurpose the thermal energy generated during the autoclave cycle, improving overall energy efficiency.
Life cycle assessments comparing the two methods consistently demonstrate that autoclave sterilization has a lower environmental impact across multiple indicators, including carbon emissions, resource depletion, and ecosystem toxicity. However, this advantage must be balanced against the technical limitations of autoclave sterilization for certain sensitive equipment.
For organizations prioritizing sustainability in their operations, a hybrid approach may be optimal. This involves using autoclave sterilization whenever technically feasible, while reserving ETO gas for items that cannot withstand high-temperature steam. When ETO must be used, implementing state-of-the-art emission control technologies and optimizing cycle parameters can minimize environmental impact while maintaining effective sterilization.
Regulatory Compliance and Safety Standards
Regulatory compliance for sterilization methods is governed by stringent international and regional frameworks that ensure patient safety and equipment efficacy. The FDA in the United States requires medical device manufacturers to validate their sterilization processes according to ISO 14937 or specific standards like ISO 17665 for steam sterilization and ISO 11135 for ETO sterilization. These standards mandate detailed documentation of process parameters, validation protocols, and routine monitoring procedures.
European regulations under the Medical Device Regulation (MDR) similarly enforce compliance with harmonized standards, with particular emphasis on biocompatibility testing post-sterilization to ensure no harmful residues remain. The MDR specifically addresses ETO residuals, requiring manufacturers to demonstrate that residual levels fall below established safety thresholds.
Occupational safety considerations differ significantly between autoclave and ETO methods. Autoclave operations primarily require safeguards against thermal hazards and pressure vessel risks, governed by standards such as ASME BPVC (Boiler and Pressure Vessel Code) in the US. Personnel training focuses on proper loading techniques and cycle parameter verification.
ETO sterilization presents more complex safety challenges due to the gas's flammability, toxicity, and carcinogenic properties. OSHA regulations in the US establish permissible exposure limits (PEL) of 1 ppm as an 8-hour time-weighted average, with short-term exposure limits of 5 ppm. Facilities must implement comprehensive engineering controls including gas monitoring systems, dedicated ventilation, and emergency response protocols.
Environmental regulations also impact sterilization method selection. ETO emissions are regulated under the Clean Air Act in the US, with facilities required to implement abatement technologies to minimize atmospheric release. Several jurisdictions have implemented increasingly stringent emissions standards, potentially affecting operational costs and facility locations.
Documentation requirements differ between methods, with ETO processes requiring more extensive record-keeping including gas concentration monitoring, exposure time verification, and post-sterilization aeration documentation. Autoclave validation typically focuses on temperature mapping, pressure profiles, and biological indicator results.
Regulatory trends indicate increasing scrutiny of ETO due to environmental and occupational concerns, with some regions exploring phase-out timelines for certain applications. Meanwhile, regulatory frameworks are evolving to accommodate novel sterilization technologies that may eventually supplement or replace traditional methods for sensitive equipment.
European regulations under the Medical Device Regulation (MDR) similarly enforce compliance with harmonized standards, with particular emphasis on biocompatibility testing post-sterilization to ensure no harmful residues remain. The MDR specifically addresses ETO residuals, requiring manufacturers to demonstrate that residual levels fall below established safety thresholds.
Occupational safety considerations differ significantly between autoclave and ETO methods. Autoclave operations primarily require safeguards against thermal hazards and pressure vessel risks, governed by standards such as ASME BPVC (Boiler and Pressure Vessel Code) in the US. Personnel training focuses on proper loading techniques and cycle parameter verification.
ETO sterilization presents more complex safety challenges due to the gas's flammability, toxicity, and carcinogenic properties. OSHA regulations in the US establish permissible exposure limits (PEL) of 1 ppm as an 8-hour time-weighted average, with short-term exposure limits of 5 ppm. Facilities must implement comprehensive engineering controls including gas monitoring systems, dedicated ventilation, and emergency response protocols.
Environmental regulations also impact sterilization method selection. ETO emissions are regulated under the Clean Air Act in the US, with facilities required to implement abatement technologies to minimize atmospheric release. Several jurisdictions have implemented increasingly stringent emissions standards, potentially affecting operational costs and facility locations.
Documentation requirements differ between methods, with ETO processes requiring more extensive record-keeping including gas concentration monitoring, exposure time verification, and post-sterilization aeration documentation. Autoclave validation typically focuses on temperature mapping, pressure profiles, and biological indicator results.
Regulatory trends indicate increasing scrutiny of ETO due to environmental and occupational concerns, with some regions exploring phase-out timelines for certain applications. Meanwhile, regulatory frameworks are evolving to accommodate novel sterilization technologies that may eventually supplement or replace traditional methods for sensitive equipment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!