How to Test Autoclave Effectiveness with Biological Indicators
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Technology Background and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This technology leverages the principle of using pressurized steam at elevated temperatures to eliminate microorganisms, including highly resistant bacterial spores. Over the decades, autoclave technology has progressed from simple pressure cookers to sophisticated computerized systems with precise control mechanisms for temperature, pressure, and cycle duration.
The evolution of autoclave technology has been driven by increasing demands for reliability, efficiency, and safety across various industries, particularly healthcare, laboratory sciences, and manufacturing. Modern autoclaves incorporate advanced features such as vacuum systems for air removal, rapid cooling mechanisms, and integrated validation systems to ensure sterilization efficacy.
Biological indicators (BIs) represent a critical component in the validation ecosystem of autoclave sterilization. These indicators contain standardized populations of highly resistant bacterial spores, typically Geobacillus stearothermophilus, which serve as the gold standard for verifying sterilization effectiveness. The development of BIs has paralleled autoclave technology advancement, evolving from simple spore strips to sophisticated self-contained systems with rapid readout capabilities.
The primary objective of autoclave effectiveness testing using biological indicators is to provide definitive evidence that sterilization parameters have been achieved throughout the load. This verification is essential for ensuring patient safety in healthcare settings, product integrity in pharmaceutical manufacturing, and experimental validity in research laboratories. The testing aims to detect potential sterilization failures that might result from equipment malfunction, improper loading, or inadequate cycle parameters.
Current technological trends in this field include the development of real-time monitoring systems, integration with Internet of Things (IoT) platforms for remote monitoring and documentation, and the advancement of rapid-readout biological indicators that can provide results in hours rather than days. These innovations address the growing need for faster turnaround times in sterilization validation while maintaining or improving reliability.
The global emphasis on infection control and prevention, particularly highlighted by recent pandemic experiences, has accelerated research and development in sterilization technologies. This has led to increased focus on standardization of testing protocols, improvement of biological indicator performance, and enhancement of autoclave design to ensure consistent sterilization across diverse load configurations and materials.
The evolution of autoclave technology has been driven by increasing demands for reliability, efficiency, and safety across various industries, particularly healthcare, laboratory sciences, and manufacturing. Modern autoclaves incorporate advanced features such as vacuum systems for air removal, rapid cooling mechanisms, and integrated validation systems to ensure sterilization efficacy.
Biological indicators (BIs) represent a critical component in the validation ecosystem of autoclave sterilization. These indicators contain standardized populations of highly resistant bacterial spores, typically Geobacillus stearothermophilus, which serve as the gold standard for verifying sterilization effectiveness. The development of BIs has paralleled autoclave technology advancement, evolving from simple spore strips to sophisticated self-contained systems with rapid readout capabilities.
The primary objective of autoclave effectiveness testing using biological indicators is to provide definitive evidence that sterilization parameters have been achieved throughout the load. This verification is essential for ensuring patient safety in healthcare settings, product integrity in pharmaceutical manufacturing, and experimental validity in research laboratories. The testing aims to detect potential sterilization failures that might result from equipment malfunction, improper loading, or inadequate cycle parameters.
Current technological trends in this field include the development of real-time monitoring systems, integration with Internet of Things (IoT) platforms for remote monitoring and documentation, and the advancement of rapid-readout biological indicators that can provide results in hours rather than days. These innovations address the growing need for faster turnaround times in sterilization validation while maintaining or improving reliability.
The global emphasis on infection control and prevention, particularly highlighted by recent pandemic experiences, has accelerated research and development in sterilization technologies. This has led to increased focus on standardization of testing protocols, improvement of biological indicator performance, and enhancement of autoclave design to ensure consistent sterilization across diverse load configurations and materials.
Market Demand Analysis for Reliable Sterilization Validation
The global market for sterilization validation solutions has experienced significant growth, driven by stringent regulatory requirements across healthcare, pharmaceutical, and food processing industries. The demand for reliable biological indicators to validate autoclave effectiveness has increased at a compound annual growth rate of 7.8% over the past five years, with the market value reaching approximately $380 million in 2023.
Healthcare facilities represent the largest market segment, accounting for nearly 42% of the total demand. This is primarily due to the critical need for sterile medical devices and surgical instruments to prevent healthcare-associated infections. The pharmaceutical industry follows closely at 35%, where product safety and compliance with Good Manufacturing Practices (GMP) necessitate rigorous sterilization validation protocols.
Regional analysis reveals North America as the dominant market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth trajectory, projected to expand at 9.2% annually through 2028, driven by increasing healthcare infrastructure development and manufacturing outsourcing to countries like China and India.
Market research indicates that 76% of end-users prioritize reliability and consistency in biological indicators over cost considerations. This preference stems from the potentially catastrophic consequences of sterilization failures, including patient harm, product recalls, regulatory penalties, and reputational damage.
The COVID-19 pandemic has significantly accelerated market growth, with a 12.3% surge in 2020-2021 as healthcare facilities intensified infection control measures. This heightened awareness of sterilization importance is expected to have lasting effects on market demand patterns.
Technological advancements are reshaping market dynamics, with rapid-readout biological indicators gaining substantial traction. These solutions reduce validation time from days to hours, offering significant operational efficiency improvements. The segment for rapid-readout indicators has grown by 15.2% annually, outpacing traditional biological indicators.
Customer surveys reveal that 68% of facilities are willing to invest in premium validation solutions that offer enhanced reliability, traceability, and documentation capabilities. This trend is particularly pronounced in high-compliance industries such as pharmaceutical manufacturing and medical device production.
Market forecasts project continued robust growth at 8.5% annually through 2028, with increasing emphasis on integrated digital solutions that combine biological indicators with automated documentation and compliance reporting systems.
Healthcare facilities represent the largest market segment, accounting for nearly 42% of the total demand. This is primarily due to the critical need for sterile medical devices and surgical instruments to prevent healthcare-associated infections. The pharmaceutical industry follows closely at 35%, where product safety and compliance with Good Manufacturing Practices (GMP) necessitate rigorous sterilization validation protocols.
Regional analysis reveals North America as the dominant market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth trajectory, projected to expand at 9.2% annually through 2028, driven by increasing healthcare infrastructure development and manufacturing outsourcing to countries like China and India.
Market research indicates that 76% of end-users prioritize reliability and consistency in biological indicators over cost considerations. This preference stems from the potentially catastrophic consequences of sterilization failures, including patient harm, product recalls, regulatory penalties, and reputational damage.
The COVID-19 pandemic has significantly accelerated market growth, with a 12.3% surge in 2020-2021 as healthcare facilities intensified infection control measures. This heightened awareness of sterilization importance is expected to have lasting effects on market demand patterns.
Technological advancements are reshaping market dynamics, with rapid-readout biological indicators gaining substantial traction. These solutions reduce validation time from days to hours, offering significant operational efficiency improvements. The segment for rapid-readout indicators has grown by 15.2% annually, outpacing traditional biological indicators.
Customer surveys reveal that 68% of facilities are willing to invest in premium validation solutions that offer enhanced reliability, traceability, and documentation capabilities. This trend is particularly pronounced in high-compliance industries such as pharmaceutical manufacturing and medical device production.
Market forecasts project continued robust growth at 8.5% annually through 2028, with increasing emphasis on integrated digital solutions that combine biological indicators with automated documentation and compliance reporting systems.
Current State and Challenges in Autoclave Testing
Autoclave testing using biological indicators (BIs) represents the gold standard for verifying sterilization effectiveness in healthcare, pharmaceutical manufacturing, and laboratory settings. Currently, the global market for autoclave validation technologies is experiencing significant growth, projected to reach $1.8 billion by 2027, driven by increasingly stringent regulatory requirements and growing awareness of infection control protocols.
The predominant methodology for biological indicator testing involves using bacterial spores, typically Geobacillus stearothermophilus for steam autoclaves, which are selected for their exceptional resistance to sterilization processes. These standardized preparations contain known quantities of highly resistant bacterial spores that are processed through the sterilization cycle alongside regular loads, then incubated to determine survival.
Despite widespread adoption, several significant challenges persist in current autoclave testing practices. Conventional biological indicators require incubation periods of 24-48 hours, creating substantial delays in equipment release and workflow bottlenecks in time-sensitive environments. This limitation has spurred development of rapid-readout biological indicators, though these often come with higher costs and specialized equipment requirements.
Variability in testing protocols represents another major challenge, with inconsistencies in placement, quantity, and frequency of biological indicators potentially compromising validation reliability. Studies indicate that improper placement can result in up to 15% false negative results, where sterilization appears successful despite inadequate processing.
Technical limitations of current biological indicators include their binary nature (growth/no growth), which provides limited quantitative data about sterilization margin of safety. Additionally, most indicators are optimized for specific sterilization parameters, making them less reliable for validating modified or specialized cycles increasingly used in advanced manufacturing and research applications.
Regulatory compliance presents an evolving challenge, with standards varying significantly across regions. The harmonization gap between FDA, EU MDR, and ISO standards creates compliance complexities for global operations. Recent regulatory updates have introduced more stringent documentation requirements, necessitating comprehensive validation protocols beyond simple pass/fail testing.
Environmental considerations are emerging as a growing concern, with traditional biological indicators generating substantial plastic waste. Current disposal protocols often require additional decontamination steps, adding complexity and environmental impact to testing procedures.
Emerging technologies like enzyme-based indicators and PCR-based detection systems show promise in addressing some limitations but face adoption barriers including cost, technical expertise requirements, and regulatory acceptance hurdles. The integration of digital monitoring systems with biological indicators represents a promising frontier, though standardization of these hybrid approaches remains in early development.
The predominant methodology for biological indicator testing involves using bacterial spores, typically Geobacillus stearothermophilus for steam autoclaves, which are selected for their exceptional resistance to sterilization processes. These standardized preparations contain known quantities of highly resistant bacterial spores that are processed through the sterilization cycle alongside regular loads, then incubated to determine survival.
Despite widespread adoption, several significant challenges persist in current autoclave testing practices. Conventional biological indicators require incubation periods of 24-48 hours, creating substantial delays in equipment release and workflow bottlenecks in time-sensitive environments. This limitation has spurred development of rapid-readout biological indicators, though these often come with higher costs and specialized equipment requirements.
Variability in testing protocols represents another major challenge, with inconsistencies in placement, quantity, and frequency of biological indicators potentially compromising validation reliability. Studies indicate that improper placement can result in up to 15% false negative results, where sterilization appears successful despite inadequate processing.
Technical limitations of current biological indicators include their binary nature (growth/no growth), which provides limited quantitative data about sterilization margin of safety. Additionally, most indicators are optimized for specific sterilization parameters, making them less reliable for validating modified or specialized cycles increasingly used in advanced manufacturing and research applications.
Regulatory compliance presents an evolving challenge, with standards varying significantly across regions. The harmonization gap between FDA, EU MDR, and ISO standards creates compliance complexities for global operations. Recent regulatory updates have introduced more stringent documentation requirements, necessitating comprehensive validation protocols beyond simple pass/fail testing.
Environmental considerations are emerging as a growing concern, with traditional biological indicators generating substantial plastic waste. Current disposal protocols often require additional decontamination steps, adding complexity and environmental impact to testing procedures.
Emerging technologies like enzyme-based indicators and PCR-based detection systems show promise in addressing some limitations but face adoption barriers including cost, technical expertise requirements, and regulatory acceptance hurdles. The integration of digital monitoring systems with biological indicators represents a promising frontier, though standardization of these hybrid approaches remains in early development.
Current Methodologies for Biological Indicator Testing
01 Biological indicators for sterilization validation
Biological indicators are used to validate sterilization processes by containing resistant microorganisms that are more difficult to kill than typical pathogens. These indicators provide direct evidence of a sterilization process's effectiveness by demonstrating the destruction of these test organisms. They typically consist of bacterial spores on carriers that are exposed to sterilization processes, then cultured to determine if any organisms survived, thus confirming sterilization effectiveness.- Biological indicators for sterilization validation: Biological indicators are used to validate sterilization processes by containing resistant microorganisms that are more difficult to kill than typical pathogens. These indicators provide direct evidence of a sterilization process's effectiveness by demonstrating the destruction of these test organisms. They typically consist of bacterial spores on carriers that are exposed to sterilization processes, then cultured to determine if any organisms survived, thus confirming sterilization effectiveness.
- Rapid readout biological indicator systems: Advanced biological indicator systems provide faster results through rapid readout technologies. These systems use enzyme detection methods, fluorescence detection, or other biochemical markers to indicate sterilization effectiveness in hours rather than days required by traditional growth-based methods. The rapid readout systems maintain accuracy while significantly reducing the time needed to validate sterilization processes, allowing for quicker release of sterilized products and equipment.
- Monitoring systems for biological indicators: Automated monitoring systems have been developed to track and analyze biological indicator results. These systems incorporate sensors, imaging technology, and data processing capabilities to objectively evaluate indicator results, reducing human error in interpretation. The monitoring systems can be integrated with facility management software to provide real-time sterilization validation data, maintain electronic records of sterilization cycles, and generate compliance reports for regulatory purposes.
- Dual and multi-parameter biological indicators: Advanced biological indicators incorporate multiple testing parameters in a single device to provide comprehensive sterilization validation. These indicators may combine traditional spore testing with chemical indicators or include multiple organism types to validate different aspects of the sterilization process simultaneously. By monitoring multiple parameters, these indicators provide more robust validation of sterilization effectiveness across various potential failure modes and process conditions.
- Process-specific biological indicators: Specialized biological indicators have been developed for specific sterilization methods including steam, ethylene oxide, hydrogen peroxide, radiation, and dry heat. These indicators contain microorganisms particularly resistant to the specific sterilization method being validated and are designed to function optimally under those process conditions. The carriers, packaging, and recovery media are all tailored to the particular sterilization process, ensuring accurate assessment of sterilization effectiveness for that specific method.
02 Rapid readout biological indicator systems
Advanced biological indicator systems provide faster results through rapid readout technologies. These systems use enzyme detection methods or fluorescence-based techniques to indicate sterilization effectiveness within hours instead of days required by traditional growth-based methods. The rapid readout systems detect enzymatic activity or metabolic products from test organisms, allowing for quicker release of sterilized materials and improved workflow efficiency in healthcare and manufacturing settings.Expand Specific Solutions03 Monitoring and control systems for biological indicators
Automated monitoring and control systems enhance the reliability of biological indicator testing. These systems incorporate electronic sensors, data logging capabilities, and software algorithms to track sterilization parameters and biological indicator results. The integration of biological indicators with monitoring systems allows for real-time assessment of sterilization processes, automatic documentation of results, and alert mechanisms for process failures, improving overall quality assurance in sterilization procedures.Expand Specific Solutions04 Novel biological indicator compositions and carriers
Innovative biological indicator compositions feature improved spore carriers, protective packaging, and specialized formulations that enhance reliability and consistency. These advancements include new carrier materials that optimize spore recovery, protective barriers that prevent contamination while allowing sterilant penetration, and specialized formulations that improve stability and shelf-life. The novel compositions also include dual-species indicators that provide more comprehensive validation of different sterilization methods.Expand Specific Solutions05 Process-specific biological indicators
Specialized biological indicators are designed for specific sterilization methods such as steam, ethylene oxide, hydrogen peroxide, radiation, or dry heat. These indicators contain microorganisms particularly resistant to the specific sterilization method being validated. The process-specific design ensures accurate assessment of sterilization effectiveness for each unique method, with tailored incubation conditions, growth media, and detection systems optimized for the particular sterilization process and its target microorganisms.Expand Specific Solutions
Key Players in Sterilization Validation Industry
The biological indicator testing market for autoclave effectiveness is in a mature growth phase, with an expanding global market driven by increasing healthcare regulations and infection control standards. Key players include established companies like STERIS Inc., 3M Innovative Properties, and Terragene LLC, who dominate with comprehensive biological indicator portfolios. Emerging competitors such as Verrix LLC and SGM Biotech are introducing innovative technologies to challenge traditional methods. The technology shows high maturity with standardized protocols, though innovation continues in rapid readout systems and digital integration. Regional players like Shinva Medical Instrument Co. and Fedegari Autoclavi SpA are strengthening their positions through specialized autoclave-indicator compatibility solutions.
American Sterilizer Co.
Technical Solution: American Sterilizer Co. (now part of STERIS) has developed comprehensive biological indicator systems for autoclave validation featuring their Attest line of products. Their technology utilizes highly resistant Geobacillus stearothermophilus spores with precisely controlled population counts and D-values specifically calibrated for steam sterilization processes. Their self-contained biological indicators incorporate specialized growth media formulated to optimize recovery of heat-stressed spores, enhancing detection sensitivity. American Sterilizer's system includes both traditional culture-based indicators requiring 24-48 hour incubation and rapid readout technology that provides results in as little as 1-3 hours through detection of enzymatic activity from surviving spores. Their Process Challenge Devices simulate worst-case sterilization scenarios by creating barriers to steam penetration, ensuring that successful sterilization of these devices indicates effective processing of the entire load. The company has also developed integrated documentation systems that automatically record test parameters, results, and operator information, creating comprehensive audit trails for regulatory compliance.
Strengths: Long-established technology with extensive validation history and regulatory acceptance; comprehensive product range addresses different autoclave types and load configurations; integrated systems approach simplifies implementation. Weaknesses: Some products still rely on traditional incubation methods requiring longer time to results; system components from different generations may have varying compatibility; requires specific incubators and readers for optimal performance.
TERRAGENE LLC
Technical Solution: TERRAGENE has pioneered innovative biological indicator systems for autoclave validation featuring their proprietary Super Rapid Readout Technology. Their BIs contain precisely calibrated populations of Geobacillus stearothermophilus spores with D-values specifically engineered to match different sterilization parameters. The company's flagship product, the Mini-Bio system, integrates fluorescence detection technology that identifies the presence of an enzyme produced by surviving spores, providing results in just 20 minutes for steam sterilization processes. TERRAGENE's dual-readout indicators offer both rapid fluorescence results and traditional color-change confirmation, ensuring maximum reliability. Their BIs are designed with specialized recovery media that neutralizes potential inhibitory substances that might interfere with accurate results. TERRAGENE has also developed Process Challenge Devices (PCDs) that simulate worst-case scenarios by placing biological indicators within specially designed barriers that create challenging conditions for sterilant penetration, ensuring that if these indicators are successfully sterilized, the entire load can be considered sterile.
Strengths: Ultra-rapid readout technology (as fast as 20 minutes) significantly improves workflow efficiency; dual-readout technology provides redundant verification; comprehensive validation documentation system meets international regulatory standards. Weaknesses: Specialized readers and incubators required for rapid results increase initial capital investment; system requires careful maintenance and calibration to ensure accuracy.
Core Technical Principles of Biological Indicators
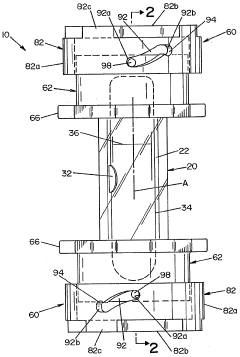
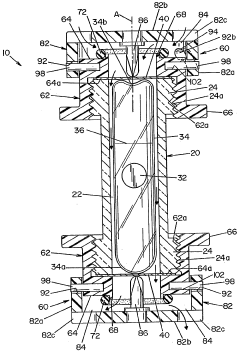
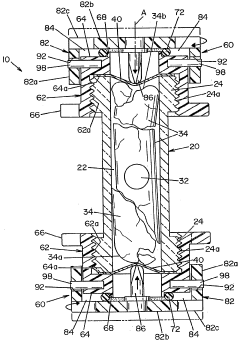
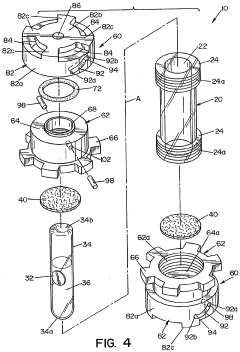
Sefl-contained biological indicator
PatentWO2005009484A2
Innovation
- A self-contained biological indicator with a tubular casing and cap assembly that houses a microorganism-inoculated element and a frangible ampule containing a culture medium, allowing for pressure-activated contact between the microorganisms and the medium, while providing a sealed environment to prevent contamination.
Device for activating a self-contained biological indicator
PatentActiveUS20090311739A1
Innovation
- A device with a first lever arm and a second lever arm that moves to deform the casing, fracturing the ampule and exposing microorganisms to the growth-promoting medium, allowing for easy activation and sealing of the indicator.
Regulatory Standards and Compliance Requirements
Compliance with regulatory standards is a critical aspect of autoclave validation processes across healthcare, pharmaceutical, and laboratory settings. The FDA in the United States mandates adherence to specific guidelines for steam sterilization processes, particularly through 21 CFR Part 11 and 21 CFR Part 820, which establish requirements for electronic records and quality system regulations respectively. These regulations necessitate thorough documentation of biological indicator testing procedures and results.
In Europe, the EN ISO 17665 standard specifically addresses the development, validation, and routine control of moist heat sterilization processes. This standard requires comprehensive validation protocols that include biological indicators as a critical component of performance qualification. Similarly, the European Medical Device Regulation (MDR) imposes stringent requirements on sterilization validation for medical devices, with biological indicators serving as essential tools for demonstrating compliance.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed the ANSI/AAMI ST79 guideline, which provides detailed recommendations for steam sterilization and sterility assurance in healthcare facilities. This guideline specifies the frequency of biological indicator testing, placement techniques, and interpretation of results, serving as a benchmark for best practices in autoclave monitoring.
For laboratory settings, the Clinical and Laboratory Standards Institute (CLSI) offers guidance documents that outline standardized approaches to biological indicator testing. These documents emphasize the importance of using appropriate biological indicators that match the intended sterilization parameters and load configurations.
International standards such as ISO 11138 series specifically address biological indicators for sterilization processes. ISO 11138-1 provides general requirements, while ISO 11138-3 focuses specifically on biological indicators for moist heat sterilization processes. These standards define the performance requirements, production, labeling, and testing methodologies for biological indicators, ensuring consistency and reliability in sterilization validation.
Compliance documentation requirements typically include maintaining records of biological indicator lot numbers, expiration dates, test results, and any corrective actions taken in response to failed tests. Many regulatory frameworks require retention of these records for specified periods, often ranging from two to five years depending on the jurisdiction and application context.
Regular audits by regulatory bodies may include review of biological indicator testing protocols and results, making it essential for organizations to establish robust quality management systems that incorporate biological indicator testing as a key component of their sterilization validation strategy.
In Europe, the EN ISO 17665 standard specifically addresses the development, validation, and routine control of moist heat sterilization processes. This standard requires comprehensive validation protocols that include biological indicators as a critical component of performance qualification. Similarly, the European Medical Device Regulation (MDR) imposes stringent requirements on sterilization validation for medical devices, with biological indicators serving as essential tools for demonstrating compliance.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed the ANSI/AAMI ST79 guideline, which provides detailed recommendations for steam sterilization and sterility assurance in healthcare facilities. This guideline specifies the frequency of biological indicator testing, placement techniques, and interpretation of results, serving as a benchmark for best practices in autoclave monitoring.
For laboratory settings, the Clinical and Laboratory Standards Institute (CLSI) offers guidance documents that outline standardized approaches to biological indicator testing. These documents emphasize the importance of using appropriate biological indicators that match the intended sterilization parameters and load configurations.
International standards such as ISO 11138 series specifically address biological indicators for sterilization processes. ISO 11138-1 provides general requirements, while ISO 11138-3 focuses specifically on biological indicators for moist heat sterilization processes. These standards define the performance requirements, production, labeling, and testing methodologies for biological indicators, ensuring consistency and reliability in sterilization validation.
Compliance documentation requirements typically include maintaining records of biological indicator lot numbers, expiration dates, test results, and any corrective actions taken in response to failed tests. Many regulatory frameworks require retention of these records for specified periods, often ranging from two to five years depending on the jurisdiction and application context.
Regular audits by regulatory bodies may include review of biological indicator testing protocols and results, making it essential for organizations to establish robust quality management systems that incorporate biological indicator testing as a key component of their sterilization validation strategy.
Quality Assurance Protocols for Sterilization Processes
Quality assurance protocols for sterilization processes represent a critical component in healthcare, pharmaceutical, and laboratory settings where sterility is paramount. These protocols establish standardized procedures to validate that sterilization equipment, particularly autoclaves, consistently achieves complete microbial inactivation. The foundation of these protocols rests on systematic testing methodologies that provide objective evidence of sterilization efficacy.
Biological indicators (BIs) serve as the gold standard for autoclave validation, containing highly resistant bacterial spores, typically Geobacillus stearothermophilus, which are more difficult to kill than common pathogens. When properly implemented, quality assurance protocols specify precise placement of these indicators within the most challenging locations of the autoclave chamber, ensuring that if sterilization conditions reach these areas, the entire load can be considered sterile.
Comprehensive protocols dictate specific testing frequencies based on risk assessment, ranging from daily validation in critical healthcare settings to weekly or monthly testing in research laboratories. Documentation requirements form another essential element, mandating detailed records of each test cycle, including date, time, temperature, pressure parameters, and BI results, creating an auditable trail of sterilization efficacy.
The protocols also establish clear acceptance criteria and response procedures. A passing result requires complete inactivation of all biological indicators, while any positive growth necessitates immediate corrective actions, including equipment inspection, recalibration, and reprocessing of all potentially affected items. This systematic approach ensures patient safety and experimental integrity.
Modern quality assurance frameworks increasingly incorporate parametric release concepts, where sterilization cycles can be validated through critical physical parameters alongside biological testing. This dual approach provides redundant verification systems that enhance reliability while potentially streamlining operational workflows.
International standards, including those from organizations like the Association for the Advancement of Medical Instrumentation (AAMI) and the International Organization for Standardization (ISO), inform these protocols, ensuring global consistency in sterilization validation practices. These standards continue to evolve as new technologies and methodologies emerge, requiring facilities to regularly update their quality assurance protocols to maintain compliance and effectiveness.
Implementation challenges often include resource constraints, training requirements, and the need for specialized expertise in microbiology and sterilization science. Successful quality assurance programs address these challenges through comprehensive staff education, clear procedural documentation, and dedicated resources for testing and validation activities.
Biological indicators (BIs) serve as the gold standard for autoclave validation, containing highly resistant bacterial spores, typically Geobacillus stearothermophilus, which are more difficult to kill than common pathogens. When properly implemented, quality assurance protocols specify precise placement of these indicators within the most challenging locations of the autoclave chamber, ensuring that if sterilization conditions reach these areas, the entire load can be considered sterile.
Comprehensive protocols dictate specific testing frequencies based on risk assessment, ranging from daily validation in critical healthcare settings to weekly or monthly testing in research laboratories. Documentation requirements form another essential element, mandating detailed records of each test cycle, including date, time, temperature, pressure parameters, and BI results, creating an auditable trail of sterilization efficacy.
The protocols also establish clear acceptance criteria and response procedures. A passing result requires complete inactivation of all biological indicators, while any positive growth necessitates immediate corrective actions, including equipment inspection, recalibration, and reprocessing of all potentially affected items. This systematic approach ensures patient safety and experimental integrity.
Modern quality assurance frameworks increasingly incorporate parametric release concepts, where sterilization cycles can be validated through critical physical parameters alongside biological testing. This dual approach provides redundant verification systems that enhance reliability while potentially streamlining operational workflows.
International standards, including those from organizations like the Association for the Advancement of Medical Instrumentation (AAMI) and the International Organization for Standardization (ISO), inform these protocols, ensuring global consistency in sterilization validation practices. These standards continue to evolve as new technologies and methodologies emerge, requiring facilities to regularly update their quality assurance protocols to maintain compliance and effectiveness.
Implementation challenges often include resource constraints, training requirements, and the need for specialized expertise in microbiology and sterilization science. Successful quality assurance programs address these challenges through comprehensive staff education, clear procedural documentation, and dedicated resources for testing and validation activities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!