Autoclave Hygiene Maintenance: Achieving Compliance
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Technology Background and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the 19th century when Charles Chamberland developed the first pressure steam sterilizer. This technology leverages the principle of using pressurized steam at elevated temperatures (typically 121-134°C) to eliminate microorganisms, including resistant bacterial spores, through protein denaturation and coagulation. The historical progression of autoclave technology reflects advancements in materials science, control systems, and understanding of microbial resistance mechanisms.
The contemporary autoclave landscape encompasses diverse designs tailored to specific applications, ranging from small tabletop units for dental practices to large industrial systems for pharmaceutical manufacturing. Recent technological innovations have focused on enhancing energy efficiency, reducing cycle times, improving validation processes, and integrating advanced monitoring systems to ensure sterilization efficacy while maintaining compliance with increasingly stringent regulatory standards.
Current market trends indicate a growing emphasis on sustainability in autoclave operations, with manufacturers developing systems that minimize water consumption, reduce energy usage, and incorporate eco-friendly materials. Additionally, the integration of IoT capabilities and smart monitoring systems represents a significant advancement, enabling real-time process verification, predictive maintenance, and comprehensive data logging for regulatory compliance.
The primary objective of modern autoclave hygiene maintenance is to achieve consistent, verifiable sterilization results while maintaining compliance with applicable standards such as ISO 17665, EN 285, and industry-specific regulations. This necessitates a multifaceted approach encompassing proper equipment design, validated operational protocols, regular maintenance schedules, and comprehensive documentation systems.
Technical challenges in this domain include biofilm formation in water systems, mineral scaling in steam generators, gasket degradation affecting chamber sealing, and ensuring uniform steam penetration throughout complex loads. These challenges are compounded by the increasing complexity of medical devices and materials requiring sterilization, many of which contain heat-sensitive components or intricate geometries that impede steam access.
Looking forward, the trajectory of autoclave technology development aims to address these challenges through innovations in chamber design, steam generation systems, load configuration optimization, and advanced cycle parameters. The ultimate goal is to establish sterilization processes that consistently achieve the required Sterility Assurance Level (SAL) of 10^-6 (one-in-a-million probability of a non-sterile unit) while minimizing resource consumption, reducing cycle times, and accommodating increasingly complex medical devices and materials.
The contemporary autoclave landscape encompasses diverse designs tailored to specific applications, ranging from small tabletop units for dental practices to large industrial systems for pharmaceutical manufacturing. Recent technological innovations have focused on enhancing energy efficiency, reducing cycle times, improving validation processes, and integrating advanced monitoring systems to ensure sterilization efficacy while maintaining compliance with increasingly stringent regulatory standards.
Current market trends indicate a growing emphasis on sustainability in autoclave operations, with manufacturers developing systems that minimize water consumption, reduce energy usage, and incorporate eco-friendly materials. Additionally, the integration of IoT capabilities and smart monitoring systems represents a significant advancement, enabling real-time process verification, predictive maintenance, and comprehensive data logging for regulatory compliance.
The primary objective of modern autoclave hygiene maintenance is to achieve consistent, verifiable sterilization results while maintaining compliance with applicable standards such as ISO 17665, EN 285, and industry-specific regulations. This necessitates a multifaceted approach encompassing proper equipment design, validated operational protocols, regular maintenance schedules, and comprehensive documentation systems.
Technical challenges in this domain include biofilm formation in water systems, mineral scaling in steam generators, gasket degradation affecting chamber sealing, and ensuring uniform steam penetration throughout complex loads. These challenges are compounded by the increasing complexity of medical devices and materials requiring sterilization, many of which contain heat-sensitive components or intricate geometries that impede steam access.
Looking forward, the trajectory of autoclave technology development aims to address these challenges through innovations in chamber design, steam generation systems, load configuration optimization, and advanced cycle parameters. The ultimate goal is to establish sterilization processes that consistently achieve the required Sterility Assurance Level (SAL) of 10^-6 (one-in-a-million probability of a non-sterile unit) while minimizing resource consumption, reducing cycle times, and accommodating increasingly complex medical devices and materials.
Market Demand Analysis for Compliant Autoclave Systems
The global autoclave market is experiencing significant growth, driven primarily by increasing regulatory requirements for sterilization in healthcare facilities, laboratories, and pharmaceutical manufacturing. Market research indicates the global medical autoclave market was valued at approximately $1.9 billion in 2022 and is projected to reach $2.8 billion by 2030, growing at a CAGR of around 5.7% during the forecast period. This growth trajectory underscores the expanding demand for compliant autoclave systems across various sectors.
Healthcare facilities represent the largest market segment, accounting for over 40% of the total market share. The COVID-19 pandemic has further accelerated this demand, as healthcare providers worldwide have heightened their focus on infection control protocols. Regulatory bodies such as the FDA, CDC, and international counterparts have strengthened compliance requirements, creating a robust market for advanced autoclave systems that can meet these stringent standards.
The pharmaceutical and biotechnology sectors are emerging as rapidly growing segments within the autoclave market. With increasing R&D activities and the expansion of biopharmaceutical manufacturing, these industries require sophisticated autoclave systems that can maintain sterility assurance levels (SAL) of 10^-6 or better. This specialized requirement is driving innovation in autoclave technology, particularly in validation and monitoring systems.
Regional analysis reveals that North America currently dominates the market with approximately 35% share, followed closely by Europe at 30%. However, the Asia-Pacific region is expected to witness the fastest growth rate, with China and India leading the expansion due to their rapidly developing healthcare infrastructure and increasing adoption of international sterilization standards.
Customer demand patterns indicate a clear shift toward autoclave systems with integrated compliance features. End-users are increasingly seeking systems with automated documentation, real-time monitoring capabilities, and validation protocols that align with regulatory frameworks such as ISO 17665, EN 285, and AAMI ST79. This trend is particularly pronounced in hospitals and pharmaceutical manufacturing facilities where non-compliance can result in significant operational disruptions and financial penalties.
Market surveys reveal that approximately 78% of healthcare facility managers consider compliance features as "very important" or "critical" when purchasing new autoclave systems. The willingness to pay premium prices for systems with advanced compliance capabilities has increased by 23% over the past five years, indicating a value-driven market transformation rather than purely cost-driven purchasing decisions.
Healthcare facilities represent the largest market segment, accounting for over 40% of the total market share. The COVID-19 pandemic has further accelerated this demand, as healthcare providers worldwide have heightened their focus on infection control protocols. Regulatory bodies such as the FDA, CDC, and international counterparts have strengthened compliance requirements, creating a robust market for advanced autoclave systems that can meet these stringent standards.
The pharmaceutical and biotechnology sectors are emerging as rapidly growing segments within the autoclave market. With increasing R&D activities and the expansion of biopharmaceutical manufacturing, these industries require sophisticated autoclave systems that can maintain sterility assurance levels (SAL) of 10^-6 or better. This specialized requirement is driving innovation in autoclave technology, particularly in validation and monitoring systems.
Regional analysis reveals that North America currently dominates the market with approximately 35% share, followed closely by Europe at 30%. However, the Asia-Pacific region is expected to witness the fastest growth rate, with China and India leading the expansion due to their rapidly developing healthcare infrastructure and increasing adoption of international sterilization standards.
Customer demand patterns indicate a clear shift toward autoclave systems with integrated compliance features. End-users are increasingly seeking systems with automated documentation, real-time monitoring capabilities, and validation protocols that align with regulatory frameworks such as ISO 17665, EN 285, and AAMI ST79. This trend is particularly pronounced in hospitals and pharmaceutical manufacturing facilities where non-compliance can result in significant operational disruptions and financial penalties.
Market surveys reveal that approximately 78% of healthcare facility managers consider compliance features as "very important" or "critical" when purchasing new autoclave systems. The willingness to pay premium prices for systems with advanced compliance capabilities has increased by 23% over the past five years, indicating a value-driven market transformation rather than purely cost-driven purchasing decisions.
Current Challenges in Autoclave Hygiene Maintenance
The autoclave hygiene maintenance landscape faces significant challenges that impede compliance with increasingly stringent regulatory standards. Healthcare facilities, laboratories, and industrial settings struggle with biofilm formation in autoclave chambers and piping systems, which proves resistant to standard cleaning protocols. These biofilms harbor microorganisms that can compromise sterilization efficacy and potentially contaminate processed items, presenting a critical risk to patient safety and product integrity.
Water quality issues represent another persistent challenge, as mineral deposits and scale buildup from hard water reduce autoclave efficiency and accelerate component deterioration. These deposits create protective environments for microorganisms and can obstruct steam penetration during sterilization cycles, leading to incomplete sterilization despite cycle completion indicators suggesting otherwise.
Documentation and validation processes remain problematic across many facilities. Manual record-keeping systems are prone to human error, inconsistency, and data gaps that complicate compliance verification during audits. The transition to digital monitoring systems, while beneficial, introduces challenges related to data security, system integration, and staff training requirements that many organizations are ill-equipped to address.
Maintenance scheduling presents operational difficulties as facilities balance the competing demands of equipment availability and thorough preventive maintenance. Many institutions operate with limited redundancy in sterilization capacity, creating pressure to minimize downtime that often results in abbreviated maintenance procedures or extended intervals between comprehensive servicing.
Staff training deficiencies compound these technical challenges. High turnover rates in technical positions and inadequate continuing education programs leave many facilities with personnel who lack comprehensive understanding of autoclave operation principles, maintenance requirements, and compliance standards. This knowledge gap manifests in procedural shortcuts and improper documentation that undermine compliance efforts.
Resource constraints further exacerbate these issues, particularly in smaller healthcare facilities and research laboratories. Limited budgets for equipment upgrades, maintenance contracts, and specialized cleaning agents force compromises that impact hygiene standards. Many facilities operate with aging autoclave equipment that lacks modern self-diagnostic capabilities and automated cleaning systems, making compliance more labor-intensive and less reliable.
Regulatory complexity adds another layer of difficulty, as facilities must navigate overlapping and sometimes contradictory standards from multiple oversight bodies. The interpretation of these requirements varies across institutions, creating inconsistent implementation practices and compliance approaches that complicate industry-wide standardization efforts.
Water quality issues represent another persistent challenge, as mineral deposits and scale buildup from hard water reduce autoclave efficiency and accelerate component deterioration. These deposits create protective environments for microorganisms and can obstruct steam penetration during sterilization cycles, leading to incomplete sterilization despite cycle completion indicators suggesting otherwise.
Documentation and validation processes remain problematic across many facilities. Manual record-keeping systems are prone to human error, inconsistency, and data gaps that complicate compliance verification during audits. The transition to digital monitoring systems, while beneficial, introduces challenges related to data security, system integration, and staff training requirements that many organizations are ill-equipped to address.
Maintenance scheduling presents operational difficulties as facilities balance the competing demands of equipment availability and thorough preventive maintenance. Many institutions operate with limited redundancy in sterilization capacity, creating pressure to minimize downtime that often results in abbreviated maintenance procedures or extended intervals between comprehensive servicing.
Staff training deficiencies compound these technical challenges. High turnover rates in technical positions and inadequate continuing education programs leave many facilities with personnel who lack comprehensive understanding of autoclave operation principles, maintenance requirements, and compliance standards. This knowledge gap manifests in procedural shortcuts and improper documentation that undermine compliance efforts.
Resource constraints further exacerbate these issues, particularly in smaller healthcare facilities and research laboratories. Limited budgets for equipment upgrades, maintenance contracts, and specialized cleaning agents force compromises that impact hygiene standards. Many facilities operate with aging autoclave equipment that lacks modern self-diagnostic capabilities and automated cleaning systems, making compliance more labor-intensive and less reliable.
Regulatory complexity adds another layer of difficulty, as facilities must navigate overlapping and sometimes contradictory standards from multiple oversight bodies. The interpretation of these requirements varies across institutions, creating inconsistent implementation practices and compliance approaches that complicate industry-wide standardization efforts.
Current Hygiene Maintenance Protocols and Solutions
01 Automated cleaning and sterilization systems
Automated systems for cleaning and sterilizing autoclaves help maintain hygiene standards by reducing human error and ensuring consistent results. These systems can include programmable cleaning cycles, automated detergent dispensing, and monitoring capabilities that track cleaning parameters. The automation helps in maintaining proper hygiene by ensuring all surfaces are adequately cleaned and sterilized according to predetermined protocols.- Automated cleaning and sterilization systems: Automated systems for cleaning and sterilizing autoclaves help maintain hygiene standards by reducing human error and ensuring consistent results. These systems can include programmable cleaning cycles, automated detergent dispensing, and monitoring capabilities that track cleaning parameters. The automation helps in maintaining proper hygiene by ensuring all surfaces are adequately cleaned and sterilized according to predetermined protocols, which is essential for preventing contamination and ensuring equipment longevity.
- Chemical cleaning agents and solutions: Specialized chemical cleaning agents and solutions are formulated specifically for autoclave maintenance. These include descaling agents to remove mineral deposits, enzymatic cleaners to break down organic matter, and antimicrobial solutions to prevent biofilm formation. The proper selection and application of these chemicals is crucial for maintaining autoclave hygiene, as they help remove stubborn residues and contaminants that could affect sterilization efficacy and equipment performance.
- Monitoring and validation systems: Monitoring and validation systems are essential for ensuring autoclave hygiene maintenance meets required standards. These systems include sensors and indicators that track parameters such as temperature, pressure, and cycle completion. Advanced systems may incorporate digital logging, real-time monitoring, and alert mechanisms for deviations from set parameters. Regular validation using biological and chemical indicators helps verify that sterilization processes are effective and that the autoclave remains in a hygienic condition.
- Physical cleaning tools and accessories: Specialized physical cleaning tools and accessories are designed for effective autoclave maintenance. These include brushes with specific shapes to reach difficult areas, non-abrasive scrubbers that won't damage sensitive components, and steam cleaning attachments. Proper tools ensure thorough cleaning of chambers, pipes, filters, and gaskets without causing damage to the equipment. Regular use of these tools helps prevent buildup of debris and contaminants that could compromise sterilization effectiveness.
- Preventive maintenance protocols: Comprehensive preventive maintenance protocols are essential for maintaining autoclave hygiene. These protocols include scheduled inspections, regular cleaning routines, component replacement schedules, and documentation procedures. Preventive maintenance helps identify potential issues before they affect hygiene standards or equipment performance. Well-established protocols ensure consistent cleaning practices, proper waste disposal, and regular verification of sterilization effectiveness, all contributing to maintaining optimal autoclave hygiene conditions.
02 Chemical cleaning agents and solutions
Specialized chemical cleaning agents and solutions are formulated specifically for autoclave maintenance. These include descaling agents to remove mineral deposits, enzymatic cleaners to break down organic matter, and antimicrobial solutions that prevent biofilm formation. The proper selection and application of these chemicals ensure effective removal of contaminants and prevent corrosion of autoclave components, extending the equipment's lifespan while maintaining hygiene standards.Expand Specific Solutions03 Monitoring and validation systems
Monitoring and validation systems are essential for ensuring autoclave hygiene maintenance. These systems include sensors and indicators that track parameters such as temperature, pressure, and cycle duration. They can also include biological and chemical indicators to verify sterilization effectiveness. Real-time monitoring allows for immediate detection of deviations from established parameters, ensuring that hygiene standards are consistently met and documented for compliance purposes.Expand Specific Solutions04 Structural design improvements for hygiene
Innovative structural designs in autoclaves facilitate better hygiene maintenance. These include smooth, crevice-free interior surfaces that prevent biofilm formation, removable components for thorough cleaning, and optimized water circulation pathways. Some designs incorporate self-cleaning features or materials resistant to microbial adhesion. These structural improvements make routine cleaning more effective and help prevent contamination buildup in hard-to-reach areas.Expand Specific Solutions05 Maintenance protocols and schedules
Comprehensive maintenance protocols and schedules are crucial for autoclave hygiene. These include daily, weekly, and monthly maintenance tasks such as chamber cleaning, gasket inspection, filter replacement, and water quality testing. Standardized protocols ensure consistent cleaning practices and help identify potential issues before they compromise sterilization effectiveness. Regular maintenance not only preserves hygiene standards but also extends equipment lifespan and reduces operational downtime.Expand Specific Solutions
Leading Manufacturers and Service Providers Analysis
The autoclave hygiene maintenance market is in a mature growth phase, characterized by established protocols and technologies, with a global market size estimated at $2.5 billion and growing steadily at 5-7% annually. The competitive landscape features diversified players across medical, pharmaceutical, and industrial sectors. Leading companies like Olympus Corp. and Shinva Medical Instrument dominate with comprehensive sterilization solutions, while specialized firms such as Veltek Associates focus on contamination control for pharmaceutical applications. The technology maturity varies by application, with medical autoclave technology being highly advanced through innovations from Eschmann Holdings and Q Med Innovations in tracking systems, while industrial applications continue to evolve with companies like Boeing and YKK Corp. implementing customized solutions for specialized manufacturing environments.
Olympus Corp.
Technical Solution: Olympus has developed an advanced autoclave hygiene maintenance system that integrates real-time monitoring technology with automated cleaning processes. Their solution employs proprietary EndoALPHA system which connects sterilization equipment to a centralized management platform, allowing for continuous monitoring of critical parameters including temperature, pressure, and humidity during the sterilization cycle. The system incorporates RFID tracking for instruments, ensuring complete traceability throughout the decontamination process. Olympus's compliance solution features automated documentation that generates comprehensive reports on sterilization cycles, maintenance activities, and validation tests, which are securely stored in a cloud-based platform accessible for regulatory inspections. Their technology also includes predictive maintenance algorithms that analyze performance patterns to identify potential issues before they lead to compliance failures[1][3].
Strengths: Comprehensive integration with existing hospital systems, excellent traceability through RFID technology, and strong data management capabilities for regulatory compliance. Weaknesses: Higher implementation costs compared to standalone solutions, requires significant staff training, and may have compatibility issues with non-Olympus equipment.
Veltek Associates, Inc.
Technical Solution: Veltek Associates has developed the SMA (Sterilization Monitoring and Assurance) system specifically designed for autoclave hygiene compliance in pharmaceutical and healthcare settings. Their technology integrates physical, chemical, and biological monitoring into a unified platform that ensures comprehensive validation of sterilization processes. The SMA system employs distributed sensor networks throughout the autoclave chamber to detect temperature variations and steam penetration inconsistencies that might compromise sterilization efficacy. Their proprietary VHP (Vaporized Hydrogen Peroxide) integration allows for enhanced sterilization in low-temperature applications while maintaining material compatibility. Veltek's compliance solution includes Core Monitoring Technology that continuously tracks critical parameters against predefined acceptance criteria and automatically flags deviations for immediate intervention. The system features SimpliAmp rapid biological indicators that provide results in hours rather than days, allowing for faster release of sterilized materials while maintaining compliance with FDA and EU GMP requirements[5][7]. Their data management platform, ComplianceTracker, automatically generates audit-ready reports and maintains electronic records with 21 CFR Part 11 compliant electronic signatures.
Strengths: Exceptional contamination control expertise, comprehensive validation documentation capabilities, and specialized solutions for aseptic processing environments. Weaknesses: Higher initial investment compared to basic systems, requires specialized technical knowledge for optimal configuration, and more complex validation processes.
Key Innovations in Autoclave Cleaning Technologies
Process challenge device for a high-pressure steam sterilizer and sheet for a challenge device
PatentInactiveUS20070264683A1
Innovation
- A process challenge device comprising steam permeable bodies and a holder, where the steam permeable bodies form a cavity with an indicator that changes appearance upon exposure to a predetermined temperature, allowing for reusable testing without damaging the components and enabling user-selectable indicators.
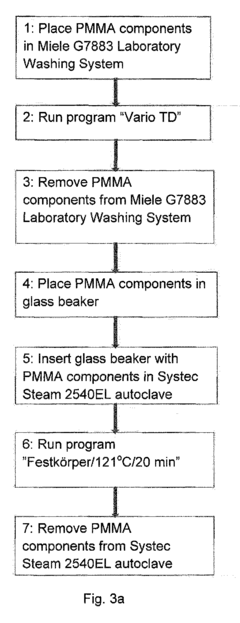
Sterilisation method of dental components comprising polymethyl methacrylate
PatentInactiveEP2698171A1
Innovation
- Steam sterilization of thermoset PMMA dental components at temperatures between 121°C and 134°C for up to 30 minutes, which surprisingly does not degrade the material and maintains its mechanical and biocompatibility properties.
Regulatory Compliance Framework for Medical Sterilization
The regulatory landscape for medical sterilization is complex and multifaceted, encompassing international standards, national regulations, and industry-specific guidelines. For autoclave hygiene maintenance, compliance begins with understanding the hierarchical structure of these regulations. At the international level, ISO 17665 establishes the requirements for the development, validation, and routine control of moist heat sterilization processes for medical devices. This standard serves as the foundation for many national regulatory frameworks.
In the United States, the Food and Drug Administration (FDA) enforces regulations through 21 CFR Part 820, which outlines quality system requirements for medical device manufacturers, including sterilization validation. The Centers for Disease Control and Prevention (CDC) provides additional guidelines through their "Guideline for Disinfection and Sterilization in Healthcare Facilities." Similarly, the European Union's Medical Device Regulation (MDR) and the European standard EN 285 specifically address large steam sterilizers used in healthcare.
Compliance requirements typically include documentation of sterilization cycles, regular calibration of autoclave equipment, validation protocols, and routine testing procedures. The Association for the Advancement of Medical Instrumentation (AAMI) has developed comprehensive standards such as AAMI ST79, which provides detailed guidance on steam sterilization and sterility assurance in healthcare facilities.
Risk-based approaches have become increasingly important in regulatory frameworks. This involves identifying critical control points in the sterilization process, implementing appropriate monitoring systems, and establishing corrective action protocols. Regulatory bodies now emphasize the importance of process validation over simple end-product testing, requiring facilities to demonstrate that their sterilization processes consistently produce sterile products.
Record-keeping requirements form a significant component of compliance frameworks. Facilities must maintain detailed logs of sterilization cycles, including parameters such as temperature, pressure, and exposure time. These records serve as evidence of compliance during regulatory inspections and audits. Many jurisdictions require retention of these records for specified periods, often ranging from two to ten years depending on the application and regulatory jurisdiction.
Training and competency assessment of personnel involved in sterilization processes is another critical aspect of regulatory compliance. Staff must be adequately trained in autoclave operation, maintenance procedures, and quality control protocols. Documentation of this training is typically required as part of the overall compliance framework.
In the United States, the Food and Drug Administration (FDA) enforces regulations through 21 CFR Part 820, which outlines quality system requirements for medical device manufacturers, including sterilization validation. The Centers for Disease Control and Prevention (CDC) provides additional guidelines through their "Guideline for Disinfection and Sterilization in Healthcare Facilities." Similarly, the European Union's Medical Device Regulation (MDR) and the European standard EN 285 specifically address large steam sterilizers used in healthcare.
Compliance requirements typically include documentation of sterilization cycles, regular calibration of autoclave equipment, validation protocols, and routine testing procedures. The Association for the Advancement of Medical Instrumentation (AAMI) has developed comprehensive standards such as AAMI ST79, which provides detailed guidance on steam sterilization and sterility assurance in healthcare facilities.
Risk-based approaches have become increasingly important in regulatory frameworks. This involves identifying critical control points in the sterilization process, implementing appropriate monitoring systems, and establishing corrective action protocols. Regulatory bodies now emphasize the importance of process validation over simple end-product testing, requiring facilities to demonstrate that their sterilization processes consistently produce sterile products.
Record-keeping requirements form a significant component of compliance frameworks. Facilities must maintain detailed logs of sterilization cycles, including parameters such as temperature, pressure, and exposure time. These records serve as evidence of compliance during regulatory inspections and audits. Many jurisdictions require retention of these records for specified periods, often ranging from two to ten years depending on the application and regulatory jurisdiction.
Training and competency assessment of personnel involved in sterilization processes is another critical aspect of regulatory compliance. Staff must be adequately trained in autoclave operation, maintenance procedures, and quality control protocols. Documentation of this training is typically required as part of the overall compliance framework.
Risk Management Strategies for Autoclave Operations
Effective risk management is essential for maintaining safe and compliant autoclave operations in healthcare and laboratory settings. Organizations must implement comprehensive risk assessment protocols that identify potential hazards throughout the autoclave lifecycle, from installation to decommissioning. These assessments should evaluate mechanical failures, operator errors, and contamination risks that could compromise sterilization efficacy or workplace safety.
A hierarchical approach to risk control measures follows the established safety hierarchy: elimination, substitution, engineering controls, administrative controls, and personal protective equipment. For autoclaves specifically, engineering controls might include pressure relief valves and automatic shut-off mechanisms, while administrative controls encompass standard operating procedures and regular maintenance schedules.
Preventive maintenance represents a cornerstone of risk management strategy. Organizations should develop structured maintenance programs with clearly defined responsibilities, documentation requirements, and verification procedures. These programs must address critical components such as door seals, pressure vessels, and control systems that directly impact sterilization performance and safety.
Contingency planning for autoclave failures is equally important. Facilities should establish backup sterilization options, emergency shutdown procedures, and clear communication protocols. This planning ensures minimal disruption to operations while maintaining compliance with regulatory standards during equipment malfunctions.
Staff training constitutes another vital risk management component. Comprehensive training programs should cover normal operations, troubleshooting, emergency procedures, and compliance requirements. Regular competency assessments help ensure operators maintain necessary skills and knowledge to safely operate autoclaves and respond appropriately to abnormal conditions.
Documentation and record-keeping systems provide the evidence base for risk management effectiveness. These systems should track maintenance activities, validation results, operator certifications, and incident reports. Modern facilities increasingly implement digital tracking systems that enable real-time monitoring and automated alerts for maintenance requirements or compliance issues.
Regular risk management reviews complete the cycle of continuous improvement. Organizations should establish formal processes to evaluate incident trends, near-misses, and technological advancements. These reviews inform updates to risk assessments and control measures, ensuring risk management strategies evolve alongside changing operational conditions and regulatory requirements.
A hierarchical approach to risk control measures follows the established safety hierarchy: elimination, substitution, engineering controls, administrative controls, and personal protective equipment. For autoclaves specifically, engineering controls might include pressure relief valves and automatic shut-off mechanisms, while administrative controls encompass standard operating procedures and regular maintenance schedules.
Preventive maintenance represents a cornerstone of risk management strategy. Organizations should develop structured maintenance programs with clearly defined responsibilities, documentation requirements, and verification procedures. These programs must address critical components such as door seals, pressure vessels, and control systems that directly impact sterilization performance and safety.
Contingency planning for autoclave failures is equally important. Facilities should establish backup sterilization options, emergency shutdown procedures, and clear communication protocols. This planning ensures minimal disruption to operations while maintaining compliance with regulatory standards during equipment malfunctions.
Staff training constitutes another vital risk management component. Comprehensive training programs should cover normal operations, troubleshooting, emergency procedures, and compliance requirements. Regular competency assessments help ensure operators maintain necessary skills and knowledge to safely operate autoclaves and respond appropriately to abnormal conditions.
Documentation and record-keeping systems provide the evidence base for risk management effectiveness. These systems should track maintenance activities, validation results, operator certifications, and incident reports. Modern facilities increasingly implement digital tracking systems that enable real-time monitoring and automated alerts for maintenance requirements or compliance issues.
Regular risk management reviews complete the cycle of continuous improvement. Organizations should establish formal processes to evaluate incident trends, near-misses, and technological advancements. These reviews inform updates to risk assessments and control measures, ensuring risk management strategies evolve alongside changing operational conditions and regulatory requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!