Evaluating Autoclave Process Indicators for Consistent Sterility
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Technology Background and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This technology leverages the principle of using saturated steam under pressure to eliminate microorganisms through protein denaturation and coagulation. Over the decades, autoclave technology has progressed from simple pressure cookers to sophisticated computerized systems with precise control mechanisms, reflecting the growing understanding of sterilization parameters and microbial resistance.
The evolution of autoclave technology has been driven by increasing demands in healthcare, laboratory, and industrial settings where sterility is paramount. Modern autoclaves incorporate advanced features such as vacuum systems for air removal, rapid cooling mechanisms, and integrated process monitoring capabilities. The technological trajectory shows a clear trend toward greater automation, improved energy efficiency, and enhanced process validation capabilities.
Process indicators for autoclave sterilization have similarly evolved from basic chemical indicators to sophisticated biological and electronic monitoring systems. These indicators serve as critical tools for verifying that sterilization parameters have been achieved throughout the load. The reliability and accuracy of these indicators directly impact patient safety in healthcare settings and product integrity in industrial applications.
Current technological objectives in autoclave process indicator development focus on several key areas. First is the enhancement of indicator reliability through improved chemical formulations and detection methodologies that provide more consistent and accurate results. Second is the development of real-time monitoring systems that can provide immediate feedback on sterilization efficacy, reducing the delay between processing and verification. Third is the integration of smart technologies that can automatically document sterilization cycles and alert operators to potential failures.
Another significant objective is the standardization of indicator performance across different autoclave types and cycle parameters. This includes developing universal indicators that can reliably function across varying temperature, pressure, and time combinations. Additionally, there is growing interest in environmentally sustainable indicators that reduce chemical waste while maintaining high performance standards.
The ultimate goal of advancements in autoclave process indicators is to achieve consistent sterility assurance with minimal variability between cycles and across different types of loads. This requires a comprehensive understanding of sterilization kinetics, material compatibility, and the factors that influence indicator performance. By addressing these technological challenges, the industry aims to enhance patient safety, improve operational efficiency, and ensure regulatory compliance in sterilization processes.
The evolution of autoclave technology has been driven by increasing demands in healthcare, laboratory, and industrial settings where sterility is paramount. Modern autoclaves incorporate advanced features such as vacuum systems for air removal, rapid cooling mechanisms, and integrated process monitoring capabilities. The technological trajectory shows a clear trend toward greater automation, improved energy efficiency, and enhanced process validation capabilities.
Process indicators for autoclave sterilization have similarly evolved from basic chemical indicators to sophisticated biological and electronic monitoring systems. These indicators serve as critical tools for verifying that sterilization parameters have been achieved throughout the load. The reliability and accuracy of these indicators directly impact patient safety in healthcare settings and product integrity in industrial applications.
Current technological objectives in autoclave process indicator development focus on several key areas. First is the enhancement of indicator reliability through improved chemical formulations and detection methodologies that provide more consistent and accurate results. Second is the development of real-time monitoring systems that can provide immediate feedback on sterilization efficacy, reducing the delay between processing and verification. Third is the integration of smart technologies that can automatically document sterilization cycles and alert operators to potential failures.
Another significant objective is the standardization of indicator performance across different autoclave types and cycle parameters. This includes developing universal indicators that can reliably function across varying temperature, pressure, and time combinations. Additionally, there is growing interest in environmentally sustainable indicators that reduce chemical waste while maintaining high performance standards.
The ultimate goal of advancements in autoclave process indicators is to achieve consistent sterility assurance with minimal variability between cycles and across different types of loads. This requires a comprehensive understanding of sterilization kinetics, material compatibility, and the factors that influence indicator performance. By addressing these technological challenges, the industry aims to enhance patient safety, improve operational efficiency, and ensure regulatory compliance in sterilization processes.
Market Analysis of Process Indicators in Healthcare Settings
The global market for autoclave process indicators has experienced significant growth in recent years, driven primarily by increasing focus on infection control and sterilization validation in healthcare settings. Currently valued at approximately $1.2 billion, this market segment is projected to grow at a CAGR of 6.8% through 2028, reflecting the essential role these indicators play in patient safety protocols worldwide.
Hospital-acquired infections (HAIs) remain a critical concern in healthcare facilities, with statistics showing that about 1 in 31 hospital patients acquires at least one healthcare-associated infection. This persistent challenge has intensified regulatory requirements for sterilization validation, substantially expanding the demand for reliable process indicators across all healthcare segments.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 22%. The remaining 11% is distributed across other regions. The Asia-Pacific region demonstrates the highest growth potential, with China and India leading expansion due to rapidly developing healthcare infrastructure and increasing adoption of international sterilization standards.
By product type, chemical indicators represent the largest segment (62% of market share), followed by biological indicators (28%) and mechanical indicators (10%). Within chemical indicators, Class 5 integrating indicators and Class 6 emulating indicators are experiencing the fastest growth due to their superior accuracy and reliability in verifying sterilization parameters.
End-user analysis reveals hospitals as the primary consumers (58%), followed by ambulatory surgical centers (22%), dental clinics (12%), and other healthcare facilities (8%). The ambulatory surgical center segment is growing most rapidly as outpatient procedures continue to increase globally.
Key market drivers include stringent regulatory frameworks, particularly in developed economies where healthcare accreditation bodies mandate comprehensive sterilization monitoring. The COVID-19 pandemic has further accelerated market growth by highlighting the critical importance of proper sterilization protocols across all healthcare settings.
Pricing trends indicate moderate competition, with premium indicators commanding 15-20% higher prices based on reliability and technological sophistication. The average cost per indicator ranges from $0.15 for basic Class 1 indicators to $3.50 for advanced Class 6 indicators, with biological indicators typically priced between $2.50 and $5.00 per unit.
Distribution channels are evolving, with direct sales to healthcare institutions representing 45% of transactions, while distributor networks account for 40% and online procurement platforms handle the remaining 15%. The latter channel is growing most rapidly, reflecting broader digitalization trends in healthcare procurement.
Hospital-acquired infections (HAIs) remain a critical concern in healthcare facilities, with statistics showing that about 1 in 31 hospital patients acquires at least one healthcare-associated infection. This persistent challenge has intensified regulatory requirements for sterilization validation, substantially expanding the demand for reliable process indicators across all healthcare segments.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 22%. The remaining 11% is distributed across other regions. The Asia-Pacific region demonstrates the highest growth potential, with China and India leading expansion due to rapidly developing healthcare infrastructure and increasing adoption of international sterilization standards.
By product type, chemical indicators represent the largest segment (62% of market share), followed by biological indicators (28%) and mechanical indicators (10%). Within chemical indicators, Class 5 integrating indicators and Class 6 emulating indicators are experiencing the fastest growth due to their superior accuracy and reliability in verifying sterilization parameters.
End-user analysis reveals hospitals as the primary consumers (58%), followed by ambulatory surgical centers (22%), dental clinics (12%), and other healthcare facilities (8%). The ambulatory surgical center segment is growing most rapidly as outpatient procedures continue to increase globally.
Key market drivers include stringent regulatory frameworks, particularly in developed economies where healthcare accreditation bodies mandate comprehensive sterilization monitoring. The COVID-19 pandemic has further accelerated market growth by highlighting the critical importance of proper sterilization protocols across all healthcare settings.
Pricing trends indicate moderate competition, with premium indicators commanding 15-20% higher prices based on reliability and technological sophistication. The average cost per indicator ranges from $0.15 for basic Class 1 indicators to $3.50 for advanced Class 6 indicators, with biological indicators typically priced between $2.50 and $5.00 per unit.
Distribution channels are evolving, with direct sales to healthcare institutions representing 45% of transactions, while distributor networks account for 40% and online procurement platforms handle the remaining 15%. The latter channel is growing most rapidly, reflecting broader digitalization trends in healthcare procurement.
Current Challenges in Autoclave Indicator Technology
Despite significant advancements in autoclave sterilization technology, several persistent challenges continue to plague indicator systems used to verify sterility. The most prominent issue remains the reliability and consistency of chemical indicators across varying autoclave conditions. Current chemical indicators often demonstrate inconsistent color changes when exposed to different combinations of temperature, pressure, and humidity, leading to ambiguous results that compromise sterility assurance.
Integration challenges between indicator systems and modern autoclave equipment represent another significant hurdle. Many indicators were designed for traditional autoclave models and struggle to provide accurate readings in newer, more sophisticated sterilization systems that utilize rapid cycling or alternative sterilant distribution methods. This compatibility gap creates uncertainty in sterility verification processes, particularly in high-throughput medical environments.
The sensitivity threshold of current indicators presents a critical limitation. Many commercially available indicators fail to detect subtle deviations in sterilization parameters that could potentially compromise sterility. This detection gap is particularly concerning for complex medical devices with intricate geometries where steam penetration may be inconsistent, yet indicators might still suggest successful sterilization.
Environmental sustainability concerns have emerged as autoclave facilities face increasing pressure to reduce waste. Traditional single-use indicators contribute significantly to medical waste, yet alternatives with reduced environmental impact often demonstrate lower reliability or shorter shelf-life, creating a difficult trade-off between sustainability and performance.
Standardization issues persist across global markets, with different regions adopting varying requirements for indicator performance. This regulatory fragmentation complicates manufacturing processes and validation protocols, particularly for healthcare facilities operating across multiple jurisdictions. The lack of harmonized standards creates confusion regarding which indicators provide truly reliable sterility assurance.
Digital integration capabilities remain underdeveloped in most current indicator technologies. While healthcare facilities increasingly adopt electronic record systems, most sterilization indicators still require manual interpretation and documentation, creating inefficiencies and potential for human error in record-keeping. The gap between analog indicators and digital healthcare infrastructure represents a significant barrier to workflow optimization.
Cost considerations continue to influence indicator selection, often at the expense of performance. Healthcare facilities frequently face budget constraints that lead to compromises in indicator quality or frequency of use, potentially jeopardizing sterility assurance. This economic pressure has slowed adoption of more advanced indicator technologies that might offer superior performance but at higher price points.
Integration challenges between indicator systems and modern autoclave equipment represent another significant hurdle. Many indicators were designed for traditional autoclave models and struggle to provide accurate readings in newer, more sophisticated sterilization systems that utilize rapid cycling or alternative sterilant distribution methods. This compatibility gap creates uncertainty in sterility verification processes, particularly in high-throughput medical environments.
The sensitivity threshold of current indicators presents a critical limitation. Many commercially available indicators fail to detect subtle deviations in sterilization parameters that could potentially compromise sterility. This detection gap is particularly concerning for complex medical devices with intricate geometries where steam penetration may be inconsistent, yet indicators might still suggest successful sterilization.
Environmental sustainability concerns have emerged as autoclave facilities face increasing pressure to reduce waste. Traditional single-use indicators contribute significantly to medical waste, yet alternatives with reduced environmental impact often demonstrate lower reliability or shorter shelf-life, creating a difficult trade-off between sustainability and performance.
Standardization issues persist across global markets, with different regions adopting varying requirements for indicator performance. This regulatory fragmentation complicates manufacturing processes and validation protocols, particularly for healthcare facilities operating across multiple jurisdictions. The lack of harmonized standards creates confusion regarding which indicators provide truly reliable sterility assurance.
Digital integration capabilities remain underdeveloped in most current indicator technologies. While healthcare facilities increasingly adopt electronic record systems, most sterilization indicators still require manual interpretation and documentation, creating inefficiencies and potential for human error in record-keeping. The gap between analog indicators and digital healthcare infrastructure represents a significant barrier to workflow optimization.
Cost considerations continue to influence indicator selection, often at the expense of performance. Healthcare facilities frequently face budget constraints that lead to compromises in indicator quality or frequency of use, potentially jeopardizing sterility assurance. This economic pressure has slowed adoption of more advanced indicator technologies that might offer superior performance but at higher price points.
Evaluation Methods for Autoclave Process Indicators
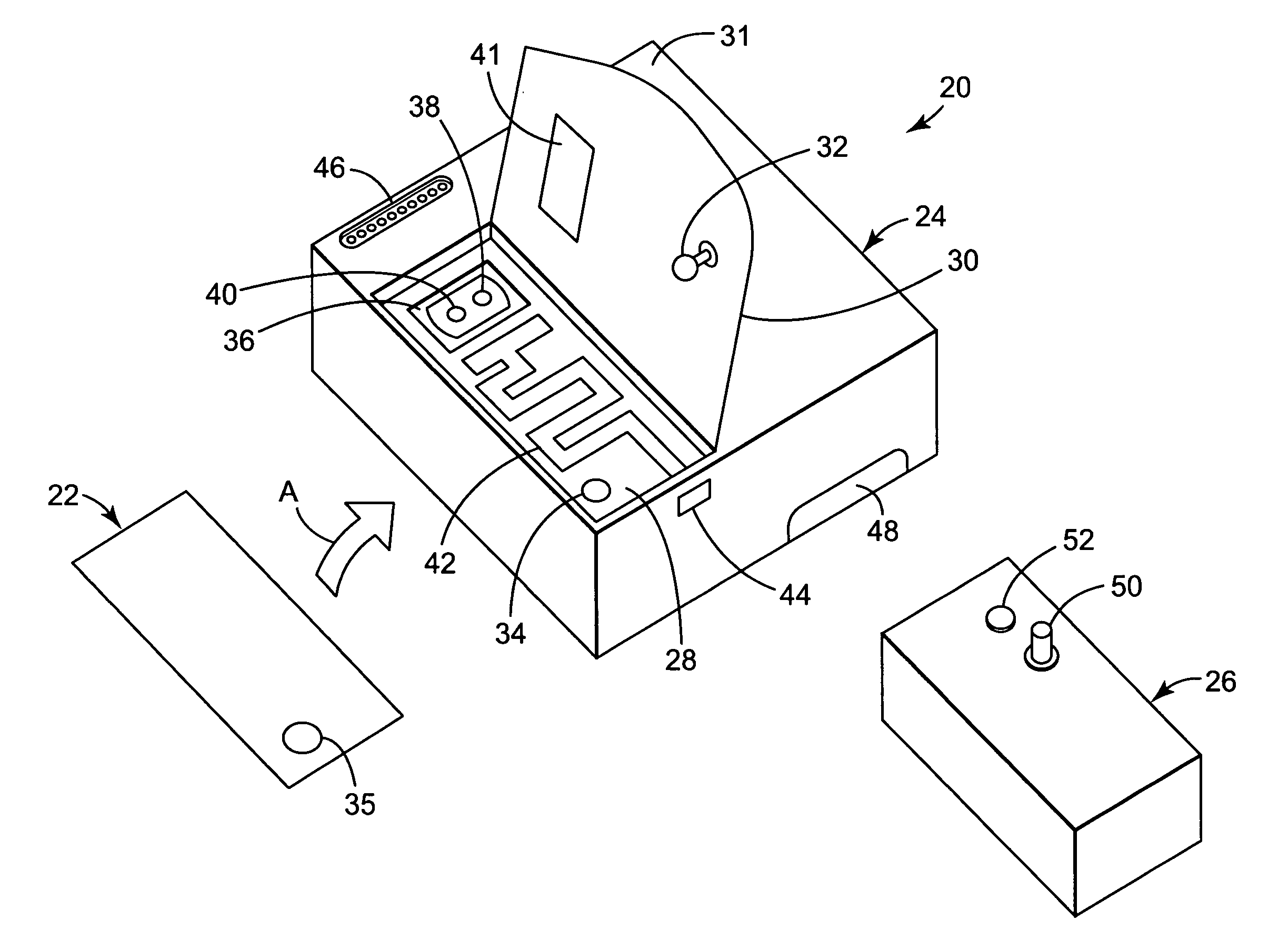
01 Chemical indicators for autoclave sterilization
Chemical indicators are used in autoclave processes to verify that sterilization conditions have been met. These indicators typically change color or physical state when exposed to specific temperature, pressure, and time conditions required for effective sterilization. They provide immediate visual confirmation that items have been processed through the sterilization cycle, helping to ensure consistent sterility assurance.- Chemical indicators for autoclave sterilization: Chemical indicators are used to verify that sterilization conditions have been met in autoclaves. These indicators change color or physical state when exposed to specific temperature, pressure, and time conditions, providing visual confirmation of the sterilization process. They can be integrated into packaging materials or used as separate strips, helping to ensure consistent sterility across different loads and cycles.
- Biological indicators for sterility validation: Biological indicators contain resistant bacterial spores that are more difficult to kill than most pathogens. These indicators provide the most reliable method for confirming sterilization effectiveness by demonstrating the actual killing of test organisms. After the autoclave cycle, the biological indicators are incubated to determine if any spores survived, thus validating the sterility assurance level of the process.
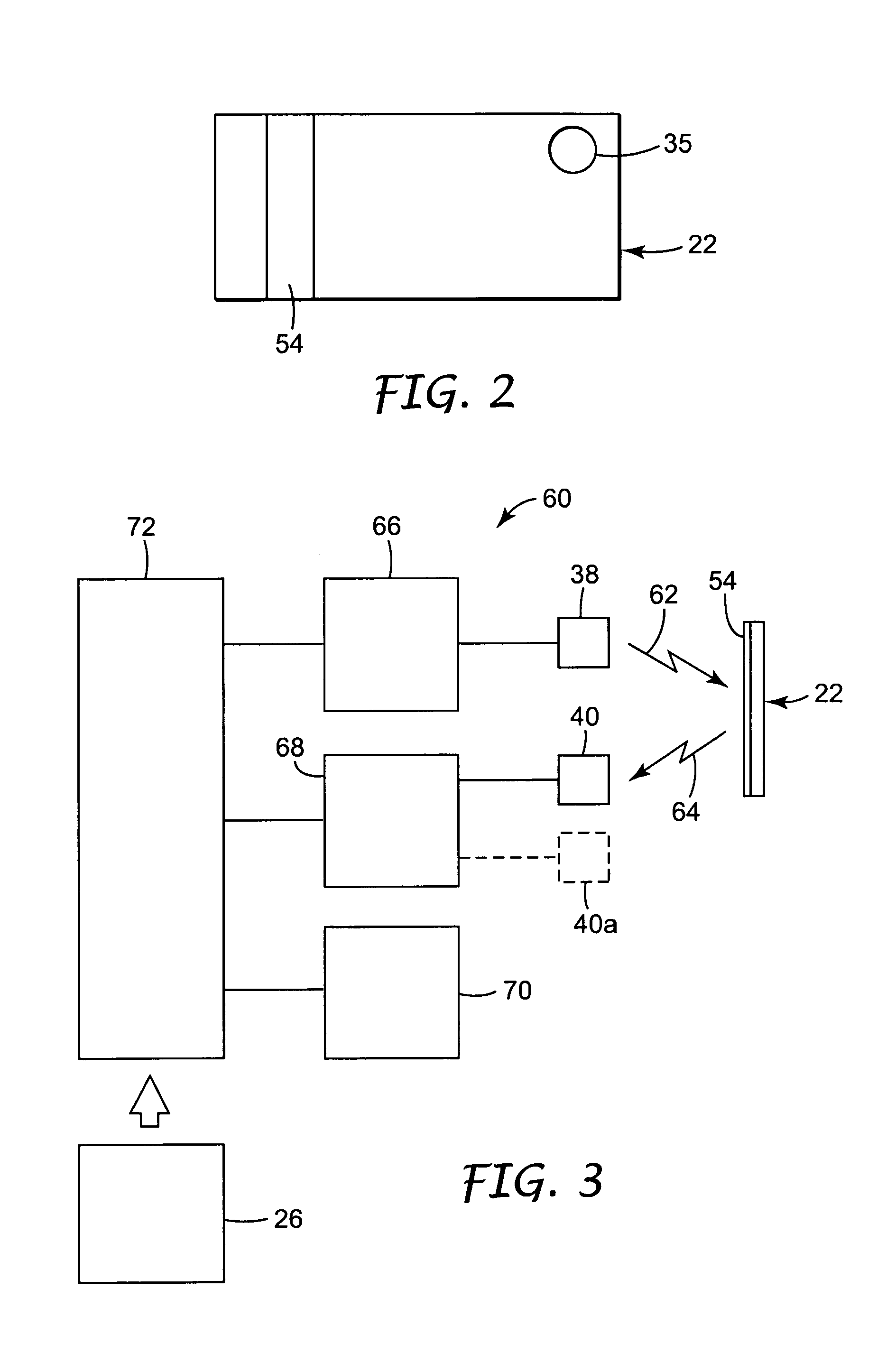
- Electronic monitoring systems for autoclave processes: Electronic monitoring systems provide real-time data on critical parameters throughout the sterilization cycle. These systems use sensors to track temperature, pressure, time, and other variables, storing this information for documentation and analysis. Advanced systems may include wireless connectivity, automated alerts for cycle failures, and integration with facility management systems to ensure consistent sterility validation.
- Integrated sterilization indicator systems: Integrated systems combine multiple indicator types (chemical, biological, and electronic) to provide comprehensive sterility assurance. These systems offer redundant verification methods and can include software for data management, trend analysis, and compliance reporting. By integrating different indicator technologies, these systems provide higher confidence in sterilization consistency across various load configurations and cycle parameters.
- Packaging and process challenge devices: Specialized packaging materials and process challenge devices (PCDs) are designed to test the most difficult sterilization scenarios. These devices simulate worst-case conditions by creating barriers to steam penetration or by positioning indicators in the most difficult-to-sterilize locations. PCDs help validate that even the most challenging items in a load have been properly sterilized, ensuring consistent sterility throughout the entire autoclave chamber.
02 Biological indicators for sterility validation
Biological indicators contain viable microorganisms with high resistance to the sterilization process. They are used to validate autoclave performance by confirming the ability to kill these test organisms. When the most resistant microorganisms are killed, it provides assurance that all other less resistant pathogens have also been eliminated, thus confirming effective sterilization and consistent sterility.Expand Specific Solutions03 Electronic monitoring systems for autoclave processes
Electronic monitoring systems provide real-time data on critical parameters during the autoclave sterilization process. These systems track temperature, pressure, time, and other variables throughout the cycle, ensuring that all parameters remain within specified ranges. The data can be stored, analyzed, and used for documentation purposes, providing reliable evidence of consistent sterility achievement across multiple sterilization cycles.Expand Specific Solutions04 Integrated indicator systems for sterility assurance
Integrated indicator systems combine multiple monitoring technologies to provide comprehensive sterility assurance. These systems may incorporate chemical indicators, biological indicators, and electronic monitoring in a unified approach. By using complementary methods, these integrated systems offer redundant verification of sterilization effectiveness, enhancing reliability and consistency in sterility assurance for autoclave processes.Expand Specific Solutions05 Packaging and placement strategies for process indicators
The effectiveness of autoclave process indicators depends significantly on their packaging and strategic placement within loads. Indicators should be positioned in the most challenging locations for steam penetration, such as the center of dense packs or within lumened devices. Proper packaging ensures that indicators accurately reflect the sterilization conditions experienced by the most difficult-to-sterilize items, thereby providing meaningful data about consistent sterility achievement.Expand Specific Solutions
Leading Manufacturers in Sterilization Indicator Industry
The autoclave process indicator market is currently in a growth phase, with increasing demand driven by stringent sterilization requirements in healthcare and pharmaceutical industries. The global market size is estimated to exceed $300 million, expanding at a CAGR of 6-7%. Technologically, the field shows varying maturity levels, with established players like 3M Innovative Properties, American Sterilizer Co. (STERIS), and Tuttnauer leading innovation with advanced chemical and biological indicators. Emerging competitors include Shinva Medical Instrument and Fedegari Autoclavi, who are introducing digital monitoring solutions. Regional players like GKE GmbH and O&M Halyard are specializing in niche applications, while companies like Ethicon and Olympus are integrating sterilization monitoring into broader medical device ecosystems, creating a competitive landscape balanced between global leaders and specialized innovators.
3M Innovative Properties Co.
Technical Solution: 3M has developed advanced chemical indicator technologies for autoclave sterilization monitoring that utilize proprietary color-change chemistry. Their Class 5 integrating indicators incorporate multiple critical parameters including time, temperature, and steam quality to provide accurate sterilization verification. The technology features specially formulated inks that undergo precise, calibrated color transitions when exposed to specific sterilization conditions. 3M's system includes both external and internal indicators with standardized performance requirements that meet or exceed ISO 11140 standards. Their indicators are designed with moving-front technology that provides progressive visual feedback throughout the sterilization cycle, allowing for immediate interpretation of results without additional equipment. The company has also developed electronic monitoring systems that integrate with their chemical indicators to provide comprehensive documentation and traceability of sterilization processes.
Strengths: High precision color-change chemistry with proven reliability across various autoclave conditions; comprehensive product range covering different sterilization parameters; strong integration with electronic documentation systems. Weaknesses: Higher cost compared to basic indicators; requires proper training for accurate interpretation; some indicators have limited shelf life requiring careful inventory management.
GKE GmbH
Technical Solution: GKE has developed specialized Batch Monitoring Systems (BMS) for autoclave process validation that combine chemical and biological indicators with innovative Process Challenge Devices (PCDs). Their technology features multi-variable indicators that respond to all critical sterilization parameters including temperature, time, steam quality, and non-condensable gases. GKE's indicators utilize proprietary chemical formulations that provide graduated responses rather than simple pass/fail results, allowing for quantitative assessment of sterilization margin. Their system includes specialized Hollow Load Process Challenge Devices that simulate worst-case scenarios for steam penetration into complex medical instruments with narrow lumens. GKE has also developed compact Bio-Compact PCDs that combine chemical indicators with biological spore tests in a single device, providing comprehensive validation with minimal materials. Their indicators are designed with strict adherence to ISO 11140 standards and include tamper-evident features to ensure result integrity. GKE's technology also includes specialized indicators for detecting superheated steam conditions that can lead to failed sterilization despite meeting temperature requirements.
Strengths: Highly specialized indicators for different load configurations and sterilization challenges; quantitative assessment capabilities beyond simple pass/fail; comprehensive range of PCDs for different applications. Weaknesses: More complex interpretation required compared to basic indicators; higher cost for specialized PCDs; requires more extensive staff training for proper implementation and interpretation.
Critical Patents in Sterilization Monitoring Technology
Saturated steam sterilization device and process having improved sterilization reliability and temperature control
PatentInactiveUS20220062458A9
Innovation
- A sterilization device and method using saturated steam with a novel heat control system that accurately monitors and controls temperature and pressure to ensure reliable and reproducible sterilization, capable of sterilizing dental handpieces without damaging the turbine assembly and achieving a sterilization cycle time of approximately 23 minutes or less.
Electronic reader for sterilization monitors
PatentInactiveUS7122150B2
Innovation
- A system comprising a reusable reader and disposable sterilization sensor that undergoes an optical change upon exposure to sterilization conditions, allowing remote assessment through an illumination source, color sensor, interpretation circuit, and communication circuit, enabling reliable and cost-effective verification without opening the package.
Regulatory Standards for Sterilization Validation
The regulatory landscape for sterilization validation is complex and multifaceted, with several international and regional standards governing the validation of autoclave processes. The primary regulatory framework is established by ISO 17665-1:2006, which specifies requirements for the development, validation, and routine control of moist heat sterilization processes for medical devices. This standard provides comprehensive guidelines for ensuring that sterilization processes consistently achieve the required sterility assurance level (SAL).
In the United States, the Food and Drug Administration (FDA) enforces compliance with sterilization validation through 21 CFR Part 820 (Quality System Regulation), which requires manufacturers to validate processes that cannot be fully verified by subsequent inspection and testing. The FDA also recognizes ANSI/AAMI ST79 as a consensus standard for steam sterilization in healthcare facilities, providing detailed requirements for biological indicators, chemical indicators, and physical monitors.
The European regulatory framework is governed by EN 285 for large steam sterilizers and EN 13060 for small steam sterilizers, both of which specify performance requirements and test methods. Additionally, the Medical Device Regulation (MDR 2017/745) mandates that manufacturers implement appropriate validation protocols for sterilization processes as part of their quality management systems.
Process indicators for autoclave sterilization are classified according to ISO 11140 series, which categorizes chemical indicators into six classes based on their performance characteristics. Class 5 and Class 6 indicators are particularly relevant for validation purposes, as they respond to all critical parameters of the sterilization process (time, temperature, and presence of steam).
Regulatory bodies also specify the frequency of validation activities. Initial qualification (IQ, OQ, PQ) must be performed before routine use, with periodic requalification required at defined intervals. The frequency of these revalidations varies by jurisdiction but typically ranges from annually to every three years, with additional validation required after significant changes to equipment or processes.
Documentation requirements are equally stringent, with regulatory standards mandating comprehensive records of validation protocols, acceptance criteria, test results, and any deviations or corrective actions. These records must be maintained for a period that exceeds the expected lifetime of the sterilized products, typically a minimum of five years after the last product was manufactured.
Recent regulatory trends indicate an increasing focus on process analytical technology (PAT) and real-time monitoring systems that can provide continuous verification of critical parameters during the sterilization cycle, potentially reducing the reliance on traditional biological and chemical indicators for routine monitoring while maintaining or enhancing sterility assurance.
In the United States, the Food and Drug Administration (FDA) enforces compliance with sterilization validation through 21 CFR Part 820 (Quality System Regulation), which requires manufacturers to validate processes that cannot be fully verified by subsequent inspection and testing. The FDA also recognizes ANSI/AAMI ST79 as a consensus standard for steam sterilization in healthcare facilities, providing detailed requirements for biological indicators, chemical indicators, and physical monitors.
The European regulatory framework is governed by EN 285 for large steam sterilizers and EN 13060 for small steam sterilizers, both of which specify performance requirements and test methods. Additionally, the Medical Device Regulation (MDR 2017/745) mandates that manufacturers implement appropriate validation protocols for sterilization processes as part of their quality management systems.
Process indicators for autoclave sterilization are classified according to ISO 11140 series, which categorizes chemical indicators into six classes based on their performance characteristics. Class 5 and Class 6 indicators are particularly relevant for validation purposes, as they respond to all critical parameters of the sterilization process (time, temperature, and presence of steam).
Regulatory bodies also specify the frequency of validation activities. Initial qualification (IQ, OQ, PQ) must be performed before routine use, with periodic requalification required at defined intervals. The frequency of these revalidations varies by jurisdiction but typically ranges from annually to every three years, with additional validation required after significant changes to equipment or processes.
Documentation requirements are equally stringent, with regulatory standards mandating comprehensive records of validation protocols, acceptance criteria, test results, and any deviations or corrective actions. These records must be maintained for a period that exceeds the expected lifetime of the sterilized products, typically a minimum of five years after the last product was manufactured.
Recent regulatory trends indicate an increasing focus on process analytical technology (PAT) and real-time monitoring systems that can provide continuous verification of critical parameters during the sterilization cycle, potentially reducing the reliance on traditional biological and chemical indicators for routine monitoring while maintaining or enhancing sterility assurance.
Risk Assessment in Sterility Assurance Systems
Risk assessment forms a critical foundation in sterility assurance systems, particularly when evaluating autoclave process indicators. The systematic identification, analysis, and prioritization of potential failure modes enables healthcare facilities and manufacturers to implement appropriate control measures that ensure consistent sterility outcomes. When assessing risks in autoclave sterilization processes, organizations must consider multiple factors including equipment reliability, operator training, environmental conditions, and the specific characteristics of items being sterilized.
The risk assessment framework for autoclave process indicators typically follows a structured methodology that begins with hazard identification. This involves cataloging all potential sources of contamination or sterilization failure throughout the autoclave cycle. Critical control points are then established at stages where monitoring can effectively prevent or detect sterilization failures. These points often include temperature achievement, steam penetration adequacy, and exposure time verification.
Failure Mode and Effects Analysis (FMEA) represents a particularly valuable tool in this context. By systematically evaluating what could go wrong with process indicators, the potential consequences of such failures, and their likelihood of occurrence, organizations can develop targeted mitigation strategies. For instance, the risk of false-positive readings from chemical indicators might be addressed through redundant indicator systems or complementary biological verification methods.
Quantitative risk assessment techniques further enhance sterility assurance by assigning numerical values to both the probability and severity of potential failures. This enables evidence-based prioritization of resources toward addressing the most significant risks. The Sterility Assurance Level (SAL), typically expressed as 10^-6 (a one-in-a-million probability of a non-sterile unit), serves as a key metric in this quantitative framework.
Process validation plays an integral role in risk management, requiring documented evidence that autoclave processes consistently deliver sterile products. This validation encompasses installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) phases, each addressing different aspects of system reliability. Regular revalidation schedules must be established based on risk assessment outcomes to maintain system integrity over time.
Regulatory compliance considerations significantly impact risk assessment approaches, with standards such as ISO 17665, AAMI ST79, and various regional requirements establishing minimum expectations for sterility assurance. These standards increasingly emphasize risk-based approaches that allow organizations to customize their monitoring and validation protocols according to their specific risk profiles rather than following rigid, one-size-fits-all procedures.
The risk assessment framework for autoclave process indicators typically follows a structured methodology that begins with hazard identification. This involves cataloging all potential sources of contamination or sterilization failure throughout the autoclave cycle. Critical control points are then established at stages where monitoring can effectively prevent or detect sterilization failures. These points often include temperature achievement, steam penetration adequacy, and exposure time verification.
Failure Mode and Effects Analysis (FMEA) represents a particularly valuable tool in this context. By systematically evaluating what could go wrong with process indicators, the potential consequences of such failures, and their likelihood of occurrence, organizations can develop targeted mitigation strategies. For instance, the risk of false-positive readings from chemical indicators might be addressed through redundant indicator systems or complementary biological verification methods.
Quantitative risk assessment techniques further enhance sterility assurance by assigning numerical values to both the probability and severity of potential failures. This enables evidence-based prioritization of resources toward addressing the most significant risks. The Sterility Assurance Level (SAL), typically expressed as 10^-6 (a one-in-a-million probability of a non-sterile unit), serves as a key metric in this quantitative framework.
Process validation plays an integral role in risk management, requiring documented evidence that autoclave processes consistently deliver sterile products. This validation encompasses installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) phases, each addressing different aspects of system reliability. Regular revalidation schedules must be established based on risk assessment outcomes to maintain system integrity over time.
Regulatory compliance considerations significantly impact risk assessment approaches, with standards such as ISO 17665, AAMI ST79, and various regional requirements establishing minimum expectations for sterility assurance. These standards increasingly emphasize risk-based approaches that allow organizations to customize their monitoring and validation protocols according to their specific risk profiles rather than following rigid, one-size-fits-all procedures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!