Autoclave vs. Microwave: Which Provides Better Sterilization?
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sterilization Technology Evolution and Objectives
Sterilization methods have evolved significantly over the past century, with autoclaves representing one of the earliest reliable technologies for medical and laboratory sterilization. Developed in the late 19th century, autoclave technology leverages the principles of steam under pressure to achieve high-temperature sterilization. This method has remained the gold standard in many applications due to its reliability and thoroughness in eliminating microbial contaminants, including resistant bacterial spores.
In contrast, microwave sterilization emerged in the late 20th century as an alternative approach, utilizing electromagnetic radiation to generate heat within materials. Initially developed for food applications, microwave technology has gradually expanded into medical and laboratory settings, offering potential advantages in terms of speed and energy efficiency.
The evolution of sterilization technology has been driven by several key factors: increasing demands for faster processing times in healthcare settings, growing concerns about energy consumption and environmental impact, and the need for methods compatible with heat-sensitive materials. These drivers have accelerated research into comparative effectiveness between traditional and emerging sterilization methods.
Current technological objectives in the sterilization field focus on achieving optimal balance between efficacy, efficiency, and material compatibility. Specifically, researchers aim to determine whether microwave sterilization can match or exceed the proven reliability of autoclave methods while offering advantages in processing time and energy consumption. Additional objectives include quantifying the impact of each method on different materials, particularly heat-sensitive instruments and components increasingly common in modern medical devices.
The scientific community has established clear parameters for evaluating sterilization effectiveness, including log reduction of microbial populations, penetration capabilities for different materials and packaging, cycle time requirements, and validation protocols. These metrics provide a framework for objective comparison between autoclave and microwave technologies.
Looking forward, the sterilization technology landscape aims to develop more adaptive systems that can optimize parameters based on specific load characteristics, potentially combining elements of both technologies. Integration with digital monitoring systems represents another evolutionary direction, enabling real-time verification of sterilization effectiveness and creating comprehensive documentation trails for regulatory compliance.
Understanding the historical context, current capabilities, and future objectives of both autoclave and microwave sterilization technologies provides essential groundwork for evaluating their relative merits in various applications and identifying opportunities for technological advancement in this critical field.
In contrast, microwave sterilization emerged in the late 20th century as an alternative approach, utilizing electromagnetic radiation to generate heat within materials. Initially developed for food applications, microwave technology has gradually expanded into medical and laboratory settings, offering potential advantages in terms of speed and energy efficiency.
The evolution of sterilization technology has been driven by several key factors: increasing demands for faster processing times in healthcare settings, growing concerns about energy consumption and environmental impact, and the need for methods compatible with heat-sensitive materials. These drivers have accelerated research into comparative effectiveness between traditional and emerging sterilization methods.
Current technological objectives in the sterilization field focus on achieving optimal balance between efficacy, efficiency, and material compatibility. Specifically, researchers aim to determine whether microwave sterilization can match or exceed the proven reliability of autoclave methods while offering advantages in processing time and energy consumption. Additional objectives include quantifying the impact of each method on different materials, particularly heat-sensitive instruments and components increasingly common in modern medical devices.
The scientific community has established clear parameters for evaluating sterilization effectiveness, including log reduction of microbial populations, penetration capabilities for different materials and packaging, cycle time requirements, and validation protocols. These metrics provide a framework for objective comparison between autoclave and microwave technologies.
Looking forward, the sterilization technology landscape aims to develop more adaptive systems that can optimize parameters based on specific load characteristics, potentially combining elements of both technologies. Integration with digital monitoring systems represents another evolutionary direction, enabling real-time verification of sterilization effectiveness and creating comprehensive documentation trails for regulatory compliance.
Understanding the historical context, current capabilities, and future objectives of both autoclave and microwave sterilization technologies provides essential groundwork for evaluating their relative merits in various applications and identifying opportunities for technological advancement in this critical field.
Market Analysis of Medical and Industrial Sterilization Needs
The global sterilization market is experiencing robust growth, driven by increasing healthcare expenditures, rising surgical procedures, and growing awareness of infection control. Currently valued at approximately 7.1 billion USD, the market is projected to reach 12.5 billion USD by 2027, representing a compound annual growth rate of 9.8%. Medical facilities remain the dominant consumers, accounting for nearly 60% of the total market share, while industrial applications, particularly in pharmaceuticals and food processing, constitute the remaining 40%.
Within the medical sector, hospitals and surgical centers demonstrate the highest demand for sterilization technologies, with autoclave systems historically dominating this space. However, recent trends indicate a growing interest in alternative technologies like microwave sterilization, particularly in outpatient settings and smaller clinics where space constraints and energy efficiency are critical considerations.
The industrial sterilization market presents distinct requirements compared to medical applications. Pharmaceutical manufacturing demands highly reliable and validated sterilization processes that meet stringent regulatory standards, while food processing industries prioritize technologies that preserve product quality and nutritional value. The industrial sector shows increasing receptiveness to microwave sterilization due to its reduced processing time and potential energy savings.
Regional analysis reveals significant market variations. North America and Europe currently lead in sterilization technology adoption, with mature healthcare systems and stringent regulatory frameworks. However, the Asia-Pacific region is experiencing the fastest growth rate at approximately 12.3% annually, driven by expanding healthcare infrastructure, increasing surgical volumes, and growing manufacturing bases in countries like China and India.
Consumer preferences are evolving based on several key factors. Cost-effectiveness remains paramount, with facilities increasingly evaluating total cost of ownership rather than initial investment alone. Energy efficiency has emerged as a critical consideration, particularly as healthcare and industrial facilities face mounting pressure to reduce their environmental footprint. Additionally, processing speed is gaining importance in high-throughput environments where rapid turnaround of sterilized equipment or products directly impacts operational efficiency.
Market research indicates a growing demand for hybrid sterilization solutions that combine the reliability of traditional methods like autoclaves with the speed and efficiency advantages of newer technologies like microwave sterilization. This trend suggests potential opportunities for innovative products that address the limitations of both approaches while capitalizing on their respective strengths.
Within the medical sector, hospitals and surgical centers demonstrate the highest demand for sterilization technologies, with autoclave systems historically dominating this space. However, recent trends indicate a growing interest in alternative technologies like microwave sterilization, particularly in outpatient settings and smaller clinics where space constraints and energy efficiency are critical considerations.
The industrial sterilization market presents distinct requirements compared to medical applications. Pharmaceutical manufacturing demands highly reliable and validated sterilization processes that meet stringent regulatory standards, while food processing industries prioritize technologies that preserve product quality and nutritional value. The industrial sector shows increasing receptiveness to microwave sterilization due to its reduced processing time and potential energy savings.
Regional analysis reveals significant market variations. North America and Europe currently lead in sterilization technology adoption, with mature healthcare systems and stringent regulatory frameworks. However, the Asia-Pacific region is experiencing the fastest growth rate at approximately 12.3% annually, driven by expanding healthcare infrastructure, increasing surgical volumes, and growing manufacturing bases in countries like China and India.
Consumer preferences are evolving based on several key factors. Cost-effectiveness remains paramount, with facilities increasingly evaluating total cost of ownership rather than initial investment alone. Energy efficiency has emerged as a critical consideration, particularly as healthcare and industrial facilities face mounting pressure to reduce their environmental footprint. Additionally, processing speed is gaining importance in high-throughput environments where rapid turnaround of sterilized equipment or products directly impacts operational efficiency.
Market research indicates a growing demand for hybrid sterilization solutions that combine the reliability of traditional methods like autoclaves with the speed and efficiency advantages of newer technologies like microwave sterilization. This trend suggests potential opportunities for innovative products that address the limitations of both approaches while capitalizing on their respective strengths.
Current Capabilities and Limitations of Autoclave and Microwave Methods
Autoclave sterilization represents the gold standard in medical and laboratory settings, utilizing high-pressure saturated steam at temperatures typically ranging from 121°C to 134°C. This method achieves sterilization through the combined action of heat and moisture, which effectively denatures proteins and destroys microorganisms including bacterial spores. Autoclaves offer consistent and reliable performance with documented validation protocols, making them the preferred choice for critical applications in healthcare facilities and research laboratories.
The primary strength of autoclave technology lies in its proven efficacy across a wide range of materials and contaminants. Modern autoclaves feature sophisticated control systems that monitor critical parameters such as temperature, pressure, and exposure time, ensuring sterilization cycles meet established standards. Additionally, the process generates minimal toxic residues, making it environmentally preferable to chemical sterilization methods.
However, autoclave sterilization presents several limitations. The process requires significant energy consumption and extended cycle times, typically 15-60 minutes plus additional time for heating and cooling. Heat-sensitive materials including certain plastics, electronics, and biological samples cannot withstand autoclave conditions without damage. The equipment demands substantial initial investment, regular maintenance, and dedicated utility connections, limiting deployment in resource-constrained settings.
Microwave sterilization, by contrast, operates through a fundamentally different mechanism. Rather than external heat application, microwaves generate heat through dielectric heating—the excitation of water molecules within the materials being sterilized. Commercial microwave sterilization systems typically operate at 2.45 GHz, creating rapid heating that can achieve sterilization in significantly shorter timeframes than conventional autoclaves.
The primary advantages of microwave sterilization include reduced processing times (often under 10 minutes), lower energy consumption, and minimal warm-up requirements. The technology offers particular promise for point-of-use applications in clinical settings and for field deployment where traditional infrastructure may be unavailable. Additionally, microwave systems generally require less maintenance than pressure vessel autoclaves.
Despite these benefits, microwave sterilization faces substantial technical limitations. The technology struggles with achieving uniform heating throughout the load, creating potential cold spots where microorganisms might survive. Penetration depth remains limited, restricting effective sterilization to relatively thin or porous materials. Metal objects cannot be processed using standard microwave technology, and validation protocols are less established compared to autoclave methods, creating regulatory challenges for critical applications.
Recent technological developments have attempted to address these limitations through hybrid systems combining microwave energy with pressure vessels or steam generation, though these solutions remain less widely adopted than traditional autoclaves in regulated healthcare environments.
The primary strength of autoclave technology lies in its proven efficacy across a wide range of materials and contaminants. Modern autoclaves feature sophisticated control systems that monitor critical parameters such as temperature, pressure, and exposure time, ensuring sterilization cycles meet established standards. Additionally, the process generates minimal toxic residues, making it environmentally preferable to chemical sterilization methods.
However, autoclave sterilization presents several limitations. The process requires significant energy consumption and extended cycle times, typically 15-60 minutes plus additional time for heating and cooling. Heat-sensitive materials including certain plastics, electronics, and biological samples cannot withstand autoclave conditions without damage. The equipment demands substantial initial investment, regular maintenance, and dedicated utility connections, limiting deployment in resource-constrained settings.
Microwave sterilization, by contrast, operates through a fundamentally different mechanism. Rather than external heat application, microwaves generate heat through dielectric heating—the excitation of water molecules within the materials being sterilized. Commercial microwave sterilization systems typically operate at 2.45 GHz, creating rapid heating that can achieve sterilization in significantly shorter timeframes than conventional autoclaves.
The primary advantages of microwave sterilization include reduced processing times (often under 10 minutes), lower energy consumption, and minimal warm-up requirements. The technology offers particular promise for point-of-use applications in clinical settings and for field deployment where traditional infrastructure may be unavailable. Additionally, microwave systems generally require less maintenance than pressure vessel autoclaves.
Despite these benefits, microwave sterilization faces substantial technical limitations. The technology struggles with achieving uniform heating throughout the load, creating potential cold spots where microorganisms might survive. Penetration depth remains limited, restricting effective sterilization to relatively thin or porous materials. Metal objects cannot be processed using standard microwave technology, and validation protocols are less established compared to autoclave methods, creating regulatory challenges for critical applications.
Recent technological developments have attempted to address these limitations through hybrid systems combining microwave energy with pressure vessels or steam generation, though these solutions remain less widely adopted than traditional autoclaves in regulated healthcare environments.
Comparative Analysis of Autoclave and Microwave Sterilization Protocols
01 Autoclave sterilization mechanisms and effectiveness
Autoclave sterilization utilizes high-pressure steam to eliminate microorganisms. The combination of heat, pressure, and moisture effectively destroys bacteria, viruses, fungi, and spores by denaturing proteins and disrupting cell membranes. Autoclaves typically operate at temperatures of 121-134°C under pressures of 15-30 psi, with exposure times ranging from 15-30 minutes depending on the load. This method is particularly effective for heat-resistant materials and provides reliable sterilization for medical instruments, laboratory equipment, and certain pharmaceutical products.- Autoclave sterilization mechanisms and effectiveness: Autoclave sterilization uses high-pressure steam to eliminate microorganisms. The combination of heat, pressure, and moisture effectively destroys bacteria, viruses, fungi, and spores by denaturing proteins and disrupting cell membranes. Standard autoclave cycles typically operate at 121°C for 15-30 minutes or 134°C for 3-4 minutes under pressure of 15-30 psi. This method provides reliable and complete sterilization for heat-resistant materials and is considered the gold standard for medical and laboratory equipment sterilization.
- Microwave sterilization technology advancements: Microwave sterilization utilizes electromagnetic radiation to generate heat within materials, causing rapid molecular motion and friction that destroys microorganisms. Recent advancements include precise power control systems, specialized containers that enhance microwave distribution, and combination technologies that integrate microwave with other sterilization methods. These innovations have improved sterilization effectiveness while reducing processing time and energy consumption compared to traditional methods, making microwave sterilization increasingly viable for various applications including medical devices and food products.
- Comparative effectiveness of autoclave versus microwave sterilization: Studies comparing autoclave and microwave sterilization show distinct advantages for each method. Autoclaves provide more consistent and thorough sterilization, particularly for dense materials and items with complex geometries. Microwave sterilization offers faster processing times and lower energy consumption but may result in uneven heating patterns. The effectiveness of microwave sterilization depends significantly on the material properties, moisture content, and equipment design. For critical medical applications requiring complete sterility assurance, autoclave remains superior, while microwave sterilization offers advantages for specific applications where speed and energy efficiency are priorities.
- Sterilization validation and monitoring methods: Effective sterilization requires proper validation and monitoring protocols. Biological indicators containing resistant bacterial spores are used to verify complete sterilization. Chemical indicators that change color or physical properties confirm exposure to sterilization conditions. Modern systems incorporate electronic sensors and data loggers to monitor critical parameters throughout the sterilization cycle. For autoclave sterilization, pressure, temperature, and time are monitored, while microwave systems track power output, exposure time, and temperature distribution. These validation methods ensure sterilization effectiveness and provide documentation for quality control and regulatory compliance.
- Application-specific sterilization technologies: Sterilization technologies are increasingly tailored to specific applications. Modified autoclave systems with pulsed vacuum cycles improve steam penetration for complex medical devices. Specialized microwave sterilization systems incorporate temperature control and moisture management for food preservation while maintaining nutritional quality. Hybrid systems combining multiple sterilization methods enhance effectiveness while minimizing material damage. For heat-sensitive materials, low-temperature microwave plasma sterilization offers an alternative to traditional high-temperature methods. These application-specific approaches optimize sterilization effectiveness while considering material compatibility, processing time, and energy efficiency requirements.
02 Microwave sterilization technology advancements
Microwave sterilization technologies have evolved significantly, offering rapid and efficient alternatives to traditional methods. These systems use electromagnetic waves to generate heat within materials, causing vibration of water molecules that leads to thermal inactivation of microorganisms. Advanced microwave sterilization incorporates precise power control, uniform field distribution, and specialized containers to ensure consistent results. Recent innovations include pulsed microwave systems, combination treatments with other technologies, and specialized applicators that enhance penetration and effectiveness while preserving material integrity.Expand Specific Solutions03 Comparative effectiveness of autoclave versus microwave sterilization
Studies comparing autoclave and microwave sterilization show distinct advantages for each method depending on application context. Autoclaves generally provide more reliable and complete sterilization, particularly for dense loads and heat-resistant materials, achieving higher sterility assurance levels. Microwave sterilization offers faster processing times and energy efficiency but may have limitations with certain materials and dense loads. The effectiveness of microwave sterilization depends significantly on proper power calibration, exposure time, and load configuration. For critical medical applications, autoclave sterilization remains the gold standard, while microwave methods offer advantages for specific applications where speed and energy efficiency are priorities.Expand Specific Solutions04 Monitoring and validation of sterilization processes
Effective monitoring and validation systems are crucial for ensuring sterilization efficacy in both autoclave and microwave technologies. These systems employ biological indicators containing resistant bacterial spores, chemical indicators that change color upon exposure to sterilizing conditions, and physical monitors tracking temperature, pressure, and time parameters. Advanced validation methods include parametric release protocols, real-time monitoring with integrated sensors, and automated documentation systems. Regular validation testing helps maintain quality assurance and regulatory compliance while providing verification that sterilization processes consistently achieve the required sterility assurance levels.Expand Specific Solutions05 Specialized applications and material compatibility
Different sterilization technologies show varying effectiveness depending on material properties and application requirements. Autoclave sterilization excels for surgical instruments and laboratory equipment but may damage heat-sensitive materials. Microwave sterilization offers advantages for certain plastics, some pharmaceuticals, and food products where rapid processing is beneficial. Specialized applications include the sterilization of porous materials, liquids, and complex geometries, each requiring optimized protocols. Material compatibility considerations include thermal stability, moisture sensitivity, and potential for chemical interactions during the sterilization process. Innovations in both technologies focus on expanding the range of compatible materials while maintaining sterilization effectiveness.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Sterilization
The sterilization technology market is in a mature growth phase, with an estimated global value exceeding $7 billion and steady annual growth of 5-8%. Autoclave technology represents the established standard with proven reliability across healthcare settings, while microwave sterilization emerges as a disruptive innovation offering faster processing times and energy efficiency. Leading medical equipment manufacturers like Olympus, Stryker, and Siemens dominate the autoclave segment with comprehensive solutions, while companies such as Samsung Electronics and SANYO are advancing microwave sterilization technology. Specialized players like Turbett Surgical and Ster O Wave are developing hybrid systems that combine both technologies to maximize effectiveness while addressing the increasing demand for faster, more efficient sterilization methods in healthcare facilities.
Olympus Corp.
Technical Solution: Olympus Corporation has developed specialized sterilization technologies focused on endoscopic equipment and other complex medical devices. Their approach combines traditional autoclave methods with proprietary cleaning and disinfection systems designed specifically for instruments with narrow lumens and complex geometries. Olympus has engineered specialized autoclave adapters and connectors that ensure steam penetration into all channels of endoscopic equipment. Their research has demonstrated that proper preparation and connection of instruments is critical for effective autoclave sterilization of complex devices. The company has also developed automated endoscope reprocessors that incorporate both cleaning and high-level disinfection steps prior to terminal sterilization. Olympus's validation studies have shown that their multi-step approach achieves complete elimination of microbial contamination, including resistant bacterial spores, from complex medical devices when properly implemented.
Strengths: Specialized solutions for complex medical devices; comprehensive approach that addresses both cleaning and sterilization; validated protocols for difficult-to-sterilize equipment; integration with instrument tracking systems. Weaknesses: Requires multiple processing steps; higher complexity in setup and operation; specialized training requirements for staff; longer total processing time when including all preparation steps.
Stryker Corp.
Technical Solution: Stryker Corporation has developed a comprehensive sterilization approach that integrates both autoclave and specialized low-temperature sterilization technologies. Their autoclave systems feature advanced steam distribution technology that ensures uniform penetration throughout instrument loads, with proprietary chamber designs that optimize steam flow patterns. Stryker's autoclaves incorporate rapid cooling systems that reduce overall cycle times while maintaining sterilization efficacy. The company has also developed specialized instrument trays and containers designed to maximize steam penetration while protecting delicate surgical instruments. Their sterilization validation protocols include both biological and chemical indicators, with real-time monitoring systems that provide documentation for regulatory compliance. Stryker's research has demonstrated that their autoclave technology consistently achieves sterility assurance levels (SAL) of 10^-6 for properly prepared instrument loads.
Strengths: Proven efficacy against all microorganisms including bacterial spores; compatible with most surgical instruments and materials; established technology with extensive validation data; meets regulatory requirements worldwide. Weaknesses: Not suitable for heat-sensitive devices; relatively long processing times; higher utility costs for steam generation and water consumption.
Key Patents and Scientific Literature on Sterilization Efficacy
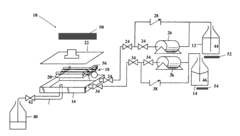
Sterilisation device and corresponding method
PatentWO2010128217A1
Innovation
- A sterilization device using wave emission, specifically S-band microwaves, that employs a temperature variation protocol involving a first rise, a decrease, and a second rise to effectively sterilize products without the need for high pressure and temperature, allowing for automatic control and safe operation.
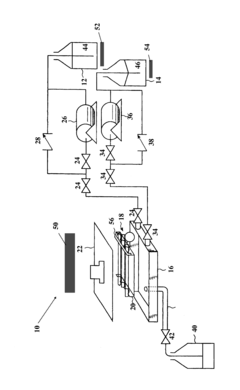
Atmospheric microwave sterilizers and methods
PatentInactiveUS20150374865A1
Innovation
- A microwave sterilizer apparatus and method that uses a sterilant fluid with a boiling point greater than 100°C, absorptive of microwave energy, and a chemical rinsant fluid to sterilize instruments at atmospheric pressure, eliminating the need for water rinsing and maintaining ambient pressure throughout the process.
Energy Efficiency and Sustainability Considerations
When comparing autoclave and microwave sterilization technologies from an energy efficiency and sustainability perspective, several critical factors must be considered. Traditional autoclaves typically consume significant amounts of energy due to their operational requirements for maintaining high temperatures and pressures over extended periods. A standard autoclave cycle may require 1-2 kWh of electricity per cycle, with additional water consumption ranging from 20-50 gallons per cycle depending on size and model. This substantial resource utilization translates to higher operational costs and environmental impact.
Microwave sterilization systems, by contrast, demonstrate notably improved energy efficiency metrics. Research indicates that microwave-based systems can achieve comparable sterilization results while consuming 60-75% less energy than conventional autoclaves. This efficiency stems from the direct heating mechanism of microwave technology, which targets water molecules within microorganisms rather than heating the entire chamber and contents indirectly.
Water consumption represents another significant sustainability consideration. Autoclaves typically require substantial water volumes for steam generation and cooling processes. Microwave sterilization dramatically reduces this water footprint, with some advanced systems operating with minimal or no water requirements beyond what is contained in the sterilization load itself.
Carbon footprint assessments further highlight the sustainability advantages of microwave technology. A comprehensive life cycle analysis conducted across multiple healthcare facilities demonstrated that transitioning from autoclave to microwave sterilization could reduce carbon emissions by approximately 50-65% per sterilization cycle, primarily due to reduced energy consumption and operational efficiency improvements.
Operational lifespan and maintenance requirements also factor into long-term sustainability evaluations. While autoclaves typically have longer operational lifespans (15-20 years with proper maintenance), they require more frequent maintenance interventions and component replacements. Microwave systems generally offer simplified maintenance protocols with fewer mechanical components subject to wear, potentially reducing waste generation from replacement parts.
Material compatibility considerations present additional sustainability implications. Certain medical instruments and materials cannot withstand repeated autoclave cycles, necessitating more frequent replacement. Microwave sterilization's gentler thermal profile may extend the usable lifespan of temperature-sensitive instruments, reducing material consumption and waste generation over time.
Recent technological advancements have further enhanced the sustainability profile of both systems. Next-generation autoclaves incorporate water recycling systems and improved insulation, while advanced microwave sterilizers feature precise power modulation to optimize energy utilization based on load characteristics. These innovations continue to narrow the sustainability gap between these competing technologies.
Microwave sterilization systems, by contrast, demonstrate notably improved energy efficiency metrics. Research indicates that microwave-based systems can achieve comparable sterilization results while consuming 60-75% less energy than conventional autoclaves. This efficiency stems from the direct heating mechanism of microwave technology, which targets water molecules within microorganisms rather than heating the entire chamber and contents indirectly.
Water consumption represents another significant sustainability consideration. Autoclaves typically require substantial water volumes for steam generation and cooling processes. Microwave sterilization dramatically reduces this water footprint, with some advanced systems operating with minimal or no water requirements beyond what is contained in the sterilization load itself.
Carbon footprint assessments further highlight the sustainability advantages of microwave technology. A comprehensive life cycle analysis conducted across multiple healthcare facilities demonstrated that transitioning from autoclave to microwave sterilization could reduce carbon emissions by approximately 50-65% per sterilization cycle, primarily due to reduced energy consumption and operational efficiency improvements.
Operational lifespan and maintenance requirements also factor into long-term sustainability evaluations. While autoclaves typically have longer operational lifespans (15-20 years with proper maintenance), they require more frequent maintenance interventions and component replacements. Microwave systems generally offer simplified maintenance protocols with fewer mechanical components subject to wear, potentially reducing waste generation from replacement parts.
Material compatibility considerations present additional sustainability implications. Certain medical instruments and materials cannot withstand repeated autoclave cycles, necessitating more frequent replacement. Microwave sterilization's gentler thermal profile may extend the usable lifespan of temperature-sensitive instruments, reducing material consumption and waste generation over time.
Recent technological advancements have further enhanced the sustainability profile of both systems. Next-generation autoclaves incorporate water recycling systems and improved insulation, while advanced microwave sterilizers feature precise power modulation to optimize energy utilization based on load characteristics. These innovations continue to narrow the sustainability gap between these competing technologies.
Regulatory Standards and Compliance Requirements
Sterilization processes in healthcare and laboratory settings are governed by stringent regulatory frameworks that ensure patient safety and reliable research outcomes. For autoclaves, the FDA in the United States has established specific guidelines under 21 CFR Part 820, which outlines quality system regulations for medical devices including sterilization equipment. Additionally, the Association for the Advancement of Medical Instrumentation (AAMI) has developed comprehensive standards such as ANSI/AAMI ST79 that provide detailed requirements for steam sterilization in healthcare facilities.
The European Union regulates sterilization equipment under the Medical Device Regulation (MDR 2017/745), which mandates CE marking for autoclaves used in medical settings. These regulations require validation of sterilization cycles, routine monitoring, and documentation of all sterilization processes to ensure traceability and compliance.
For microwave sterilization, the regulatory landscape is more complex and less established. While conventional microwaves are not FDA-approved for medical sterilization, specialized microwave sterilization systems must meet requirements under 21 CFR Part 880.6885 for liquid chemical sterilants. The International Electrotechnical Commission (IEC) provides standards for microwave equipment safety, but specific sterilization efficacy standards are still evolving.
Both sterilization methods must comply with ISO 17665 (Sterilization of health care products - Moist heat) and ISO 14937 (General requirements for characterization of a sterilizing agent and development, validation, and routine control of a sterilization process). These standards establish parameters for validation protocols, including biological indicators, chemical indicators, and physical monitoring systems.
Compliance verification differs significantly between the two technologies. Autoclaves typically utilize standardized biological indicators containing Geobacillus stearothermophilus spores, which provide a reliable measure of sterilization efficacy. Documentation requirements include cycle parameters (temperature, pressure, time), maintenance records, and calibration certificates. Microwave sterilization, however, lacks standardized biological indicators specifically designed for this technology, creating challenges for validation.
Healthcare facilities must also adhere to occupational safety regulations when operating sterilization equipment. OSHA standards in the United States and similar agencies worldwide mandate proper training, personal protective equipment, and safety protocols for staff working with high-temperature sterilization equipment and potentially hazardous chemicals used in some microwave sterilization processes.
The regulatory gap between established autoclave standards and emerging microwave sterilization technologies represents a significant consideration for healthcare facilities evaluating these options, particularly in highly regulated environments where documentation of validated processes is essential for accreditation and legal compliance.
The European Union regulates sterilization equipment under the Medical Device Regulation (MDR 2017/745), which mandates CE marking for autoclaves used in medical settings. These regulations require validation of sterilization cycles, routine monitoring, and documentation of all sterilization processes to ensure traceability and compliance.
For microwave sterilization, the regulatory landscape is more complex and less established. While conventional microwaves are not FDA-approved for medical sterilization, specialized microwave sterilization systems must meet requirements under 21 CFR Part 880.6885 for liquid chemical sterilants. The International Electrotechnical Commission (IEC) provides standards for microwave equipment safety, but specific sterilization efficacy standards are still evolving.
Both sterilization methods must comply with ISO 17665 (Sterilization of health care products - Moist heat) and ISO 14937 (General requirements for characterization of a sterilizing agent and development, validation, and routine control of a sterilization process). These standards establish parameters for validation protocols, including biological indicators, chemical indicators, and physical monitoring systems.
Compliance verification differs significantly between the two technologies. Autoclaves typically utilize standardized biological indicators containing Geobacillus stearothermophilus spores, which provide a reliable measure of sterilization efficacy. Documentation requirements include cycle parameters (temperature, pressure, time), maintenance records, and calibration certificates. Microwave sterilization, however, lacks standardized biological indicators specifically designed for this technology, creating challenges for validation.
Healthcare facilities must also adhere to occupational safety regulations when operating sterilization equipment. OSHA standards in the United States and similar agencies worldwide mandate proper training, personal protective equipment, and safety protocols for staff working with high-temperature sterilization equipment and potentially hazardous chemicals used in some microwave sterilization processes.
The regulatory gap between established autoclave standards and emerging microwave sterilization technologies represents a significant consideration for healthcare facilities evaluating these options, particularly in highly regulated environments where documentation of validated processes is essential for accreditation and legal compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!