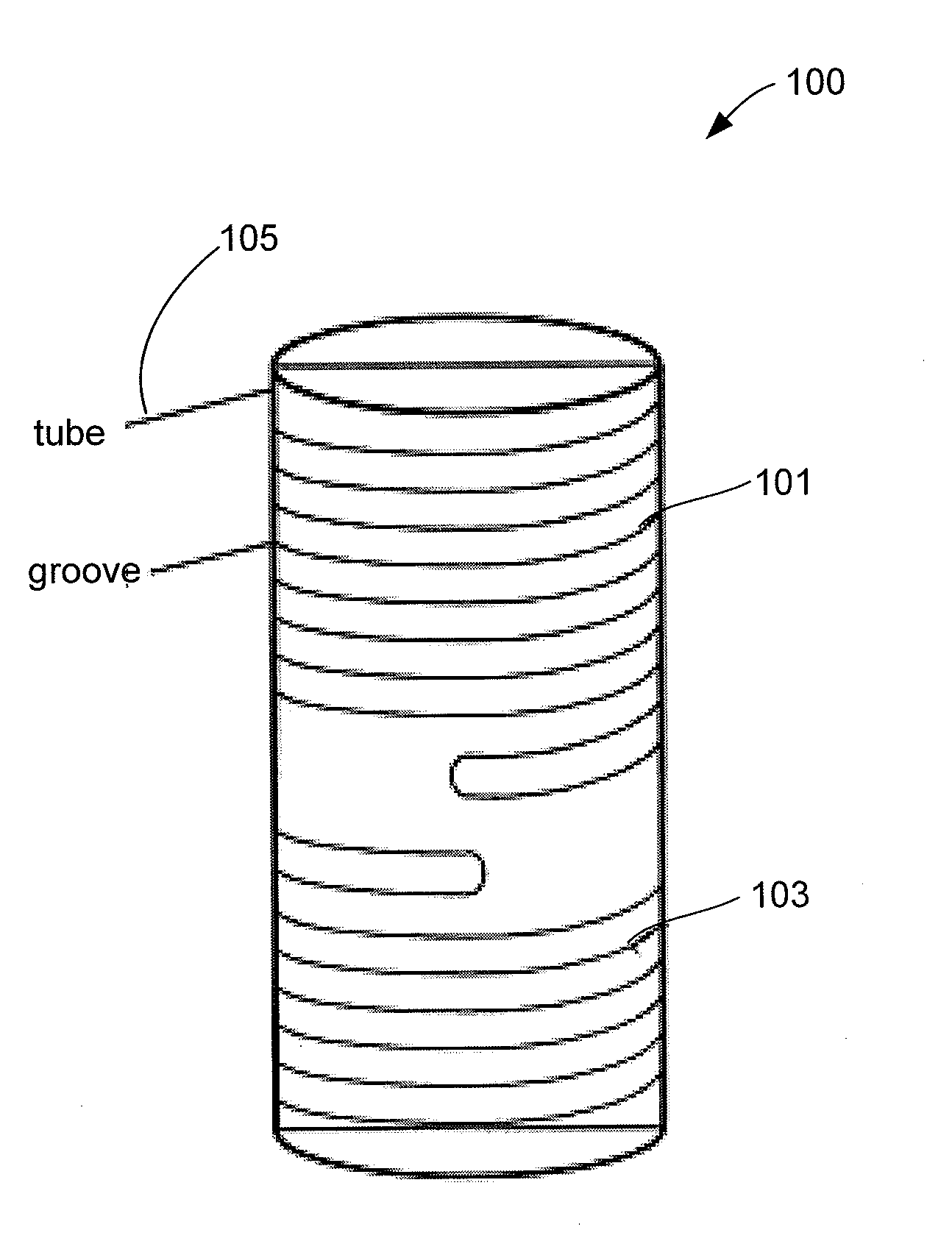
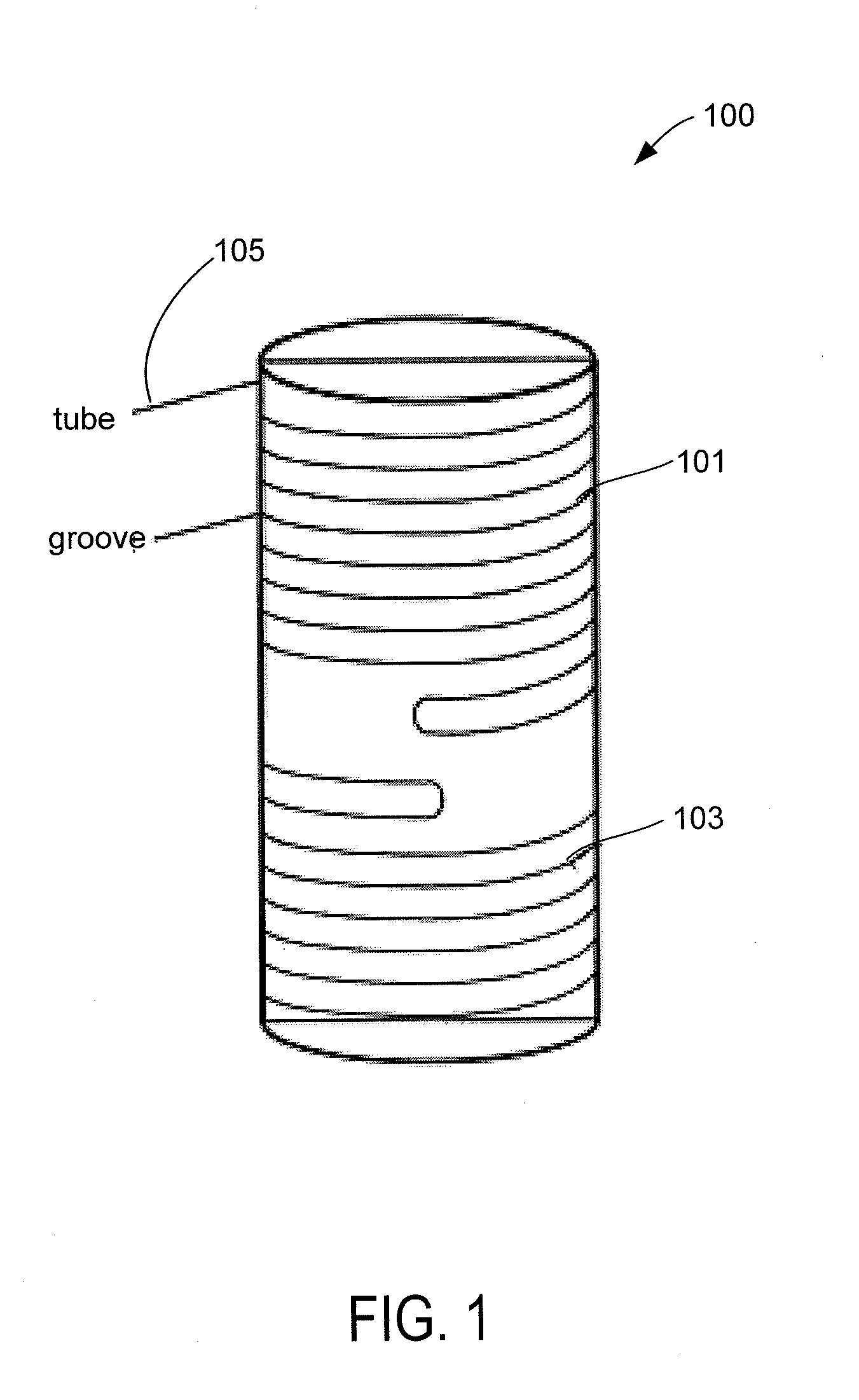
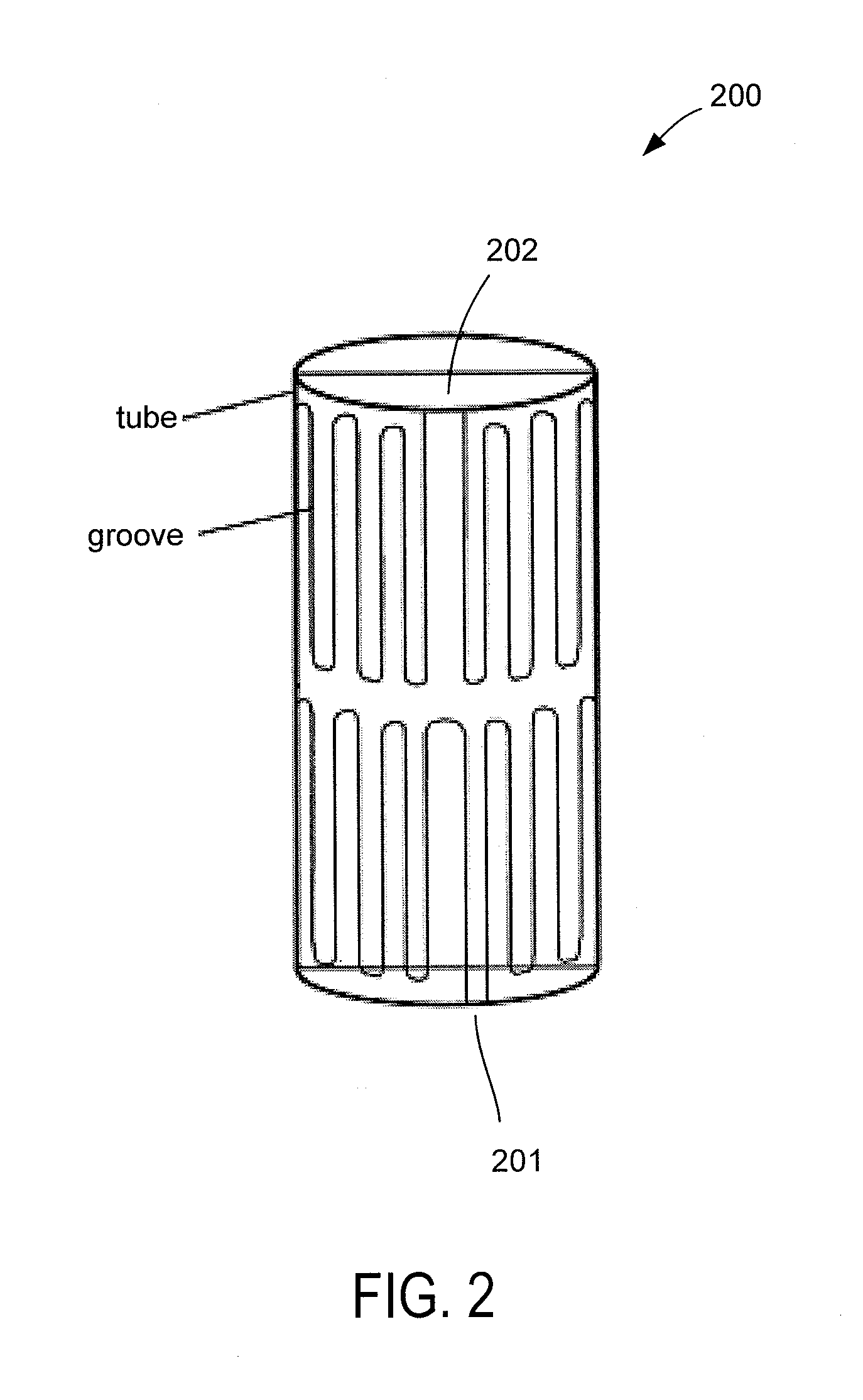
Autoclave Load Density Effects: Understanding Capacity Limits
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Sterilization Goals
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This evolution has been driven by the fundamental need to achieve complete microbial inactivation while optimizing process efficiency. Early autoclaves were simple pressure vessels with minimal controls, whereas modern systems incorporate sophisticated monitoring systems, vacuum capabilities, and precise parameter control.
The primary sterilization goal of autoclave technology remains consistent: to achieve a sterility assurance level (SAL) of 10^-6, meaning a probability of less than one in a million that a viable microorganism exists on a sterilized item. This standard is particularly critical in healthcare, pharmaceutical manufacturing, and laboratory settings where contamination can have severe consequences.
Throughout the 20th century, autoclave technology advanced from manual operation to automated systems with improved safety features and process documentation capabilities. The introduction of pre-vacuum cycles in the 1950s represented a significant breakthrough, allowing for more effective air removal and steam penetration, particularly for porous loads and hollow instruments.
Recent technological developments have focused on optimizing energy consumption, reducing cycle times, and enhancing load capacity while maintaining sterilization efficacy. The integration of microprocessor controls in the 1980s and 1990s enabled more precise monitoring of critical parameters such as temperature, pressure, and time, leading to improved process reliability and reproducibility.
Understanding load density effects has become increasingly important as healthcare facilities and manufacturers seek to maximize throughput without compromising sterilization quality. Research has demonstrated that excessive load density can create "cold spots" where steam penetration is inadequate, potentially resulting in sterilization failure. This has led to the development of specific loading configurations and validation protocols to ensure consistent results across varying load compositions.
Current sterilization goals extend beyond mere microbial inactivation to include considerations of material compatibility, environmental impact, and operational efficiency. Modern autoclave technology aims to achieve effective sterilization while minimizing water and energy consumption, reducing chemical usage, and preserving the integrity of increasingly complex medical devices and materials.
The evolution of autoclave technology continues to be shaped by regulatory requirements, such as those established by organizations like the FDA, ISO, and various pharmacopeias, which define the parameters and validation methods necessary to ensure sterilization efficacy while accommodating the challenges presented by diverse load densities and configurations.
The primary sterilization goal of autoclave technology remains consistent: to achieve a sterility assurance level (SAL) of 10^-6, meaning a probability of less than one in a million that a viable microorganism exists on a sterilized item. This standard is particularly critical in healthcare, pharmaceutical manufacturing, and laboratory settings where contamination can have severe consequences.
Throughout the 20th century, autoclave technology advanced from manual operation to automated systems with improved safety features and process documentation capabilities. The introduction of pre-vacuum cycles in the 1950s represented a significant breakthrough, allowing for more effective air removal and steam penetration, particularly for porous loads and hollow instruments.
Recent technological developments have focused on optimizing energy consumption, reducing cycle times, and enhancing load capacity while maintaining sterilization efficacy. The integration of microprocessor controls in the 1980s and 1990s enabled more precise monitoring of critical parameters such as temperature, pressure, and time, leading to improved process reliability and reproducibility.
Understanding load density effects has become increasingly important as healthcare facilities and manufacturers seek to maximize throughput without compromising sterilization quality. Research has demonstrated that excessive load density can create "cold spots" where steam penetration is inadequate, potentially resulting in sterilization failure. This has led to the development of specific loading configurations and validation protocols to ensure consistent results across varying load compositions.
Current sterilization goals extend beyond mere microbial inactivation to include considerations of material compatibility, environmental impact, and operational efficiency. Modern autoclave technology aims to achieve effective sterilization while minimizing water and energy consumption, reducing chemical usage, and preserving the integrity of increasingly complex medical devices and materials.
The evolution of autoclave technology continues to be shaped by regulatory requirements, such as those established by organizations like the FDA, ISO, and various pharmacopeias, which define the parameters and validation methods necessary to ensure sterilization efficacy while accommodating the challenges presented by diverse load densities and configurations.
Market Demand Analysis for High-Capacity Autoclaves
The global autoclave market is experiencing significant growth, driven primarily by expanding applications in aerospace, healthcare, and composite manufacturing industries. Current market valuations place the industrial autoclave sector at approximately $2.3 billion, with projections indicating a compound annual growth rate of 6.8% through 2028. This growth trajectory is particularly pronounced in the high-capacity autoclave segment, where demand is outpacing supply in several key regions.
In the aerospace sector, the transition toward composite materials for aircraft components has created substantial demand for larger autoclaves capable of processing multiple parts simultaneously. Boeing and Airbus have both increased production rates for their composite-intensive aircraft models, necessitating higher throughput in autoclave operations. This trend is expected to continue as the commercial aviation industry recovers from pandemic-related disruptions and resumes its growth trajectory.
The healthcare industry represents another significant market driver, with hospitals and medical device manufacturers seeking increased sterilization capacity. The global pandemic highlighted critical bottlenecks in medical sterilization infrastructure, prompting healthcare facilities to invest in higher-capacity autoclave systems. Market research indicates that 78% of large hospitals are planning to upgrade their sterilization capabilities within the next three years.
Composite manufacturing for renewable energy applications, particularly wind turbine blades, has emerged as a rapidly growing market segment. As wind turbine designs increase in size to improve efficiency, manufacturers require autoclaves with greater dimensions and load capacities. The renewable energy sector's expansion is projected to generate demand for approximately 150-200 new large-scale autoclaves globally by 2026.
Regional analysis reveals that Asia-Pacific represents the fastest-growing market for high-capacity autoclaves, with China and India leading industrial expansion. North America maintains the largest market share by value, primarily due to aerospace and defense applications requiring premium autoclave systems with advanced control capabilities.
Customer requirements are increasingly focused on energy efficiency and process optimization alongside raw capacity increases. Survey data from industrial users indicates that 63% consider energy consumption per processed unit as a critical factor in purchasing decisions, while 71% prioritize systems that can maximize load density without compromising quality.
The market is also witnessing growing demand for autoclave systems with enhanced monitoring capabilities and predictive maintenance features. This trend reflects broader Industry 4.0 adoption, with manufacturers seeking to optimize autoclave operations through data analytics and process automation.
In the aerospace sector, the transition toward composite materials for aircraft components has created substantial demand for larger autoclaves capable of processing multiple parts simultaneously. Boeing and Airbus have both increased production rates for their composite-intensive aircraft models, necessitating higher throughput in autoclave operations. This trend is expected to continue as the commercial aviation industry recovers from pandemic-related disruptions and resumes its growth trajectory.
The healthcare industry represents another significant market driver, with hospitals and medical device manufacturers seeking increased sterilization capacity. The global pandemic highlighted critical bottlenecks in medical sterilization infrastructure, prompting healthcare facilities to invest in higher-capacity autoclave systems. Market research indicates that 78% of large hospitals are planning to upgrade their sterilization capabilities within the next three years.
Composite manufacturing for renewable energy applications, particularly wind turbine blades, has emerged as a rapidly growing market segment. As wind turbine designs increase in size to improve efficiency, manufacturers require autoclaves with greater dimensions and load capacities. The renewable energy sector's expansion is projected to generate demand for approximately 150-200 new large-scale autoclaves globally by 2026.
Regional analysis reveals that Asia-Pacific represents the fastest-growing market for high-capacity autoclaves, with China and India leading industrial expansion. North America maintains the largest market share by value, primarily due to aerospace and defense applications requiring premium autoclave systems with advanced control capabilities.
Customer requirements are increasingly focused on energy efficiency and process optimization alongside raw capacity increases. Survey data from industrial users indicates that 63% consider energy consumption per processed unit as a critical factor in purchasing decisions, while 71% prioritize systems that can maximize load density without compromising quality.
The market is also witnessing growing demand for autoclave systems with enhanced monitoring capabilities and predictive maintenance features. This trend reflects broader Industry 4.0 adoption, with manufacturers seeking to optimize autoclave operations through data analytics and process automation.
Current Limitations in Autoclave Load Density
The current limitations in autoclave load density are primarily governed by physical, thermal, and operational constraints that impact processing efficiency and product quality. Despite advancements in autoclave technology, several critical bottlenecks persist that restrict optimal utilization of autoclave capacity.
Heat transfer dynamics represent the foremost limitation, as increasing load density creates thermal gradients within the autoclave chamber. When components are packed too densely, heat distribution becomes uneven, resulting in inconsistent curing profiles across different parts. This is particularly problematic for composite materials where precise temperature control is essential for achieving desired mechanical properties. Studies have shown that thermal shadowing effects can create temperature differentials of up to 15°C between adjacent parts in high-density loads.
Pressure distribution uniformity also deteriorates with increased load density. In aerospace and advanced composites manufacturing, uniform pressure application is crucial for void elimination and proper consolidation. Dense loading configurations can create pressure shadows where certain areas receive insufficient compaction force, leading to porosity issues and structural weaknesses in the final products.
Vacuum integrity presents another significant challenge. Higher load densities often necessitate complex vacuum bagging arrangements with multiple components sharing vacuum systems. This increases the risk of vacuum leaks and cross-contamination between parts. Industry data indicates that vacuum-related defects increase exponentially when load density exceeds 70% of theoretical maximum capacity.
Material outgassing considerations further constrain load density optimization. During cure cycles, composite materials release volatile compounds that must be effectively evacuated from the autoclave environment. Densely packed loads restrict the pathways for these volatiles to escape, potentially trapping harmful byproducts within the laminate structure and compromising mechanical performance.
Operational limitations also impact load density decisions. Increased loading complexity extends preparation time and elevates the risk of human error during setup. The economic benefits of higher density must be balanced against increased labor costs and potential quality issues. Additionally, denser loads typically require more sophisticated tooling designs to maintain part geometry during processing.
Regulatory and safety considerations impose further restrictions, particularly in industries with stringent certification requirements like aerospace and medical device manufacturing. Validation protocols often specify maximum load configurations that have been proven to deliver consistent results, limiting opportunities for density optimization without extensive requalification efforts.
Heat transfer dynamics represent the foremost limitation, as increasing load density creates thermal gradients within the autoclave chamber. When components are packed too densely, heat distribution becomes uneven, resulting in inconsistent curing profiles across different parts. This is particularly problematic for composite materials where precise temperature control is essential for achieving desired mechanical properties. Studies have shown that thermal shadowing effects can create temperature differentials of up to 15°C between adjacent parts in high-density loads.
Pressure distribution uniformity also deteriorates with increased load density. In aerospace and advanced composites manufacturing, uniform pressure application is crucial for void elimination and proper consolidation. Dense loading configurations can create pressure shadows where certain areas receive insufficient compaction force, leading to porosity issues and structural weaknesses in the final products.
Vacuum integrity presents another significant challenge. Higher load densities often necessitate complex vacuum bagging arrangements with multiple components sharing vacuum systems. This increases the risk of vacuum leaks and cross-contamination between parts. Industry data indicates that vacuum-related defects increase exponentially when load density exceeds 70% of theoretical maximum capacity.
Material outgassing considerations further constrain load density optimization. During cure cycles, composite materials release volatile compounds that must be effectively evacuated from the autoclave environment. Densely packed loads restrict the pathways for these volatiles to escape, potentially trapping harmful byproducts within the laminate structure and compromising mechanical performance.
Operational limitations also impact load density decisions. Increased loading complexity extends preparation time and elevates the risk of human error during setup. The economic benefits of higher density must be balanced against increased labor costs and potential quality issues. Additionally, denser loads typically require more sophisticated tooling designs to maintain part geometry during processing.
Regulatory and safety considerations impose further restrictions, particularly in industries with stringent certification requirements like aerospace and medical device manufacturing. Validation protocols often specify maximum load configurations that have been proven to deliver consistent results, limiting opportunities for density optimization without extensive requalification efforts.
Existing Load Optimization Strategies and Solutions
01 Optimizing load density for sterilization efficiency
The density of items loaded into an autoclave significantly impacts sterilization efficiency. Proper spacing between items ensures steam penetration and heat distribution throughout the load. Overloading can create 'cold spots' where sterilization may be incomplete, while underloading wastes energy and resources. Optimal load density configurations help achieve complete sterilization while maximizing autoclave capacity.- Optimizing load density for sterilization efficiency: The density of items loaded into an autoclave significantly impacts sterilization efficiency. Proper spacing between items ensures steam penetration and heat distribution throughout the load. Overloading can create 'cold spots' where sterilization may be incomplete, while underloading wastes energy and capacity. Optimal load density configurations help achieve complete sterilization while maximizing throughput and energy efficiency.
- Load monitoring and density measurement systems: Advanced systems for monitoring and measuring autoclave load density employ sensors and imaging technologies to assess load distribution. These systems can detect improper loading patterns, calculate actual load density, and provide feedback to operators. Some implementations use weight sensors, optical recognition, or radiofrequency identification to track items and their positioning within the autoclave chamber, ensuring optimal density is maintained for effective sterilization.
- Load density standards and guidelines for specific materials: Different materials and items require specific load density considerations in autoclave processing. Porous materials may need lower density loading to allow steam penetration, while dense materials might require specific arrangements to ensure heat transfer. Industry standards and guidelines establish recommended load densities for various material types, including medical instruments, laboratory equipment, and manufacturing components, to ensure sterilization efficacy while maintaining material integrity.
- Automated loading systems for optimal density control: Automated loading systems help achieve consistent and optimal load density in autoclaves. These systems use robotic arms, conveyor mechanisms, or specialized loading carts designed to position items with precise spacing. The automation ensures reproducible loading patterns that maximize chamber utilization while maintaining required spacing for steam circulation. Some systems incorporate pre-programmed loading configurations for different item types to optimize both density and sterilization effectiveness.
- Impact of load density on cycle parameters and validation: Load density directly affects autoclave cycle parameters including temperature distribution, pressure requirements, and cycle duration. Higher density loads typically require longer cycle times to achieve complete sterilization. Validation protocols must account for maximum load density scenarios to ensure sterilization efficacy under worst-case conditions. Testing with thermocouples placed throughout loads of varying densities helps establish appropriate cycle parameters and ensures that sterilization standards are consistently met regardless of loading variations.
02 Load density monitoring systems
Advanced monitoring systems can be used to assess and control autoclave load density. These systems employ sensors to measure parameters such as steam penetration, temperature distribution, and pressure throughout the load. Real-time monitoring allows for adjustments to ensure sterilization efficacy despite varying load densities. Some systems include alarms or automatic shutdown features if load density exceeds recommended parameters.Expand Specific Solutions03 Load configuration and arrangement techniques
Specific techniques for arranging items within an autoclave can optimize load density. These include using specialized racks, trays, or containers designed to maintain proper spacing between items. Positioning items to allow for vertical steam flow and avoiding stacking of impermeable materials improves sterilization effectiveness. Some methods involve categorizing items by material type and sterilization requirements to determine optimal arrangement patterns.Expand Specific Solutions04 Relationship between load density and sterilization parameters
Load density directly affects critical sterilization parameters such as temperature, pressure, and cycle duration. Higher density loads typically require longer cycle times or higher temperatures to achieve complete sterilization. Mathematical models and algorithms can be used to calculate the optimal sterilization parameters based on load density measurements. Understanding this relationship helps in developing efficient sterilization protocols for various load configurations.Expand Specific Solutions05 Industry-specific load density standards
Different industries have established specific standards for autoclave load density based on their particular requirements. Medical facilities follow strict guidelines for sterilizing surgical instruments, while pharmaceutical manufacturing has different standards for product sterilization. Food processing industries have their own load density requirements to ensure food safety. These standards often specify maximum load weights, configurations, and validation procedures to ensure consistent sterilization results.Expand Specific Solutions
Leading Manufacturers and Industry Competition
The autoclave load density market is currently in a growth phase, with increasing demand across aerospace, medical, and automotive sectors. Market size is expanding due to rising composite material usage and sterilization needs. Technologically, the field shows varying maturity levels, with aerospace leaders like Boeing and Airbus demonstrating advanced capabilities in managing load density effects for composite curing. Medical equipment manufacturers including Becton Dickinson, Stryker, and Olympus have developed sophisticated protocols for sterilization load optimization. Companies like LBBC Beechwood and Aerothermal Technology Group offer specialized autoclave solutions addressing capacity limits, while automotive manufacturers such as Toyota are increasingly adopting autoclave processes for advanced materials production.
The Boeing Co.
Technical Solution: Boeing has developed advanced autoclave optimization systems for composite manufacturing that precisely control load density parameters. Their approach incorporates computational fluid dynamics (CFD) modeling to simulate heat transfer and cure kinetics within densely packed autoclave loads. Boeing's proprietary AGATE (Advanced Grid Autoclave Thermal Engineering) system enables real-time monitoring of temperature gradients across complex part geometries, allowing for dynamic adjustment of cure cycles based on actual part temperatures rather than autoclave air temperature. This system incorporates machine learning algorithms that analyze historical cure data to predict optimal load configurations, maximizing throughput while maintaining quality standards. Boeing has demonstrated that their system can increase autoclave capacity utilization by up to 25% while reducing cure cycle time variations.
Strengths: Superior computational modeling capabilities allow for precise prediction of thermal behavior in complex load configurations; extensive historical data from aerospace applications provides robust validation. Weaknesses: System optimization is primarily focused on aerospace-grade composites and may require significant adaptation for other material systems; high implementation cost limits accessibility for smaller manufacturers.
Airbus Operations SAS
Technical Solution: Airbus has pioneered the SMART (Systematic Monitoring and Adaptive Regulation Technology) autoclave system that addresses load density challenges through innovative sensor networks and thermal mapping. Their approach integrates over 200 wireless sensors throughout the autoclave chamber to create 3D thermal profiles of complex load arrangements. The system employs predictive thermal modeling that accounts for part geometry, material properties, and spatial arrangement to optimize load configurations. Airbus has developed proprietary algorithms that can predict thermal shadowing effects in densely packed loads and automatically adjust heating parameters to ensure uniform curing. Their research has demonstrated that optimized load configurations can increase autoclave capacity by up to 30% while maintaining strict aerospace quality requirements. The system also incorporates digital twin technology to simulate different loading scenarios before physical implementation.
Strengths: Comprehensive sensor network provides unparalleled visibility into thermal conditions throughout the autoclave; integration with digital twin technology enables virtual testing of loading configurations. Weaknesses: System complexity requires specialized training and maintenance; high initial investment cost may be prohibitive for smaller operations.
Critical Research on Steam Penetration and Heat Transfer
Compound internally-heated high-pressure apparatus for solvothermal crystal growth
PatentWO2024020513A1
Innovation
- A compound internally-heated high-pressure apparatus with a cylindrical enclosure, a primary liner, load-bearing annular insulating members, and heating elements, allowing for radial and axial support, enabling pressures up to 500 MPa and temperatures between 200 and 900 degrees Celsius, while reducing costs and improving scalability.
Heater device and method for high pressure processing of crystalline materials
PatentInactiveUS20090320745A1
Innovation
- A scalable heater design with multiple heating elements and a dense inner tube member, combined with insulating material, forms a cylindrical structure that is electrically isolated and capable of operating within a high-pressure apparatus, maintaining structural integrity and efficiency by minimizing voids and gaps, allowing for operation at pressures up to 2 GPa and temperatures up to 1200°C.
Validation Protocols for Autoclave Load Configurations
Validation protocols for autoclave load configurations must be systematically developed to ensure sterilization efficacy while optimizing operational efficiency. These protocols begin with comprehensive load mapping studies that identify cold spots and temperature distribution patterns within various load densities. Critical parameters such as temperature uniformity, steam penetration, and heat transfer rates must be measured across different load configurations to establish validated boundaries for safe operation.
The protocol development process typically involves a three-phase approach: initial qualification testing with empty chamber verification, followed by representative load testing with biological and chemical indicators, and finally performance qualification with maximum challenge loads. Each phase must generate statistically significant data to support load configuration guidelines that balance processing capacity with sterilization assurance.
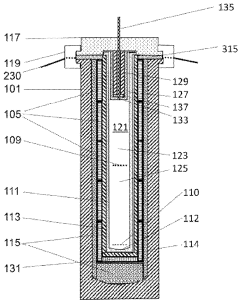
Temperature mapping using calibrated thermocouples positioned at strategic locations throughout the load is essential for protocol validation. A minimum of 10-12 measurement points is recommended for standard autoclaves, with additional sensors required for larger units or complex load arrangements. The collected temperature profiles must demonstrate that all points within the load reach and maintain the specified sterilization parameters (typically 121°C for at least 15 minutes or 134°C for 3-4 minutes for medical applications).
Load density validation requires establishing clear correlations between packing density metrics and sterilization efficacy. Modern protocols incorporate computational fluid dynamics (CFD) modeling to predict steam flow patterns and identify potential dead zones before physical testing. This approach significantly reduces the number of experimental runs required while improving protocol robustness. The validation data should establish maximum safe load densities expressed in kg/m³ or as percentage of chamber volume utilization.
Documentation requirements for validated load configurations must include detailed loading diagrams, photographs of reference configurations, weight and density specifications, and clear instructions for load arrangement. These documents become part of the master validation record and serve as operational guidelines for autoclave operators. Regular revalidation schedules must be established based on risk assessment, typically ranging from annual verification to complete revalidation every three years.
Acceptance criteria must be clearly defined in the validation protocol, including minimum temperature thresholds, maximum acceptable temperature variation across the load, and biological indicator inactivation requirements. Statistical analysis of validation data should employ appropriate methodologies such as process capability indices (Cpk) to demonstrate that the process consistently meets these criteria with adequate safety margins.
The protocol development process typically involves a three-phase approach: initial qualification testing with empty chamber verification, followed by representative load testing with biological and chemical indicators, and finally performance qualification with maximum challenge loads. Each phase must generate statistically significant data to support load configuration guidelines that balance processing capacity with sterilization assurance.
Temperature mapping using calibrated thermocouples positioned at strategic locations throughout the load is essential for protocol validation. A minimum of 10-12 measurement points is recommended for standard autoclaves, with additional sensors required for larger units or complex load arrangements. The collected temperature profiles must demonstrate that all points within the load reach and maintain the specified sterilization parameters (typically 121°C for at least 15 minutes or 134°C for 3-4 minutes for medical applications).
Load density validation requires establishing clear correlations between packing density metrics and sterilization efficacy. Modern protocols incorporate computational fluid dynamics (CFD) modeling to predict steam flow patterns and identify potential dead zones before physical testing. This approach significantly reduces the number of experimental runs required while improving protocol robustness. The validation data should establish maximum safe load densities expressed in kg/m³ or as percentage of chamber volume utilization.
Documentation requirements for validated load configurations must include detailed loading diagrams, photographs of reference configurations, weight and density specifications, and clear instructions for load arrangement. These documents become part of the master validation record and serve as operational guidelines for autoclave operators. Regular revalidation schedules must be established based on risk assessment, typically ranging from annual verification to complete revalidation every three years.
Acceptance criteria must be clearly defined in the validation protocol, including minimum temperature thresholds, maximum acceptable temperature variation across the load, and biological indicator inactivation requirements. Statistical analysis of validation data should employ appropriate methodologies such as process capability indices (Cpk) to demonstrate that the process consistently meets these criteria with adequate safety margins.
Regulatory Standards and Compliance Requirements
Regulatory compliance in autoclave operations is governed by a comprehensive framework of standards that vary across industries and jurisdictions. For medical applications, the FDA's Quality System Regulation (21 CFR Part 820) and ISO 17665 establish specific requirements for steam sterilization validation, including load density parameters. These standards mandate that healthcare facilities demonstrate consistent sterility assurance levels across varying load configurations, with particular emphasis on worst-case scenarios involving maximum density loads.
In pharmaceutical manufacturing, GMP regulations (21 CFR Parts 210 and 211) require validation of sterilization processes with documented evidence that load density variations do not compromise product quality or safety. The FDA's "Guideline on General Principles of Process Validation" specifically addresses the need to establish acceptable ranges for critical parameters such as load density and configuration.
For aerospace and composite manufacturing, industry standards like AMS 2750 and NADCAP requirements define precise specifications for temperature uniformity and pressure consistency across autoclave loads. These standards typically require more stringent documentation of load density effects on cure cycles and material properties, with tolerance ranges becoming increasingly narrow for critical aerospace components.
International standards bodies have established harmonized approaches to load density validation. The ISO 11134 and EN 285 standards provide detailed methodologies for qualifying autoclave performance under various loading conditions, including requirements for temperature mapping studies that must demonstrate uniform heat distribution regardless of load density variations.
Regulatory inspections frequently focus on documented evidence of load density validation studies. Manufacturers must maintain records demonstrating that their autoclave processes remain effective at maximum approved densities, with clear operational limits established through scientific testing. Non-compliance with these requirements can result in regulatory actions ranging from Form 483 observations to product recalls.
Recent regulatory trends indicate increasing scrutiny of load density validation, particularly for critical applications. Authorities now expect risk-based approaches that quantify the relationship between load density and critical quality attributes, with statistical analysis demonstrating process capability across the operational range. This shift reflects growing regulatory emphasis on process understanding rather than simple adherence to fixed parameters.
Compliance strategies must therefore incorporate comprehensive load density studies during initial validation, with ongoing monitoring programs to detect potential drift in process performance as equipment ages or product mixes evolve.
In pharmaceutical manufacturing, GMP regulations (21 CFR Parts 210 and 211) require validation of sterilization processes with documented evidence that load density variations do not compromise product quality or safety. The FDA's "Guideline on General Principles of Process Validation" specifically addresses the need to establish acceptable ranges for critical parameters such as load density and configuration.
For aerospace and composite manufacturing, industry standards like AMS 2750 and NADCAP requirements define precise specifications for temperature uniformity and pressure consistency across autoclave loads. These standards typically require more stringent documentation of load density effects on cure cycles and material properties, with tolerance ranges becoming increasingly narrow for critical aerospace components.
International standards bodies have established harmonized approaches to load density validation. The ISO 11134 and EN 285 standards provide detailed methodologies for qualifying autoclave performance under various loading conditions, including requirements for temperature mapping studies that must demonstrate uniform heat distribution regardless of load density variations.
Regulatory inspections frequently focus on documented evidence of load density validation studies. Manufacturers must maintain records demonstrating that their autoclave processes remain effective at maximum approved densities, with clear operational limits established through scientific testing. Non-compliance with these requirements can result in regulatory actions ranging from Form 483 observations to product recalls.
Recent regulatory trends indicate increasing scrutiny of load density validation, particularly for critical applications. Authorities now expect risk-based approaches that quantify the relationship between load density and critical quality attributes, with statistical analysis demonstrating process capability across the operational range. This shift reflects growing regulatory emphasis on process understanding rather than simple adherence to fixed parameters.
Compliance strategies must therefore incorporate comprehensive load density studies during initial validation, with ongoing monitoring programs to detect potential drift in process performance as equipment ages or product mixes evolve.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!