How to Implement Autoclave Upgrades with Minimal Disruption
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Upgrade Objectives
Autoclave technology has evolved significantly since its inception in the early 20th century, transitioning from basic pressure vessels to sophisticated computer-controlled systems. The earliest autoclaves were simple steam chambers used primarily in medical sterilization, while modern industrial autoclaves incorporate advanced materials, precise temperature and pressure controls, and automated operation systems. This evolution has been driven by increasing demands for efficiency, safety, and process optimization across various industries including aerospace, composites manufacturing, and medical device production.
The past decade has witnessed particularly rapid advancement in autoclave technology, with the integration of Industry 4.0 principles. Key developments include real-time monitoring capabilities, predictive maintenance systems, energy recovery mechanisms, and enhanced process control algorithms. These innovations have collectively improved cycle times, reduced energy consumption, and enhanced product quality while maintaining strict compliance with regulatory standards.
Current upgrade objectives focus on implementing these technological advancements with minimal operational disruption. Primary goals include improving energy efficiency by 15-30%, reducing cycle times by up to 25%, enhancing process reliability through predictive maintenance, and implementing advanced data analytics for quality control. Additionally, upgrades aim to extend equipment lifespan, reduce maintenance costs, and ensure compliance with evolving industry standards and environmental regulations.
The technical trajectory suggests continued movement toward more intelligent systems with greater automation, improved energy efficiency, and enhanced process control. Future autoclaves will likely incorporate more sophisticated sensors, machine learning algorithms for process optimization, and improved human-machine interfaces. The integration of digital twins for simulation and predictive modeling represents another significant trend in autoclave technology evolution.
Successful upgrade implementation requires balancing immediate operational needs with long-term technological objectives. This necessitates a phased approach that prioritizes critical improvements while minimizing production downtime. The ideal upgrade strategy addresses not only hardware modifications but also software enhancements, operator training, and process validation to ensure seamless transition and immediate realization of benefits.
Understanding this evolutionary context is essential for developing effective upgrade strategies that align with industry trends while addressing specific operational requirements. By recognizing both historical development patterns and future trajectories, organizations can make informed decisions about which technological improvements will deliver maximum value with minimal disruption to existing production workflows.
The past decade has witnessed particularly rapid advancement in autoclave technology, with the integration of Industry 4.0 principles. Key developments include real-time monitoring capabilities, predictive maintenance systems, energy recovery mechanisms, and enhanced process control algorithms. These innovations have collectively improved cycle times, reduced energy consumption, and enhanced product quality while maintaining strict compliance with regulatory standards.
Current upgrade objectives focus on implementing these technological advancements with minimal operational disruption. Primary goals include improving energy efficiency by 15-30%, reducing cycle times by up to 25%, enhancing process reliability through predictive maintenance, and implementing advanced data analytics for quality control. Additionally, upgrades aim to extend equipment lifespan, reduce maintenance costs, and ensure compliance with evolving industry standards and environmental regulations.
The technical trajectory suggests continued movement toward more intelligent systems with greater automation, improved energy efficiency, and enhanced process control. Future autoclaves will likely incorporate more sophisticated sensors, machine learning algorithms for process optimization, and improved human-machine interfaces. The integration of digital twins for simulation and predictive modeling represents another significant trend in autoclave technology evolution.
Successful upgrade implementation requires balancing immediate operational needs with long-term technological objectives. This necessitates a phased approach that prioritizes critical improvements while minimizing production downtime. The ideal upgrade strategy addresses not only hardware modifications but also software enhancements, operator training, and process validation to ensure seamless transition and immediate realization of benefits.
Understanding this evolutionary context is essential for developing effective upgrade strategies that align with industry trends while addressing specific operational requirements. By recognizing both historical development patterns and future trajectories, organizations can make informed decisions about which technological improvements will deliver maximum value with minimal disruption to existing production workflows.
Market Demand for Minimally Disruptive Sterilization Solutions
The global sterilization equipment market has witnessed significant growth in recent years, with the autoclave segment maintaining a dominant position. Current market valuations exceed $3 billion, with projections indicating a compound annual growth rate of 7.2% through 2028. This growth is primarily driven by stringent regulatory requirements in healthcare facilities, pharmaceutical manufacturing, and research laboratories, where maintaining sterile environments is critical for operational success and compliance.
Healthcare facilities, particularly hospitals and surgical centers, represent the largest market segment demanding minimally disruptive autoclave upgrades. These institutions operate under intense pressure to maximize facility uptime while maintaining strict sterilization standards. Traditional autoclave replacement or major overhauls typically require 3-7 days of downtime, creating substantial operational challenges and potential revenue losses estimated at $10,000-$30,000 per day for medium-sized facilities.
Pharmaceutical and biotechnology companies form another significant market segment, where production continuity is paramount. These organizations often operate under strict Good Manufacturing Practice (GMP) regulations, making sterilization equipment reliability essential. Market research indicates that 78% of pharmaceutical manufacturers prioritize solutions that can be implemented without disrupting production schedules, with 65% willing to pay premium prices for upgrades that can be completed during scheduled maintenance windows.
Research laboratories and academic institutions represent a growing market segment with unique needs. These facilities typically operate with limited budgets but require high-performance sterilization capabilities. Survey data shows that 82% of laboratory managers consider equipment downtime as a critical factor when evaluating autoclave upgrades, with 71% expressing strong interest in modular solutions that can be implemented incrementally.
The food and beverage industry has emerged as an expanding market for advanced sterilization solutions, particularly as safety regulations tighten globally. Companies in this sector report average losses of $50,000-$100,000 per day when production lines are halted for equipment upgrades, creating strong demand for solutions that minimize operational disruption.
Regional market analysis reveals that North America and Europe currently lead in adoption of minimally disruptive autoclave solutions, driven by higher regulatory standards and labor costs. However, the Asia-Pacific region shows the fastest growth rate at 9.8%, as manufacturing facilities increasingly adopt international quality standards while facing intense production pressures that make operational disruptions particularly costly.
Customer feedback consistently highlights three primary demands: reduced implementation time, minimal impact on adjacent operations, and rapid return to full operational capacity. Market surveys indicate that solutions offering implementation within 24-48 hours command premium pricing, with 68% of decision-makers ranking "minimal disruption" as their top consideration when evaluating autoclave upgrade options.
Healthcare facilities, particularly hospitals and surgical centers, represent the largest market segment demanding minimally disruptive autoclave upgrades. These institutions operate under intense pressure to maximize facility uptime while maintaining strict sterilization standards. Traditional autoclave replacement or major overhauls typically require 3-7 days of downtime, creating substantial operational challenges and potential revenue losses estimated at $10,000-$30,000 per day for medium-sized facilities.
Pharmaceutical and biotechnology companies form another significant market segment, where production continuity is paramount. These organizations often operate under strict Good Manufacturing Practice (GMP) regulations, making sterilization equipment reliability essential. Market research indicates that 78% of pharmaceutical manufacturers prioritize solutions that can be implemented without disrupting production schedules, with 65% willing to pay premium prices for upgrades that can be completed during scheduled maintenance windows.
Research laboratories and academic institutions represent a growing market segment with unique needs. These facilities typically operate with limited budgets but require high-performance sterilization capabilities. Survey data shows that 82% of laboratory managers consider equipment downtime as a critical factor when evaluating autoclave upgrades, with 71% expressing strong interest in modular solutions that can be implemented incrementally.
The food and beverage industry has emerged as an expanding market for advanced sterilization solutions, particularly as safety regulations tighten globally. Companies in this sector report average losses of $50,000-$100,000 per day when production lines are halted for equipment upgrades, creating strong demand for solutions that minimize operational disruption.
Regional market analysis reveals that North America and Europe currently lead in adoption of minimally disruptive autoclave solutions, driven by higher regulatory standards and labor costs. However, the Asia-Pacific region shows the fastest growth rate at 9.8%, as manufacturing facilities increasingly adopt international quality standards while facing intense production pressures that make operational disruptions particularly costly.
Customer feedback consistently highlights three primary demands: reduced implementation time, minimal impact on adjacent operations, and rapid return to full operational capacity. Market surveys indicate that solutions offering implementation within 24-48 hours command premium pricing, with 68% of decision-makers ranking "minimal disruption" as their top consideration when evaluating autoclave upgrade options.
Current Autoclave Upgrade Challenges and Constraints
Autoclave upgrades present significant operational challenges for manufacturing facilities, particularly in industries where these pressure vessels are critical to production processes. The primary constraint when implementing upgrades is minimizing production downtime, as each day an autoclave remains offline can result in substantial revenue losses, often exceeding $50,000-$100,000 per day in high-volume manufacturing environments.
Technical constraints further complicate upgrade initiatives. Many existing autoclaves operate with legacy control systems that lack modern communication protocols and digital interfaces. This incompatibility creates integration challenges when attempting to incorporate new sensors, control systems, or data collection capabilities. Additionally, physical space limitations within facilities often restrict the scale and scope of possible modifications, particularly when dealing with large industrial autoclaves that may be partially built into facility structures.
Regulatory compliance represents another significant hurdle. Autoclaves in medical, aerospace, and food processing industries must adhere to strict regulatory standards (FDA, FAA, ASME, etc.). Any modifications must be validated and documented to maintain compliance, adding layers of complexity and time requirements to upgrade projects. The validation process alone can extend project timelines by weeks or months.
Supply chain dependencies create additional constraints, as specialized components for industrial autoclaves often have extended lead times ranging from 8-16 weeks. This necessitates precise planning and potentially maintaining redundant systems during transition periods. The specialized nature of autoclave technology also creates workforce challenges, as technicians with expertise in both legacy and modern autoclave systems are increasingly scarce.
Budget limitations frequently force difficult trade-offs between comprehensive upgrades and targeted improvements. The high cost of complete system replacements (often $500,000 to several million dollars) pushes many organizations toward phased approaches, which can create temporary compatibility issues between new and legacy components.
Safety considerations add another dimension of complexity. Autoclaves operate under high pressure and temperature conditions, making any modification potentially hazardous if not properly engineered and tested. This necessitates comprehensive risk assessments and testing protocols that extend project timelines but cannot be circumvented.
Energy efficiency requirements increasingly influence upgrade decisions, as older autoclaves typically consume significant amounts of energy and utilities. Balancing improved efficiency with minimal disruption requires sophisticated engineering approaches that optimize system performance while working within existing infrastructure constraints.
Technical constraints further complicate upgrade initiatives. Many existing autoclaves operate with legacy control systems that lack modern communication protocols and digital interfaces. This incompatibility creates integration challenges when attempting to incorporate new sensors, control systems, or data collection capabilities. Additionally, physical space limitations within facilities often restrict the scale and scope of possible modifications, particularly when dealing with large industrial autoclaves that may be partially built into facility structures.
Regulatory compliance represents another significant hurdle. Autoclaves in medical, aerospace, and food processing industries must adhere to strict regulatory standards (FDA, FAA, ASME, etc.). Any modifications must be validated and documented to maintain compliance, adding layers of complexity and time requirements to upgrade projects. The validation process alone can extend project timelines by weeks or months.
Supply chain dependencies create additional constraints, as specialized components for industrial autoclaves often have extended lead times ranging from 8-16 weeks. This necessitates precise planning and potentially maintaining redundant systems during transition periods. The specialized nature of autoclave technology also creates workforce challenges, as technicians with expertise in both legacy and modern autoclave systems are increasingly scarce.
Budget limitations frequently force difficult trade-offs between comprehensive upgrades and targeted improvements. The high cost of complete system replacements (often $500,000 to several million dollars) pushes many organizations toward phased approaches, which can create temporary compatibility issues between new and legacy components.
Safety considerations add another dimension of complexity. Autoclaves operate under high pressure and temperature conditions, making any modification potentially hazardous if not properly engineered and tested. This necessitates comprehensive risk assessments and testing protocols that extend project timelines but cannot be circumvented.
Energy efficiency requirements increasingly influence upgrade decisions, as older autoclaves typically consume significant amounts of energy and utilities. Balancing improved efficiency with minimal disruption requires sophisticated engineering approaches that optimize system performance while working within existing infrastructure constraints.
Contemporary Upgrade Implementation Methodologies
01 Autoclave system upgrades with minimal disruption
Implementing upgrades to autoclave systems while minimizing operational disruption involves strategic planning and modular design approaches. These methods allow for incremental improvements to sterilization equipment without requiring complete system shutdowns. Techniques include parallel installation of new components, phased implementation strategies, and the use of temporary bypass systems to maintain critical operations during upgrade periods.- Autoclave system upgrades and modifications: Autoclave systems can be upgraded or modified to improve their performance, efficiency, and functionality. These upgrades may include enhancements to the control systems, pressure management, temperature regulation, and overall operational capabilities. Such modifications can minimize disruption to sterilization processes while improving the reliability and effectiveness of the autoclave equipment.
- Software updates and system maintenance with minimal disruption: Methods for implementing software updates and system maintenance in autoclave control systems with minimal operational disruption. These approaches include techniques for seamless updates, background installations, and scheduled maintenance procedures that allow for continuous operation or reduced downtime of critical sterilization equipment.
- Fault detection and recovery mechanisms: Systems and methods for detecting faults or failures in autoclave operations and implementing recovery mechanisms to minimize disruption. These technologies include monitoring systems, diagnostic tools, and automated recovery procedures that can identify potential issues before they cause significant downtime and implement corrective actions to maintain operational continuity.
- Remote monitoring and control systems: Implementation of remote monitoring and control capabilities for autoclaves to reduce operational disruptions. These systems allow for real-time monitoring, remote diagnostics, and control of autoclave functions, enabling quick response to potential issues and reducing the need for physical presence, thereby minimizing disruption to sterilization processes.
- Scheduling and resource optimization for autoclave operations: Methods and systems for optimizing the scheduling and resource allocation of autoclave operations to minimize disruption. These approaches include algorithms for efficient batch processing, predictive maintenance scheduling, and resource allocation strategies that maximize throughput while reducing downtime and operational interruptions.
02 Software updates for autoclave control systems
Software-based approaches for upgrading autoclave control systems enable functionality improvements without hardware replacement. These solutions include remote firmware updates, software patches that enhance monitoring capabilities, and digital interface upgrades that improve user experience. Advanced software implementations can provide predictive maintenance features and optimize sterilization cycles while maintaining system availability.Expand Specific Solutions03 Redundancy systems for continuous autoclave operation
Redundancy-based solutions ensure continuous autoclave operation during upgrade processes. These systems incorporate backup components, failover mechanisms, and parallel processing capabilities that maintain sterilization services while primary systems undergo modifications. Designs featuring hot-swappable modules allow for component replacement without complete system shutdown, significantly reducing operational disruption.Expand Specific Solutions04 Modular autoclave component replacement strategies
Modular design approaches enable targeted replacement of autoclave components without complete system overhauls. These strategies involve standardized interfaces between subsystems, allowing individual modules to be upgraded independently. Implementation techniques include quick-connect fittings, plug-and-play electronic components, and standardized communication protocols that facilitate seamless integration of new technologies into existing autoclave infrastructure.Expand Specific Solutions05 Automated testing and validation for autoclave upgrades
Automated testing and validation procedures minimize downtime during autoclave system upgrades. These methodologies incorporate simulation-based verification, automated regression testing, and real-time monitoring during implementation phases. Advanced validation protocols ensure that upgraded systems meet regulatory requirements and performance specifications while reducing the time required for qualification and return to service.Expand Specific Solutions
Leading Manufacturers and Service Providers in Autoclave Upgrades
The autoclave upgrade market is currently in a growth phase, characterized by increasing demand for efficient sterilization solutions across pharmaceutical, food processing, and manufacturing sectors. The global market size is estimated to exceed $2 billion, driven by stringent regulatory requirements and technological advancements. Leading players like Siemens AG and Steriflow SAS are pioneering minimally disruptive upgrade solutions through modular designs and predictive maintenance technologies. Companies such as SANYO Electric and SIG Combibloc Systems are focusing on retrofit solutions that enable phased implementation, while Neusoft Corp. is developing advanced control systems for seamless integration. The competitive landscape is evolving with specialized players like Shandong Shuangchao Biological Equipment Technology emerging with niche expertise in high-pressure sterilization technologies.
Siemens AG
Technical Solution: Siemens has developed a comprehensive Autoclave Modernization Program that focuses on minimizing operational disruption through phased implementation. Their approach includes pre-fabricated modular components that can be installed during planned maintenance windows, reducing downtime by up to 40% compared to traditional methods. The system incorporates Digital Twin technology to simulate upgrades before physical implementation, allowing for virtual testing and optimization. Siemens' solution features remote monitoring capabilities through their MindSphere IoT platform, enabling predictive maintenance and real-time performance analysis. The upgrade process includes temporary bypass systems that maintain partial production capacity during critical installation phases. Additionally, Siemens provides specialized training programs for operators to quickly adapt to new control systems, further reducing the transition period impact.
Strengths: Extensive industrial automation expertise and global service network provide comprehensive support throughout the upgrade process. Their Digital Twin technology significantly reduces implementation risks. Weaknesses: Higher initial investment compared to simpler solutions, and potential vendor lock-in with proprietary control systems that may limit future flexibility.
Steriflow SAS
Technical Solution: Steriflow SAS has pioneered a specialized "Hot-Swap" autoclave upgrade methodology designed specifically for minimal production disruption in pharmaceutical and food processing industries. Their approach centers on a proprietary parallel installation technique where new autoclave components are pre-assembled and tested adjacent to existing systems. The company's SmartControl upgrade package allows for sequential migration of control systems during non-production hours, maintaining full production capacity during working shifts. Steriflow's solution includes temporary sterilization capacity through mobile autoclave units that can be deployed during critical upgrade phases, ensuring continuous production capability. Their modular design philosophy enables targeted upgrades of specific components (control systems, steam generators, or door mechanisms) without complete system replacement. The company also provides comprehensive validation packages that accelerate regulatory approval processes following upgrades, reducing compliance-related downtime by approximately 30%.
Strengths: Specialized focus on autoclave technology provides deep domain expertise and purpose-built solutions for minimal disruption upgrades. Their mobile temporary capacity solutions ensure production continuity. Weaknesses: More limited global service network compared to larger industrial automation companies, potentially causing delays in support for international installations.
Critical Technologies for Seamless Autoclave Transitions
Flexible shape low volume autoclave and method of using it
PatentInactiveGB2464228A
Innovation
- A flexible shape low volume autoclave design featuring an elongated pressure vessel with a circumferential joint that creates an angle between portions, allowing for reduced internal volume while maintaining the ability to pressurize and heat large parts, along with a transport system for opening and closing the vessel to facilitate part insertion and removal.
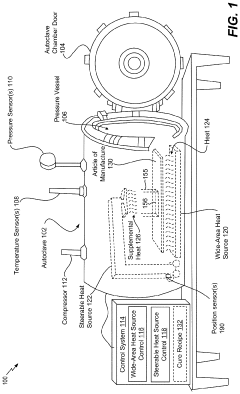
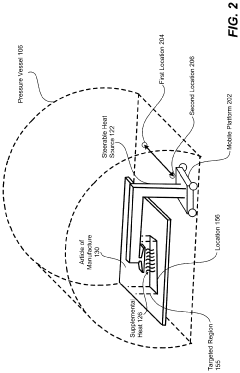
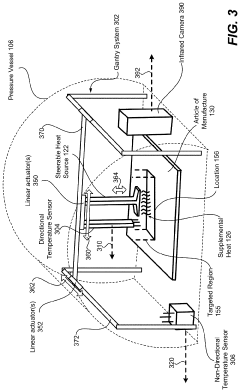
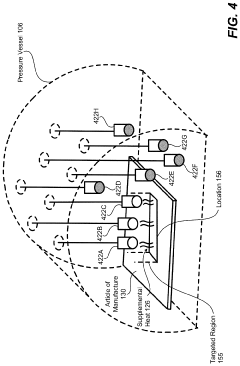
Steerable heat source
PatentActiveUS20200307035A1
Innovation
- A steerable heat source is integrated within the autoclave, coupled with a control system that directs supplemental heat to targeted regions using temperature sensors and a compressor to regulate pressure, ensuring precise temperature control and uniform heating.
Risk Management Strategies for Autoclave Upgrade Projects
Effective risk management is crucial for successful autoclave upgrade projects, particularly when minimizing operational disruption is a priority. A comprehensive risk assessment should be conducted at the project's inception, identifying potential hazards across technical, operational, and financial dimensions. This assessment must evaluate both the likelihood and potential impact of each risk, with particular attention to those that could extend downtime beyond planned windows.
Risk categorization provides structure to management efforts, typically dividing risks into equipment failure risks, timeline risks, regulatory compliance risks, and personnel safety risks. Each category requires tailored mitigation strategies and contingency plans. For equipment-related risks, maintaining critical spare parts inventory and establishing relationships with emergency service providers can significantly reduce unplanned downtime.
Schedule risk management demands robust project planning with built-in buffer periods for unexpected complications. Implementing critical path analysis helps identify activities where delays would directly impact overall project timelines. Parallel processing of certain upgrade components, where feasible, can compress schedules and provide flexibility when issues arise.
Financial risk mitigation involves detailed budgeting with contingency funds typically set at 15-20% of the total project cost. Return on investment calculations should incorporate various disruption scenarios to ensure financial viability even under sub-optimal conditions. Insurance coverage specifically tailored to upgrade projects provides additional financial protection.
Communication emerges as a critical risk management tool, with clear protocols established for various scenarios. Regular stakeholder updates maintain transparency, while dedicated communication channels ensure rapid response to emerging issues. Documentation of all risk-related decisions creates an invaluable knowledge base for future projects.
Continuous monitoring throughout the implementation phase allows for dynamic risk management. Key performance indicators should be established to trigger intervention when thresholds are approached. Post-implementation reviews capture lessons learned, contributing to organizational knowledge and improving future autoclave upgrade projects.
The most successful risk management approaches employ redundancy strategies where critical systems are concerned. These may include temporary autoclave solutions during extended upgrades or partnership arrangements with other facilities to handle processing during downtime periods. Such redundancies, while adding cost, significantly reduce the operational impact of extended disruptions.
Risk categorization provides structure to management efforts, typically dividing risks into equipment failure risks, timeline risks, regulatory compliance risks, and personnel safety risks. Each category requires tailored mitigation strategies and contingency plans. For equipment-related risks, maintaining critical spare parts inventory and establishing relationships with emergency service providers can significantly reduce unplanned downtime.
Schedule risk management demands robust project planning with built-in buffer periods for unexpected complications. Implementing critical path analysis helps identify activities where delays would directly impact overall project timelines. Parallel processing of certain upgrade components, where feasible, can compress schedules and provide flexibility when issues arise.
Financial risk mitigation involves detailed budgeting with contingency funds typically set at 15-20% of the total project cost. Return on investment calculations should incorporate various disruption scenarios to ensure financial viability even under sub-optimal conditions. Insurance coverage specifically tailored to upgrade projects provides additional financial protection.
Communication emerges as a critical risk management tool, with clear protocols established for various scenarios. Regular stakeholder updates maintain transparency, while dedicated communication channels ensure rapid response to emerging issues. Documentation of all risk-related decisions creates an invaluable knowledge base for future projects.
Continuous monitoring throughout the implementation phase allows for dynamic risk management. Key performance indicators should be established to trigger intervention when thresholds are approached. Post-implementation reviews capture lessons learned, contributing to organizational knowledge and improving future autoclave upgrade projects.
The most successful risk management approaches employ redundancy strategies where critical systems are concerned. These may include temporary autoclave solutions during extended upgrades or partnership arrangements with other facilities to handle processing during downtime periods. Such redundancies, while adding cost, significantly reduce the operational impact of extended disruptions.
Regulatory Compliance Requirements for Sterilization Equipment Modifications
Regulatory compliance for sterilization equipment modifications, particularly autoclave upgrades, involves navigating a complex framework of standards and requirements. Healthcare facilities and manufacturers must adhere to regulations from multiple governing bodies, including the FDA's Quality System Regulation (21 CFR Part 820), ISO 13485 for medical device quality management systems, and ISO 17665 for steam sterilization validation. These regulations establish the foundation for ensuring patient safety and maintaining sterility assurance levels during and after equipment modifications.
When implementing autoclave upgrades, facilities must conduct a thorough regulatory impact assessment to identify all applicable standards. This assessment should document how the proposed modifications might affect the equipment's validated state and determine what revalidation activities are necessary. The FDA requires that all changes to sterilization equipment be evaluated, verified, and validated before implementation, with comprehensive documentation maintained throughout the process.
Documentation requirements represent a significant compliance consideration. Facilities must maintain detailed records of pre-modification validation status, modification plans, risk assessments, testing protocols, and post-modification validation results. These records must demonstrate that the modified equipment continues to meet all performance specifications and regulatory requirements. The documentation package should include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols and reports.
Change control procedures constitute another critical regulatory element. A formal change control system must be implemented to evaluate proposed modifications, assess their impact on the validated state of the equipment, and ensure appropriate verification and validation activities are completed. This system should include provisions for review and approval by qualified personnel, including quality assurance representatives and, where applicable, regulatory affairs specialists.
Revalidation requirements following modifications vary based on the nature and extent of the changes. Minor modifications may require limited revalidation focused on affected components, while major modifications typically necessitate comprehensive revalidation of the entire sterilization process. The AAMI ST79 guideline provides specific recommendations for determining appropriate revalidation activities based on the type of modification implemented.
Personnel training requirements must also be addressed when implementing autoclave upgrades. Regulations require that personnel involved in operating and maintaining sterilization equipment receive appropriate training on modified systems. This training must be documented and should cover both technical operation aspects and compliance requirements associated with the upgraded equipment.
When implementing autoclave upgrades, facilities must conduct a thorough regulatory impact assessment to identify all applicable standards. This assessment should document how the proposed modifications might affect the equipment's validated state and determine what revalidation activities are necessary. The FDA requires that all changes to sterilization equipment be evaluated, verified, and validated before implementation, with comprehensive documentation maintained throughout the process.
Documentation requirements represent a significant compliance consideration. Facilities must maintain detailed records of pre-modification validation status, modification plans, risk assessments, testing protocols, and post-modification validation results. These records must demonstrate that the modified equipment continues to meet all performance specifications and regulatory requirements. The documentation package should include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols and reports.
Change control procedures constitute another critical regulatory element. A formal change control system must be implemented to evaluate proposed modifications, assess their impact on the validated state of the equipment, and ensure appropriate verification and validation activities are completed. This system should include provisions for review and approval by qualified personnel, including quality assurance representatives and, where applicable, regulatory affairs specialists.
Revalidation requirements following modifications vary based on the nature and extent of the changes. Minor modifications may require limited revalidation focused on affected components, while major modifications typically necessitate comprehensive revalidation of the entire sterilization process. The AAMI ST79 guideline provides specific recommendations for determining appropriate revalidation activities based on the type of modification implemented.
Personnel training requirements must also be addressed when implementing autoclave upgrades. Regulations require that personnel involved in operating and maintaining sterilization equipment receive appropriate training on modified systems. This training must be documented and should cover both technical operation aspects and compliance requirements associated with the upgraded equipment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!