Autoclave Control Systems: Evaluating Cycle Consistency
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Control Systems Background and Objectives
Autoclave systems have evolved significantly since their inception in the late 19th century, transitioning from basic pressure vessels to sophisticated control systems that maintain precise temperature, pressure, and time parameters. Originally developed for sterilization in medical settings, autoclaves now serve critical functions across diverse industries including aerospace, composites manufacturing, food processing, and pharmaceutical production. The technological trajectory has been marked by increasing automation, enhanced sensor capabilities, and more sophisticated control algorithms.
The fundamental principle of autoclave operation involves creating a pressurized, high-temperature environment to achieve specific material or sterilization outcomes. Modern autoclave control systems represent a convergence of mechanical engineering, thermal dynamics, pressure control technology, and advanced software systems. These integrated systems must maintain consistent cycle parameters across multiple operations to ensure product quality, safety, and regulatory compliance.
Current technological trends in autoclave control systems focus on achieving greater cycle consistency through adaptive control algorithms, real-time monitoring capabilities, and predictive maintenance features. The industry is moving toward systems that can automatically compensate for variations in load characteristics, environmental conditions, and equipment wear patterns that might otherwise compromise cycle consistency.
The primary objective of evaluating cycle consistency in autoclave control systems is to identify and quantify variations in critical process parameters across multiple operational cycles. This evaluation aims to determine whether the system can reliably reproduce the same conditions within specified tolerances, regardless of external factors or system age. Secondary objectives include identifying root causes of inconsistency, developing mitigation strategies, and establishing performance benchmarks for system optimization.
From a technical perspective, cycle consistency evaluation encompasses several key metrics: temperature uniformity throughout the chamber, pressure stability during critical phases, accurate timing of cycle segments, and repeatability of these parameters across successive operations. Advanced evaluation approaches also consider energy efficiency, resource utilization, and system responsiveness to unexpected variations.
The strategic importance of cycle consistency cannot be overstated, particularly in industries where product quality and safety directly depend on autoclave performance. In aerospace applications, for example, inconsistent curing cycles can compromise the structural integrity of composite components. In medical sterilization, cycle variations may result in inadequate pathogen elimination, creating significant health risks.
As regulatory requirements become increasingly stringent across industries, the demand for demonstrable cycle consistency has intensified, driving innovation in both control systems and evaluation methodologies. This technological evolution continues to push toward more precise, reliable, and transparent autoclave operations.
The fundamental principle of autoclave operation involves creating a pressurized, high-temperature environment to achieve specific material or sterilization outcomes. Modern autoclave control systems represent a convergence of mechanical engineering, thermal dynamics, pressure control technology, and advanced software systems. These integrated systems must maintain consistent cycle parameters across multiple operations to ensure product quality, safety, and regulatory compliance.
Current technological trends in autoclave control systems focus on achieving greater cycle consistency through adaptive control algorithms, real-time monitoring capabilities, and predictive maintenance features. The industry is moving toward systems that can automatically compensate for variations in load characteristics, environmental conditions, and equipment wear patterns that might otherwise compromise cycle consistency.
The primary objective of evaluating cycle consistency in autoclave control systems is to identify and quantify variations in critical process parameters across multiple operational cycles. This evaluation aims to determine whether the system can reliably reproduce the same conditions within specified tolerances, regardless of external factors or system age. Secondary objectives include identifying root causes of inconsistency, developing mitigation strategies, and establishing performance benchmarks for system optimization.
From a technical perspective, cycle consistency evaluation encompasses several key metrics: temperature uniformity throughout the chamber, pressure stability during critical phases, accurate timing of cycle segments, and repeatability of these parameters across successive operations. Advanced evaluation approaches also consider energy efficiency, resource utilization, and system responsiveness to unexpected variations.
The strategic importance of cycle consistency cannot be overstated, particularly in industries where product quality and safety directly depend on autoclave performance. In aerospace applications, for example, inconsistent curing cycles can compromise the structural integrity of composite components. In medical sterilization, cycle variations may result in inadequate pathogen elimination, creating significant health risks.
As regulatory requirements become increasingly stringent across industries, the demand for demonstrable cycle consistency has intensified, driving innovation in both control systems and evaluation methodologies. This technological evolution continues to push toward more precise, reliable, and transparent autoclave operations.
Market Demand Analysis for Consistent Sterilization Solutions
The global market for autoclave control systems with consistent sterilization capabilities is experiencing significant growth, driven primarily by heightened awareness of infection control across healthcare facilities, pharmaceutical manufacturing, and laboratory environments. Current market analysis indicates that healthcare-associated infections (HAIs) remain a critical concern worldwide, creating sustained demand for reliable sterilization technologies that can deliver consistent results across multiple cycles.
Healthcare facilities represent the largest market segment, with hospitals increasingly investing in advanced autoclave systems that offer improved cycle consistency and validation capabilities. This trend is particularly evident in developed markets where regulatory requirements for sterilization validation have become more stringent. The pharmaceutical industry follows closely, where Good Manufacturing Practices (GMP) compliance necessitates highly reliable sterilization processes with minimal variation between cycles.
Market research reveals that end-users are increasingly prioritizing autoclave systems that provide comprehensive data logging, real-time monitoring, and cycle consistency verification. This shift represents a move away from basic time-temperature parameters toward more sophisticated control systems that can maintain precise conditions throughout the entire sterilization cycle. The demand for these advanced features stems from both regulatory pressure and operational efficiency requirements.
Regional analysis shows varying market maturity levels. North America and Europe lead in adoption of advanced autoclave control systems, while Asia-Pacific represents the fastest-growing market due to expanding healthcare infrastructure and increasing manufacturing quality standards. Emerging economies are gradually transitioning from manual or semi-automated systems to fully automated solutions with enhanced cycle consistency capabilities.
Industry surveys indicate that customers are willing to pay premium prices for autoclave systems that can demonstrate superior cycle consistency, particularly when supported by comprehensive validation documentation. This value proposition is especially strong in critical applications such as medical device manufacturing, pharmaceutical production, and laboratory research where sterilization failure can have severe consequences.
Market forecasts suggest that demand for consistent sterilization solutions will continue to grow at a compound rate exceeding the general medical equipment market. This growth is further supported by increasing regulatory scrutiny of sterilization processes across industries and the growing complexity of materials requiring sterilization, which necessitates more precise and consistent autoclave cycles.
Customer feedback analysis reveals that key purchasing factors include reliability, validation capabilities, cycle consistency, and total cost of ownership rather than initial acquisition cost alone. This indicates a market shift toward value-based purchasing decisions where performance metrics like cycle-to-cycle consistency are becoming central evaluation criteria.
Healthcare facilities represent the largest market segment, with hospitals increasingly investing in advanced autoclave systems that offer improved cycle consistency and validation capabilities. This trend is particularly evident in developed markets where regulatory requirements for sterilization validation have become more stringent. The pharmaceutical industry follows closely, where Good Manufacturing Practices (GMP) compliance necessitates highly reliable sterilization processes with minimal variation between cycles.
Market research reveals that end-users are increasingly prioritizing autoclave systems that provide comprehensive data logging, real-time monitoring, and cycle consistency verification. This shift represents a move away from basic time-temperature parameters toward more sophisticated control systems that can maintain precise conditions throughout the entire sterilization cycle. The demand for these advanced features stems from both regulatory pressure and operational efficiency requirements.
Regional analysis shows varying market maturity levels. North America and Europe lead in adoption of advanced autoclave control systems, while Asia-Pacific represents the fastest-growing market due to expanding healthcare infrastructure and increasing manufacturing quality standards. Emerging economies are gradually transitioning from manual or semi-automated systems to fully automated solutions with enhanced cycle consistency capabilities.
Industry surveys indicate that customers are willing to pay premium prices for autoclave systems that can demonstrate superior cycle consistency, particularly when supported by comprehensive validation documentation. This value proposition is especially strong in critical applications such as medical device manufacturing, pharmaceutical production, and laboratory research where sterilization failure can have severe consequences.
Market forecasts suggest that demand for consistent sterilization solutions will continue to grow at a compound rate exceeding the general medical equipment market. This growth is further supported by increasing regulatory scrutiny of sterilization processes across industries and the growing complexity of materials requiring sterilization, which necessitates more precise and consistent autoclave cycles.
Customer feedback analysis reveals that key purchasing factors include reliability, validation capabilities, cycle consistency, and total cost of ownership rather than initial acquisition cost alone. This indicates a market shift toward value-based purchasing decisions where performance metrics like cycle-to-cycle consistency are becoming central evaluation criteria.
Current Challenges in Autoclave Cycle Consistency
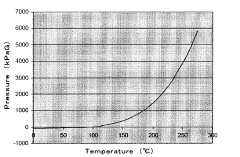
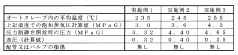
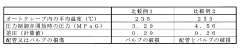
Despite significant advancements in autoclave technology, maintaining consistent cycle parameters remains a persistent challenge across various industries. Current autoclave control systems face several critical issues that impact process reliability and product quality. Temperature uniformity within the autoclave chamber continues to be problematic, with variations of 2-5°C commonly observed in industrial settings. These temperature gradients can significantly affect material properties, particularly in aerospace composite curing and medical device sterilization applications.
Pressure control systems demonstrate limitations in responding to rapid changes during critical cycle phases. Research indicates that pressure fluctuations of ±0.2 bar are common during transition periods, potentially compromising both process efficiency and end-product integrity. These fluctuations are particularly problematic in applications requiring precise pressure profiles, such as composite manufacturing where resin flow dynamics are pressure-dependent.
Data acquisition and monitoring systems present another significant challenge. Many existing systems operate with sampling rates insufficient for capturing transient phenomena, typically recording data at 1-5 second intervals. This temporal resolution gap creates blind spots in process understanding and complicates root cause analysis when cycle inconsistencies occur.
Control algorithm sophistication varies widely across equipment manufacturers, with many systems still relying on traditional PID controllers that struggle with the complex, non-linear dynamics of autoclave processes. More advanced model predictive control (MPC) implementations remain limited to high-end systems, creating a technological divide in the industry.
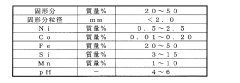
Sensor reliability and calibration drift represent another persistent issue. Studies show that thermocouple accuracy can deteriorate by up to 2°C annually without proper calibration protocols. This degradation often goes undetected until significant process deviations occur, by which time substantial product quality issues may have developed.
Integration challenges between control systems and enterprise data platforms further complicate cycle consistency efforts. Many facilities operate with isolated control systems that lack seamless data transfer capabilities, hindering comprehensive process analysis and continuous improvement initiatives.
Validation methodologies for cycle consistency also present significant challenges. Current approaches often rely on limited sampling techniques that may not adequately represent the full spectrum of process variability. Statistical process control methods applied to autoclave operations frequently suffer from insufficient data density and inappropriate baseline assumptions.
Human factors continue to influence cycle consistency, with operator variability in loading patterns, parameter selection, and maintenance practices contributing to process inconsistencies. Even in highly automated systems, the human-machine interface design can impact operational consistency through unclear visualization of critical parameters or counterintuitive control sequences.
Pressure control systems demonstrate limitations in responding to rapid changes during critical cycle phases. Research indicates that pressure fluctuations of ±0.2 bar are common during transition periods, potentially compromising both process efficiency and end-product integrity. These fluctuations are particularly problematic in applications requiring precise pressure profiles, such as composite manufacturing where resin flow dynamics are pressure-dependent.
Data acquisition and monitoring systems present another significant challenge. Many existing systems operate with sampling rates insufficient for capturing transient phenomena, typically recording data at 1-5 second intervals. This temporal resolution gap creates blind spots in process understanding and complicates root cause analysis when cycle inconsistencies occur.
Control algorithm sophistication varies widely across equipment manufacturers, with many systems still relying on traditional PID controllers that struggle with the complex, non-linear dynamics of autoclave processes. More advanced model predictive control (MPC) implementations remain limited to high-end systems, creating a technological divide in the industry.
Sensor reliability and calibration drift represent another persistent issue. Studies show that thermocouple accuracy can deteriorate by up to 2°C annually without proper calibration protocols. This degradation often goes undetected until significant process deviations occur, by which time substantial product quality issues may have developed.
Integration challenges between control systems and enterprise data platforms further complicate cycle consistency efforts. Many facilities operate with isolated control systems that lack seamless data transfer capabilities, hindering comprehensive process analysis and continuous improvement initiatives.
Validation methodologies for cycle consistency also present significant challenges. Current approaches often rely on limited sampling techniques that may not adequately represent the full spectrum of process variability. Statistical process control methods applied to autoclave operations frequently suffer from insufficient data density and inappropriate baseline assumptions.
Human factors continue to influence cycle consistency, with operator variability in loading patterns, parameter selection, and maintenance practices contributing to process inconsistencies. Even in highly automated systems, the human-machine interface design can impact operational consistency through unclear visualization of critical parameters or counterintuitive control sequences.
Current Control System Architectures and Methodologies
01 Advanced monitoring systems for autoclave cycle consistency
Advanced monitoring systems are implemented to ensure autoclave cycle consistency by continuously tracking critical parameters such as temperature, pressure, and time. These systems utilize sensors and data acquisition technologies to provide real-time feedback on cycle performance. The monitoring systems can detect deviations from programmed parameters and alert operators to potential issues, ensuring sterilization efficacy and consistency across multiple cycles.- Monitoring and control systems for autoclave cycle consistency: Advanced monitoring and control systems are essential for maintaining autoclave cycle consistency. These systems utilize sensors and controllers to continuously monitor critical parameters such as temperature, pressure, and time throughout the sterilization process. Real-time data collection and analysis enable precise control of cycle conditions, ensuring that each sterilization cycle meets predetermined specifications. These systems can automatically adjust operating parameters to maintain consistency across multiple cycles, reducing variability and enhancing reliability of the sterilization process.
- Validation and verification methods for cycle consistency: Various validation and verification methods are employed to ensure autoclave cycle consistency. These include biological indicators, chemical indicators, and physical measurements that verify sterilization effectiveness. Validation protocols involve testing under worst-case scenarios to confirm that the autoclave consistently achieves required sterilization parameters. Regular verification testing helps identify potential issues before they affect cycle consistency. Documentation of validation results provides evidence of consistent performance and compliance with regulatory standards for sterilization processes.
- Data management and analysis for cycle consistency: Effective data management systems are crucial for maintaining autoclave cycle consistency. These systems collect, store, and analyze operational data from multiple sterilization cycles to identify trends, patterns, and potential issues. Advanced analytics can detect subtle deviations in cycle parameters before they become significant problems. Historical data comparison allows for continuous improvement of cycle consistency over time. Cloud-based platforms enable remote monitoring and analysis of cycle data, facilitating prompt intervention when inconsistencies are detected.
- Automated cycle parameter adjustment technologies: Automated technologies for adjusting cycle parameters help maintain consistency across multiple sterilization cycles. These systems use algorithms to automatically modify temperature, pressure, and time settings based on load characteristics, environmental conditions, and historical performance data. Adaptive control mechanisms can compensate for variations in steam quality, power supply fluctuations, and other external factors that might affect cycle consistency. Self-learning systems continuously improve their performance by analyzing the outcomes of previous cycles and making incremental adjustments to optimize future operations.
- Hardware innovations for improving cycle consistency: Hardware innovations play a significant role in enhancing autoclave cycle consistency. These include advanced heating elements that provide more uniform temperature distribution, precision pressure regulators that maintain stable pressure conditions, and high-quality valves that ensure consistent steam flow. Improved chamber designs minimize cold spots and ensure even heat distribution throughout the load. Redundant sensor systems provide accurate measurements and enable cross-verification of critical parameters. These hardware components work together to create a more reliable and consistent sterilization environment across multiple cycles.
02 Automated control algorithms for cycle optimization
Sophisticated control algorithms are employed to optimize autoclave sterilization cycles for consistency. These algorithms dynamically adjust operating parameters based on load characteristics, environmental conditions, and sterilization requirements. By implementing PID (Proportional-Integral-Derivative) control and adaptive feedback mechanisms, these systems can maintain precise temperature and pressure profiles throughout the sterilization process, resulting in highly reproducible cycles and improved energy efficiency.Expand Specific Solutions03 Validation and documentation systems for cycle consistency
Comprehensive validation and documentation systems are integrated into autoclave control systems to verify and record cycle consistency. These systems automatically generate detailed reports of each sterilization cycle, including graphical representations of critical parameters over time. The documentation provides evidence of proper sterilization for regulatory compliance and quality assurance purposes. Validation protocols include regular testing of cycle parameters against predetermined specifications to ensure ongoing consistency.Expand Specific Solutions04 Fault detection and recovery mechanisms
Autoclave control systems incorporate sophisticated fault detection and recovery mechanisms to maintain cycle consistency even when anomalies occur. These systems continuously analyze operational data to identify potential issues before they affect sterilization efficacy. When deviations are detected, automated recovery protocols can be initiated to correct the problem or safely abort the cycle. This approach minimizes failed cycles and ensures consistent sterilization results across multiple operations.Expand Specific Solutions05 Load-responsive cycle adjustment technologies
Advanced autoclave control systems utilize load-responsive technologies that automatically adjust cycle parameters based on the specific characteristics of each load. These systems employ sensors to detect load volume, density, and composition, then modify temperature ramp rates, hold times, and pressure levels accordingly. By tailoring each cycle to the specific requirements of the load while maintaining critical sterilization parameters, these systems ensure consistent sterilization efficacy across varying load conditions.Expand Specific Solutions
Leading Manufacturers and Industry Landscape
The autoclave control systems market is currently in a growth phase, with increasing demand driven by healthcare sterilization requirements and industrial applications. The market size is expanding steadily, particularly in medical and industrial sectors, with projections indicating continued growth due to stringent sterilization regulations. Technologically, the field shows varying maturity levels across applications. Leading players like Eschmann Holdings and ASP Global Manufacturing demonstrate advanced capabilities in medical autoclaves, while industrial leaders such as Baker Hughes, Caterpillar, and Hitachi offer sophisticated control systems for industrial applications. ChromaCon and BSH Hausgeräte are advancing cycle consistency innovations, while companies like Sidel and NETSTAL focus on specialized applications requiring precise autoclave control parameters, collectively driving the technology toward greater automation and reliability.
Eschmann Holdings Ltd.
Technical Solution: Eschmann has developed advanced autoclave control systems focusing on cycle consistency validation for medical sterilization applications. Their technology employs a multi-sensor array system that continuously monitors critical parameters including temperature, pressure, and humidity throughout the sterilization cycle. The system utilizes proprietary algorithms to analyze real-time data against predefined cycle parameters, automatically detecting deviations that could compromise sterilization efficacy. Eschmann's solution incorporates a digital twin modeling approach that simulates ideal cycle conditions and compares them with actual performance, enabling predictive maintenance and cycle optimization. Their control systems feature adaptive PID (Proportional-Integral-Derivative) controllers that dynamically adjust parameters to maintain consistency across different load configurations and environmental conditions.
Strengths: Specialized expertise in medical-grade sterilization requirements with exceptional precision in maintaining cycle parameters. Their systems offer comprehensive validation documentation that meets stringent regulatory requirements for healthcare facilities. Weaknesses: Higher implementation costs compared to general industrial solutions, and their systems may require more specialized technical support for maintenance and calibration.
Baker Hughes Co.
Technical Solution: Baker Hughes has developed specialized autoclave control systems primarily focused on oil and gas industry applications where cycle consistency is critical for material testing and certification. Their solution incorporates high-temperature, high-pressure sensor arrays capable of operating in extreme conditions up to 30,000 psi and 650°F. The system features adaptive control algorithms that maintain precise temperature and pressure profiles throughout extended test cycles, some lasting several weeks. Baker Hughes' technology employs a distributed control architecture with redundant safety systems that ensure cycle integrity even during power fluctuations or component failures. Their platform includes comprehensive data logging capabilities that capture thousands of data points throughout each cycle, enabling detailed analysis of material performance under simulated downhole conditions. Additionally, their control system incorporates automated calibration routines that regularly verify sensor accuracy and control response to maintain consistent cycle parameters over extended operational periods.
Strengths: Exceptional performance in extreme pressure and temperature environments with robust safety features designed for hazardous testing conditions. Their systems offer unmatched durability and reliability for continuous operation. Weaknesses: Highly specialized for oil and gas applications with limited flexibility for adaptation to other industries, and significant cost premium compared to general-purpose autoclave control systems.
Key Patents and Innovations in Cycle Consistency
Pressure adjustment method for autoclave
PatentActiveJP2010059489A
Innovation
- A method involving a thermometer, pressure gauge, computing device, high-pressure compressor, and pressure control valve is used to control the differential pressure by setting a reference value corresponding to the height difference between the autoclave and flash vessel, adjusting the pressure to prevent evaporation and expansion.
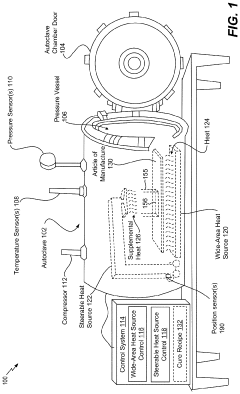
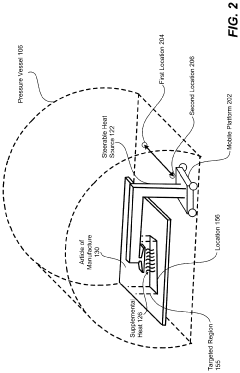
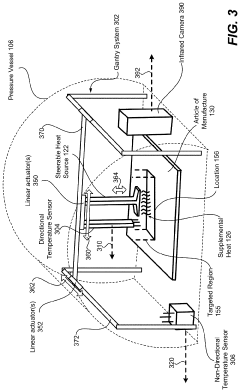
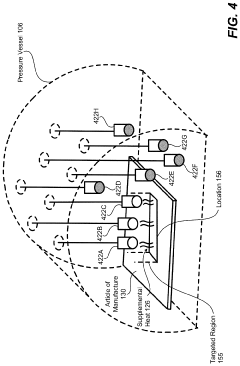
Steerable heat source
PatentActiveUS20200307035A1
Innovation
- A steerable heat source is integrated within the autoclave, coupled with a control system that directs supplemental heat to targeted regions using temperature sensors and a compressor to regulate pressure, ensuring precise temperature control and uniform heating.
Regulatory Standards and Compliance Requirements
Autoclave sterilization processes are governed by stringent regulatory frameworks that vary across regions but share common principles focused on patient safety and process validation. In the United States, the FDA's Quality System Regulation (21 CFR Part 820) establishes requirements for medical device manufacturers, with specific guidance on sterilization validation under ISO 17665 for moist heat sterilization processes. These standards mandate documented evidence that autoclave cycles consistently achieve sterility assurance levels (SAL) of 10^-6 or better, meaning a probability of less than one in a million that a viable microorganism remains after sterilization.
The European Medical Device Regulation (MDR 2017/745) imposes similar requirements through harmonized standards like EN 285 for large steam sterilizers and EN 13060 for small steam sterilizers. These standards specify precise parameters for temperature uniformity, pressure fluctuations, and cycle reproducibility that must be maintained across the autoclave chamber during operation. Notably, temperature variations must typically remain within ±1°C during the sterilization hold phase, with pressure deviations limited to ±0.3 bar.
Healthcare facilities must comply with additional standards from organizations such as the Association for the Advancement of Medical Instrumentation (AAMI) in North America and the Robert Koch Institute guidelines in Germany. These standards establish protocols for routine monitoring, including the use of biological indicators containing Geobacillus stearothermophilus spores, which must be inactivated during proper sterilization cycles.
Pharmaceutical manufacturing follows even more rigorous requirements under cGMP regulations (21 CFR Parts 210 and 211), with additional guidance from USP <1211> on sterilization and sterility assurance. These regulations require comprehensive validation protocols including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) phases to demonstrate that autoclave control systems can consistently deliver the required sterilization parameters.
Documentation requirements across all regulatory frameworks are extensive, mandating detailed records of cycle parameters, calibration data, maintenance activities, and validation studies. Modern autoclave control systems must incorporate features that facilitate this compliance through automated data logging, electronic signatures compliant with 21 CFR Part 11, and audit trail capabilities that track all parameter adjustments and system interventions.
Emerging regulatory trends indicate increasing emphasis on real-time monitoring technologies and process analytical technology (PAT) approaches that enable continuous verification of critical parameters rather than relying solely on end-point testing. This shift aligns with the FDA's quality by design initiative and represents a significant consideration for next-generation autoclave control system development.
The European Medical Device Regulation (MDR 2017/745) imposes similar requirements through harmonized standards like EN 285 for large steam sterilizers and EN 13060 for small steam sterilizers. These standards specify precise parameters for temperature uniformity, pressure fluctuations, and cycle reproducibility that must be maintained across the autoclave chamber during operation. Notably, temperature variations must typically remain within ±1°C during the sterilization hold phase, with pressure deviations limited to ±0.3 bar.
Healthcare facilities must comply with additional standards from organizations such as the Association for the Advancement of Medical Instrumentation (AAMI) in North America and the Robert Koch Institute guidelines in Germany. These standards establish protocols for routine monitoring, including the use of biological indicators containing Geobacillus stearothermophilus spores, which must be inactivated during proper sterilization cycles.
Pharmaceutical manufacturing follows even more rigorous requirements under cGMP regulations (21 CFR Parts 210 and 211), with additional guidance from USP <1211> on sterilization and sterility assurance. These regulations require comprehensive validation protocols including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) phases to demonstrate that autoclave control systems can consistently deliver the required sterilization parameters.
Documentation requirements across all regulatory frameworks are extensive, mandating detailed records of cycle parameters, calibration data, maintenance activities, and validation studies. Modern autoclave control systems must incorporate features that facilitate this compliance through automated data logging, electronic signatures compliant with 21 CFR Part 11, and audit trail capabilities that track all parameter adjustments and system interventions.
Emerging regulatory trends indicate increasing emphasis on real-time monitoring technologies and process analytical technology (PAT) approaches that enable continuous verification of critical parameters rather than relying solely on end-point testing. This shift aligns with the FDA's quality by design initiative and represents a significant consideration for next-generation autoclave control system development.
Validation Protocols and Performance Metrics
Validation protocols for autoclave control systems must be designed to systematically evaluate cycle consistency across multiple operational parameters. The establishment of comprehensive validation methodologies begins with the identification of critical process parameters (CPPs) including temperature uniformity, pressure stability, and cycle timing precision. These parameters require continuous monitoring through strategically positioned sensors that capture data from various locations within the autoclave chamber, particularly focusing on cold spots and areas with potential thermal gradients.
Performance metrics for autoclave systems should be quantifiable and reproducible, with statistical process control (SPC) methodologies applied to analyze cycle-to-cycle variations. Key performance indicators (KPIs) typically include temperature deviation tolerances (±1.0°C during sterilization phase), pressure fluctuation limits (±0.1 bar), and F0 value consistency for biological validation. The implementation of Process Capability Index (Cpk) calculations provides objective measurement of how well the autoclave process meets specification limits, with industry standards generally requiring Cpk values exceeding 1.33 for critical parameters.
Validation protocols must incorporate three sequential phases: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). The IQ verifies proper equipment installation and calibration of control instruments. OQ confirms that the system operates as designed across its operational range, while PQ demonstrates consistent performance under actual production conditions. Each qualification phase requires predetermined acceptance criteria and documented evidence of compliance.
Modern validation approaches increasingly utilize Process Analytical Technology (PAT) frameworks that enable real-time monitoring and adaptive control. These systems employ multivariate data analysis techniques to detect subtle deviations in cycle parameters before they reach critical thresholds. The implementation of digital twins for autoclave systems further enhances validation capabilities by allowing virtual simulation of process variations and their potential impacts on sterilization efficacy.
Risk-based validation strategies, aligned with ICH Q9 guidelines, prioritize testing resources toward parameters with highest potential impact on product quality and safety. This approach incorporates Failure Mode and Effects Analysis (FMEA) to identify critical control points and establish appropriate monitoring frequencies. The validation protocol should also include challenge testing with biological indicators positioned at worst-case locations to verify sterilization effectiveness under minimum acceptable conditions.
Performance metrics for autoclave systems should be quantifiable and reproducible, with statistical process control (SPC) methodologies applied to analyze cycle-to-cycle variations. Key performance indicators (KPIs) typically include temperature deviation tolerances (±1.0°C during sterilization phase), pressure fluctuation limits (±0.1 bar), and F0 value consistency for biological validation. The implementation of Process Capability Index (Cpk) calculations provides objective measurement of how well the autoclave process meets specification limits, with industry standards generally requiring Cpk values exceeding 1.33 for critical parameters.
Validation protocols must incorporate three sequential phases: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). The IQ verifies proper equipment installation and calibration of control instruments. OQ confirms that the system operates as designed across its operational range, while PQ demonstrates consistent performance under actual production conditions. Each qualification phase requires predetermined acceptance criteria and documented evidence of compliance.
Modern validation approaches increasingly utilize Process Analytical Technology (PAT) frameworks that enable real-time monitoring and adaptive control. These systems employ multivariate data analysis techniques to detect subtle deviations in cycle parameters before they reach critical thresholds. The implementation of digital twins for autoclave systems further enhances validation capabilities by allowing virtual simulation of process variations and their potential impacts on sterilization efficacy.
Risk-based validation strategies, aligned with ICH Q9 guidelines, prioritize testing resources toward parameters with highest potential impact on product quality and safety. This approach incorporates Failure Mode and Effects Analysis (FMEA) to identify critical control points and establish appropriate monitoring frequencies. The validation protocol should also include challenge testing with biological indicators positioned at worst-case locations to verify sterilization effectiveness under minimum acceptable conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!