Autoclave Instrument Sterilization: Achieving Seamless Integration
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Background and Objectives
Autoclave sterilization has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This technology has become a cornerstone in medical facilities, laboratories, and various industries where microbial contamination poses significant risks. The fundamental principle of using pressurized steam to achieve high temperatures capable of destroying microorganisms has remained consistent, though the implementation has grown increasingly sophisticated.
The evolution of autoclave technology has progressed from simple pressure cooker-like devices to highly automated systems with precise control mechanisms. Early autoclaves required manual operation and monitoring, while contemporary models incorporate computerized controls, validation systems, and integration capabilities. This progression reflects broader technological trends toward automation, connectivity, and data-driven operation in medical and laboratory equipment.
Current autoclave systems typically operate at temperatures of 121-134°C under pressure of 15-30 psi, conditions that effectively eliminate bacteria, viruses, fungi, and spores. However, the integration of these systems into modern healthcare workflows presents significant challenges, particularly regarding efficiency, monitoring, and documentation compliance with increasingly stringent regulatory requirements.
The primary objective of seamless autoclave integration is to create systems that function as cohesive components within larger operational ecosystems rather than as isolated units. This integration aims to enhance sterilization process reliability, improve operational efficiency, reduce human error, and ensure comprehensive documentation for regulatory compliance. Specifically, integration goals include real-time monitoring capabilities, automated record-keeping, predictive maintenance alerts, and interoperability with inventory management systems.
Another critical objective is addressing the environmental impact of autoclave operations, which traditionally consume significant amounts of water and energy. Modern integration approaches seek to optimize resource utilization through intelligent cycle management, water recycling systems, and energy recovery mechanisms. These improvements align with broader sustainability initiatives in healthcare and laboratory settings.
The technological trajectory points toward "smart" autoclave systems that leverage Internet of Things (IoT) connectivity, artificial intelligence for cycle optimization, and blockchain for secure documentation. These advancements promise to transform autoclaves from standalone sterilization equipment into networked assets that contribute to overall operational intelligence and quality assurance.
As healthcare facilities and laboratories increasingly adopt digital transformation strategies, achieving seamless autoclave integration represents not merely a technical enhancement but a fundamental shift in how sterilization processes are conceptualized, implemented, and managed within complex operational environments.
The evolution of autoclave technology has progressed from simple pressure cooker-like devices to highly automated systems with precise control mechanisms. Early autoclaves required manual operation and monitoring, while contemporary models incorporate computerized controls, validation systems, and integration capabilities. This progression reflects broader technological trends toward automation, connectivity, and data-driven operation in medical and laboratory equipment.
Current autoclave systems typically operate at temperatures of 121-134°C under pressure of 15-30 psi, conditions that effectively eliminate bacteria, viruses, fungi, and spores. However, the integration of these systems into modern healthcare workflows presents significant challenges, particularly regarding efficiency, monitoring, and documentation compliance with increasingly stringent regulatory requirements.
The primary objective of seamless autoclave integration is to create systems that function as cohesive components within larger operational ecosystems rather than as isolated units. This integration aims to enhance sterilization process reliability, improve operational efficiency, reduce human error, and ensure comprehensive documentation for regulatory compliance. Specifically, integration goals include real-time monitoring capabilities, automated record-keeping, predictive maintenance alerts, and interoperability with inventory management systems.
Another critical objective is addressing the environmental impact of autoclave operations, which traditionally consume significant amounts of water and energy. Modern integration approaches seek to optimize resource utilization through intelligent cycle management, water recycling systems, and energy recovery mechanisms. These improvements align with broader sustainability initiatives in healthcare and laboratory settings.
The technological trajectory points toward "smart" autoclave systems that leverage Internet of Things (IoT) connectivity, artificial intelligence for cycle optimization, and blockchain for secure documentation. These advancements promise to transform autoclaves from standalone sterilization equipment into networked assets that contribute to overall operational intelligence and quality assurance.
As healthcare facilities and laboratories increasingly adopt digital transformation strategies, achieving seamless autoclave integration represents not merely a technical enhancement but a fundamental shift in how sterilization processes are conceptualized, implemented, and managed within complex operational environments.
Market Demand Analysis for Integrated Sterilization Solutions
The global market for integrated autoclave sterilization solutions is experiencing robust growth, driven primarily by increasing healthcare expenditures and stringent infection control regulations across medical facilities worldwide. Current market analysis indicates that the medical sterilization equipment market is projected to reach $8.6 billion by 2027, with autoclave systems representing approximately 35% of this market share. The compound annual growth rate (CAGR) for integrated autoclave solutions specifically is estimated at 6.8%, outpacing the broader sterilization equipment market.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, are demonstrating heightened demand for seamlessly integrated sterilization solutions that can improve operational efficiency while maintaining compliance with evolving regulatory standards. A recent survey of 500 hospital administrators revealed that 78% consider integration capabilities as "very important" or "critical" when evaluating new sterilization equipment purchases.
The demand is particularly pronounced in regions with rapidly expanding healthcare infrastructure, such as Asia-Pacific and Latin America, where new facility construction presents opportunities for implementing integrated systems from the ground up. In mature markets like North America and Europe, the replacement cycle for aging sterilization equipment is driving adoption of next-generation integrated solutions.
Key market drivers include the rising incidence of healthcare-associated infections (HAIs), which cost healthcare systems billions annually and have prompted stricter sterilization protocols. Additionally, staffing shortages in central sterile processing departments have accelerated interest in automated, integrated solutions that can reduce manual intervention and human error while improving throughput.
From an end-user perspective, demand segmentation shows hospitals accounting for 62% of the market, followed by ambulatory surgical centers (18%), dental clinics (12%), and pharmaceutical/biotechnology companies (8%). Within hospitals, departments performing high-volume surgical procedures demonstrate the strongest demand for integrated sterilization solutions.
Customer requirements are evolving beyond basic sterilization functionality to encompass comprehensive integration with inventory management systems, electronic health records, and quality assurance protocols. The ability to provide real-time sterilization cycle data, instrument tracking, and automated documentation is increasingly viewed as essential rather than optional.
Market research indicates willingness to pay premium prices for solutions offering demonstrable improvements in workflow efficiency, with 65% of procurement decision-makers citing potential labor savings as justification for higher capital expenditures on advanced integrated systems. This represents a significant shift from traditional purchasing patterns that prioritized initial acquisition costs over total cost of ownership.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, are demonstrating heightened demand for seamlessly integrated sterilization solutions that can improve operational efficiency while maintaining compliance with evolving regulatory standards. A recent survey of 500 hospital administrators revealed that 78% consider integration capabilities as "very important" or "critical" when evaluating new sterilization equipment purchases.
The demand is particularly pronounced in regions with rapidly expanding healthcare infrastructure, such as Asia-Pacific and Latin America, where new facility construction presents opportunities for implementing integrated systems from the ground up. In mature markets like North America and Europe, the replacement cycle for aging sterilization equipment is driving adoption of next-generation integrated solutions.
Key market drivers include the rising incidence of healthcare-associated infections (HAIs), which cost healthcare systems billions annually and have prompted stricter sterilization protocols. Additionally, staffing shortages in central sterile processing departments have accelerated interest in automated, integrated solutions that can reduce manual intervention and human error while improving throughput.
From an end-user perspective, demand segmentation shows hospitals accounting for 62% of the market, followed by ambulatory surgical centers (18%), dental clinics (12%), and pharmaceutical/biotechnology companies (8%). Within hospitals, departments performing high-volume surgical procedures demonstrate the strongest demand for integrated sterilization solutions.
Customer requirements are evolving beyond basic sterilization functionality to encompass comprehensive integration with inventory management systems, electronic health records, and quality assurance protocols. The ability to provide real-time sterilization cycle data, instrument tracking, and automated documentation is increasingly viewed as essential rather than optional.
Market research indicates willingness to pay premium prices for solutions offering demonstrable improvements in workflow efficiency, with 65% of procurement decision-makers citing potential labor savings as justification for higher capital expenditures on advanced integrated systems. This represents a significant shift from traditional purchasing patterns that prioritized initial acquisition costs over total cost of ownership.
Current Challenges in Autoclave Integration Technology
Despite significant advancements in autoclave technology, healthcare facilities continue to face substantial challenges in achieving seamless integration of instrument sterilization systems. The primary obstacle remains the lack of standardized communication protocols between autoclave equipment and broader hospital management systems. This fragmentation creates inefficiencies in workflow and increases the risk of sterilization process failures.
Interoperability issues persist as autoclave manufacturers often develop proprietary systems that cannot easily communicate with equipment from other vendors. Healthcare facilities utilizing diverse equipment face significant integration barriers, requiring costly custom middleware solutions or manual documentation processes that introduce human error risks.
Physical space constraints present another critical challenge, particularly in older healthcare facilities not designed for modern sterilization requirements. Limited floor space, inadequate utility connections, and insufficient ventilation systems complicate the installation of integrated autoclave systems. These spatial limitations often force compromises in workflow design and equipment selection.
Energy management remains problematic as autoclave systems are significant consumers of electricity, water, and steam. Integration with building management systems for optimized resource utilization is technically complex and often overlooked. The resulting inefficiencies contribute to higher operational costs and environmental impact, contradicting healthcare sustainability initiatives.
Data security concerns have intensified as sterilization systems become increasingly connected. The integration of autoclave systems with hospital networks creates potential cybersecurity vulnerabilities that could compromise patient data or even sterilization processes themselves. Many facilities lack comprehensive security protocols specifically addressing these medical device integration points.
Regulatory compliance adds another layer of complexity, with requirements varying significantly across regions and healthcare settings. Documentation systems must integrate seamlessly with autoclave equipment to ensure proper cycle recording, validation, and traceability. Current solutions often require redundant data entry, increasing administrative burden and error potential.
Staff training represents a persistent challenge, as integrated systems demand higher technical competency from sterile processing personnel. The complexity of modern integrated autoclave systems requires specialized knowledge that exceeds traditional sterilization training. This knowledge gap contributes to underutilization of advanced features and potential procedural errors.
Cost justification remains difficult despite the clear operational benefits of fully integrated systems. Healthcare administrators struggle to quantify return on investment for integration projects, particularly when existing manual processes appear functional. This economic barrier often results in piecemeal implementation that fails to deliver comprehensive benefits.
Interoperability issues persist as autoclave manufacturers often develop proprietary systems that cannot easily communicate with equipment from other vendors. Healthcare facilities utilizing diverse equipment face significant integration barriers, requiring costly custom middleware solutions or manual documentation processes that introduce human error risks.
Physical space constraints present another critical challenge, particularly in older healthcare facilities not designed for modern sterilization requirements. Limited floor space, inadequate utility connections, and insufficient ventilation systems complicate the installation of integrated autoclave systems. These spatial limitations often force compromises in workflow design and equipment selection.
Energy management remains problematic as autoclave systems are significant consumers of electricity, water, and steam. Integration with building management systems for optimized resource utilization is technically complex and often overlooked. The resulting inefficiencies contribute to higher operational costs and environmental impact, contradicting healthcare sustainability initiatives.
Data security concerns have intensified as sterilization systems become increasingly connected. The integration of autoclave systems with hospital networks creates potential cybersecurity vulnerabilities that could compromise patient data or even sterilization processes themselves. Many facilities lack comprehensive security protocols specifically addressing these medical device integration points.
Regulatory compliance adds another layer of complexity, with requirements varying significantly across regions and healthcare settings. Documentation systems must integrate seamlessly with autoclave equipment to ensure proper cycle recording, validation, and traceability. Current solutions often require redundant data entry, increasing administrative burden and error potential.
Staff training represents a persistent challenge, as integrated systems demand higher technical competency from sterile processing personnel. The complexity of modern integrated autoclave systems requires specialized knowledge that exceeds traditional sterilization training. This knowledge gap contributes to underutilization of advanced features and potential procedural errors.
Cost justification remains difficult despite the clear operational benefits of fully integrated systems. Healthcare administrators struggle to quantify return on investment for integration projects, particularly when existing manual processes appear functional. This economic barrier often results in piecemeal implementation that fails to deliver comprehensive benefits.
Current Integration Solutions for Autoclave Systems
01 Automated sterilization systems with integrated monitoring
Modern autoclave systems incorporate automated monitoring and control features that enable seamless integration with hospital information systems. These systems provide real-time tracking of sterilization parameters, automatic documentation of cycles, and validation of sterilization effectiveness. The integration allows for improved workflow efficiency, reduced human error, and enhanced compliance with sterilization standards through continuous monitoring of critical parameters like temperature, pressure, and exposure time.- Automated sterilization systems with integrated monitoring: Modern autoclave systems incorporate automated monitoring and control features that seamlessly integrate with instrument processing workflows. These systems include real-time tracking of sterilization parameters, automated documentation of cycle data, and integration with hospital information systems. The technology ensures consistent sterilization results while minimizing manual intervention and providing complete traceability of the sterilization process for medical instruments.
- Sterilization validation and quality assurance integration: Advanced autoclave systems feature integrated validation protocols that ensure sterilization effectiveness through continuous monitoring and verification. These systems incorporate biological indicators, chemical indicators, and physical parameter monitoring that seamlessly integrate with quality management systems. The integration allows for automatic documentation of sterilization efficacy, alerts for cycle failures, and compliance with regulatory standards for medical device reprocessing.
- Instrument tracking and workflow optimization: Seamless integration of RFID and barcode technologies with autoclave systems enables comprehensive tracking of individual instruments throughout the sterilization process. These tracking systems integrate with inventory management software to optimize instrument availability, reduce processing delays, and ensure proper sterilization protocols are followed for specific instrument types. The technology creates a digital chain of custody that improves efficiency in sterile processing departments.
- Design innovations for improved sterilization efficiency: Novel autoclave chamber designs and loading systems facilitate more efficient sterilization of complex medical instruments. These innovations include specialized racks, holders, and chamber configurations that ensure steam penetration to all instrument surfaces while protecting delicate components. The designs integrate seamlessly with instrument sets and trays to maximize load capacity while maintaining sterilization efficacy and reducing cycle times.
- Material compatibility and sterilization process integration: Advanced formulations and materials have been developed to ensure compatibility between sterilization processes and sensitive medical instruments. These innovations include specialized lubricants, coatings, and packaging materials that withstand autoclave conditions while preserving instrument functionality. The materials integrate seamlessly with both the sterilization process and subsequent instrument use, extending instrument life while maintaining sterility assurance.
02 Instrument tracking and inventory management integration
Sterilization systems with integrated RFID or barcode technology enable seamless tracking of medical instruments throughout the sterilization process. These systems automatically record instrument information, sterilization history, and usage data, which can be integrated with hospital inventory management systems. This integration ensures proper instrument rotation, reduces loss, provides complete traceability from sterilization to patient use, and helps maintain compliance with regulatory requirements for medical device reprocessing.Expand Specific Solutions03 Sterilization process validation and quality assurance
Advanced autoclave systems incorporate integrated validation technologies that ensure sterilization effectiveness through continuous monitoring and documentation. These systems use biological indicators, chemical integrators, and physical parameter monitoring to verify that proper sterilization conditions have been achieved. The integration of these validation methods with digital documentation systems provides seamless quality assurance, enabling healthcare facilities to maintain consistent sterilization standards and demonstrate regulatory compliance through comprehensive, automated record-keeping.Expand Specific Solutions04 Network connectivity and remote monitoring capabilities
Modern autoclave systems feature network connectivity that enables remote monitoring and control of sterilization processes. These systems can be integrated with facility-wide networks, allowing authorized personnel to monitor sterilization cycles, receive alerts about cycle completion or failures, and access sterilization records from any connected device. This seamless integration improves operational efficiency by enabling real-time oversight of multiple sterilization units, facilitating preventive maintenance scheduling, and supporting data-driven decision making for sterilization department management.Expand Specific Solutions05 Specialized sterilization formulations and materials
Innovative sterilization formulations and materials have been developed to enhance autoclave effectiveness while ensuring compatibility with various medical instruments. These specialized formulations include sterilization indicators that change color when proper conditions are achieved, protective wrapping materials that maintain sterility while allowing steam penetration, and instrument coatings that resist degradation during repeated sterilization cycles. The seamless integration of these materials with autoclave systems improves sterilization efficacy, extends instrument life, and provides visual confirmation of successful sterilization.Expand Specific Solutions
Leading Manufacturers and Competitive Landscape
The autoclave instrument sterilization market is in a growth phase, driven by increasing demand for infection control in healthcare settings. The market size is expanding due to rising surgical procedures globally and stringent sterilization regulations. Technologically, the field is evolving from basic sterilization to integrated systems, with varying maturity levels among key players. Companies like Olympus Corp. and Karl Storz are leading with advanced integration capabilities, while Shinva Medical and Mocom (Cefla SC) are developing specialized sterilization solutions. Emerging players such as Turbett Surgical are introducing innovations in instrument organization during sterilization. The competitive landscape shows a mix of established medical device manufacturers and specialized sterilization technology providers working toward seamless integration of sterilization processes into broader healthcare workflows.
Turbett Surgical, Inc.
Technical Solution: Turbett Surgical has developed an innovative container system that enables seamless integration of autoclave sterilization into surgical workflows. Their patented technology allows for the sterilization of complete surgical trays with instruments arranged in the exact configuration needed for procedures. The system features specialized containers that maintain sterility while allowing steam penetration during the autoclave process. Their solution eliminates the need for traditional blue wrap, reducing waste and improving efficiency. The containers are designed with specialized filters and valve systems that ensure proper steam penetration while maintaining a sterile barrier post-sterilization. This technology significantly reduces instrument preparation time by up to 80% compared to conventional methods.
Strengths: Eliminates blue wrap waste, reduces preparation time, and maintains instrument organization throughout the sterilization process. Weaknesses: Requires initial investment in proprietary container systems and potential workflow adjustments during implementation.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva Medical has pioneered integrated autoclave sterilization systems with their comprehensive medical sterilization equipment portfolio. Their technology focuses on full-cycle sterilization management with digital tracking capabilities. Their autoclaves feature advanced PLC control systems that enable precise monitoring of critical parameters including temperature, pressure, and sterilization time. Shinva's systems incorporate RFID tracking technology to monitor instruments throughout the sterilization process, ensuring complete traceability. Their latest models include automated loading/unloading systems that integrate with hospital inventory management software, creating a seamless workflow from decontamination through sterilization to storage and distribution. The company has also developed specialized sterilization containers with integrated sensors that verify sterilization parameters have been met.
Strengths: Comprehensive sterilization ecosystem with digital integration capabilities and established market presence in Asia. Weaknesses: Less market penetration in Western markets compared to European and American competitors.
Key Technical Innovations in Sterilization Integration
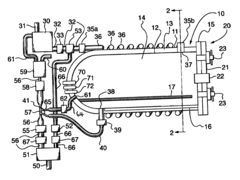
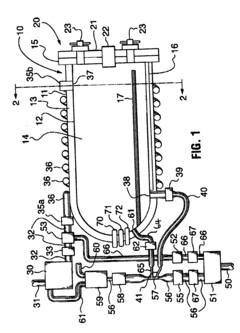
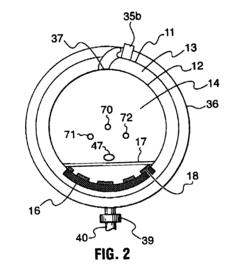
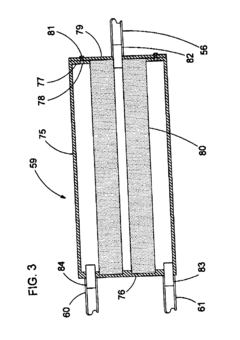
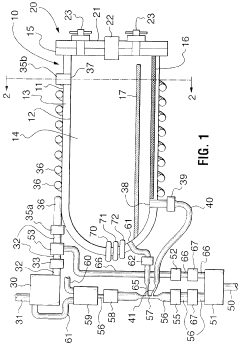
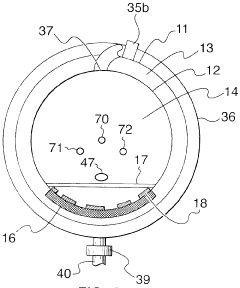
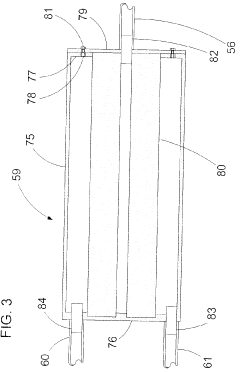
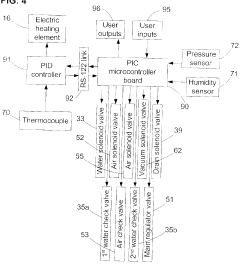
Fixed vacuum-insulated saturated steam autoclave
PatentInactiveUS20060057021A1
Innovation
- A double-walled vacuum-sealed vessel with a self-contained water supply and heat-conductive metal tubing, combined with a PID temperature controller and PIC microprocessor, allows for precise temperature control and rapid steam generation and removal, using concurrent positive and negative air pressures to ensure thorough sterilization and efficient operation.
Fixed vacuum-insulated saturated steam autoclave
PatentInactiveCA2559406A1
Innovation
- A double-walled vacuum-sealed vessel with a self-contained water supply and airflow system, using positive and negative air pressures to rapidly generate and remove steam, combined with a PID temperature controller and PIC microprocessor for precise temperature control, ensures a consistent sterilization environment within a portable, insulated chamber.
Standards and Compliance Requirements for Medical Sterilization
Medical device sterilization is governed by stringent regulatory frameworks that ensure patient safety and infection control. For autoclave instrument sterilization, compliance with international standards is non-negotiable. The primary regulatory bodies include the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and similar authorities in other regions, all of which enforce specific requirements for sterilization processes.
ISO 17665 stands as the cornerstone standard for moist heat sterilization, providing comprehensive guidelines for the development, validation, and routine control of sterilization processes. This standard requires that autoclave cycles be validated through biological indicators, chemical indicators, and physical measurements to ensure sterility assurance levels (SAL) of 10^-6, meaning a one-in-a-million probability of a viable microorganism surviving the process.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed complementary standards, including AAMI ST79, which offers detailed guidance on steam sterilization in healthcare facilities. These standards specify parameters such as temperature ranges (typically 121-134°C), pressure levels, and exposure times necessary for effective sterilization of different instrument types.
Documentation requirements represent another critical aspect of compliance. Healthcare facilities must maintain detailed records of each sterilization cycle, including time, temperature, pressure readings, and results of biological and chemical indicators. These records serve as evidence of compliance during regulatory inspections and are essential for traceability in case of infection outbreaks.
Equipment qualification is equally important, with standards requiring installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) for all autoclaves. Regular calibration and preventive maintenance must be documented to demonstrate ongoing compliance with performance specifications.
Recent regulatory trends have emphasized the integration of sterilization data into hospital information systems, requiring secure electronic record-keeping that complies with data integrity standards. This shift towards digital documentation necessitates cybersecurity measures to protect sterilization records from unauthorized access or modification.
Environmental considerations have also gained prominence in recent standards updates, with requirements for water conservation, energy efficiency, and proper management of sterilization byproducts. Facilities must demonstrate compliance with local environmental regulations while maintaining sterilization efficacy.
For seamless integration of autoclave sterilization into healthcare workflows, systems must be designed to meet these multifaceted compliance requirements while remaining user-friendly and efficient. This balance between regulatory adherence and operational practicality represents one of the central challenges in modern medical sterilization technology.
ISO 17665 stands as the cornerstone standard for moist heat sterilization, providing comprehensive guidelines for the development, validation, and routine control of sterilization processes. This standard requires that autoclave cycles be validated through biological indicators, chemical indicators, and physical measurements to ensure sterility assurance levels (SAL) of 10^-6, meaning a one-in-a-million probability of a viable microorganism surviving the process.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed complementary standards, including AAMI ST79, which offers detailed guidance on steam sterilization in healthcare facilities. These standards specify parameters such as temperature ranges (typically 121-134°C), pressure levels, and exposure times necessary for effective sterilization of different instrument types.
Documentation requirements represent another critical aspect of compliance. Healthcare facilities must maintain detailed records of each sterilization cycle, including time, temperature, pressure readings, and results of biological and chemical indicators. These records serve as evidence of compliance during regulatory inspections and are essential for traceability in case of infection outbreaks.
Equipment qualification is equally important, with standards requiring installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) for all autoclaves. Regular calibration and preventive maintenance must be documented to demonstrate ongoing compliance with performance specifications.
Recent regulatory trends have emphasized the integration of sterilization data into hospital information systems, requiring secure electronic record-keeping that complies with data integrity standards. This shift towards digital documentation necessitates cybersecurity measures to protect sterilization records from unauthorized access or modification.
Environmental considerations have also gained prominence in recent standards updates, with requirements for water conservation, energy efficiency, and proper management of sterilization byproducts. Facilities must demonstrate compliance with local environmental regulations while maintaining sterilization efficacy.
For seamless integration of autoclave sterilization into healthcare workflows, systems must be designed to meet these multifaceted compliance requirements while remaining user-friendly and efficient. This balance between regulatory adherence and operational practicality represents one of the central challenges in modern medical sterilization technology.
Energy Efficiency and Sustainability Considerations
The energy consumption of autoclave sterilization processes represents a significant operational cost for healthcare facilities and laboratories. Traditional autoclave systems are notorious for their high energy demands, primarily due to the extensive heating requirements to reach and maintain sterilization temperatures (typically 121-134°C) and the subsequent cooling phases. Modern autoclave designs are increasingly incorporating energy recovery systems that capture and reuse heat from exhaust steam, significantly reducing overall energy consumption by up to 25-30% compared to conventional models.
Water conservation has emerged as another critical sustainability consideration in autoclave operations. Conventional autoclaves can consume between 50-150 gallons of water per cycle, primarily for cooling and vacuum generation. Advanced water recirculation systems now enable facilities to reduce water usage by up to 90%, representing both environmental and economic benefits. These systems collect, filter, and reuse water across multiple sterilization cycles, dramatically reducing the resource footprint of sterilization operations.
The integration of smart control systems has revolutionized energy management in autoclave operations. Intelligent cycle optimization algorithms analyze load characteristics and automatically adjust cycle parameters to minimize energy and water consumption while maintaining sterilization efficacy. These systems can reduce cycle times by 15-20% and energy consumption by up to 40% compared to fixed-parameter cycles, particularly for partial loads or specialized instruments.
Carbon footprint reduction strategies for autoclave operations extend beyond the equipment itself to encompass facility-wide integration. Heat recovery systems can capture waste heat from sterilization processes to supplement building heating systems or preheat incoming water for subsequent cycles. Some healthcare facilities have reported annual energy savings of $10,000-30,000 through such integration approaches, with corresponding reductions in greenhouse gas emissions.
Lifecycle assessment studies indicate that the environmental impact of autoclave sterilization is heavily influenced by operational practices rather than manufacturing or disposal phases. Implementing preventive maintenance programs ensures optimal energy efficiency throughout the equipment lifecycle, while proper load organization and cycle selection significantly reduce unnecessary resource consumption. Training programs focused on sustainable operation practices have demonstrated reductions in energy use by 10-15% without capital investment.
The transition toward renewable energy sources presents another avenue for improving the sustainability profile of autoclave operations. Solar thermal systems can provide supplementary heating for water used in sterilization processes, while facilities with access to renewable electricity can significantly reduce the carbon intensity of their sterilization operations. Several healthcare institutions have successfully integrated autoclave systems into their broader renewable energy strategies, achieving carbon neutrality for their sterilization departments.
Water conservation has emerged as another critical sustainability consideration in autoclave operations. Conventional autoclaves can consume between 50-150 gallons of water per cycle, primarily for cooling and vacuum generation. Advanced water recirculation systems now enable facilities to reduce water usage by up to 90%, representing both environmental and economic benefits. These systems collect, filter, and reuse water across multiple sterilization cycles, dramatically reducing the resource footprint of sterilization operations.
The integration of smart control systems has revolutionized energy management in autoclave operations. Intelligent cycle optimization algorithms analyze load characteristics and automatically adjust cycle parameters to minimize energy and water consumption while maintaining sterilization efficacy. These systems can reduce cycle times by 15-20% and energy consumption by up to 40% compared to fixed-parameter cycles, particularly for partial loads or specialized instruments.
Carbon footprint reduction strategies for autoclave operations extend beyond the equipment itself to encompass facility-wide integration. Heat recovery systems can capture waste heat from sterilization processes to supplement building heating systems or preheat incoming water for subsequent cycles. Some healthcare facilities have reported annual energy savings of $10,000-30,000 through such integration approaches, with corresponding reductions in greenhouse gas emissions.
Lifecycle assessment studies indicate that the environmental impact of autoclave sterilization is heavily influenced by operational practices rather than manufacturing or disposal phases. Implementing preventive maintenance programs ensures optimal energy efficiency throughout the equipment lifecycle, while proper load organization and cycle selection significantly reduce unnecessary resource consumption. Training programs focused on sustainable operation practices have demonstrated reductions in energy use by 10-15% without capital investment.
The transition toward renewable energy sources presents another avenue for improving the sustainability profile of autoclave operations. Solar thermal systems can provide supplementary heating for water used in sterilization processes, while facilities with access to renewable electricity can significantly reduce the carbon intensity of their sterilization operations. Several healthcare institutions have successfully integrated autoclave systems into their broader renewable energy strategies, achieving carbon neutrality for their sterilization departments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!