Autoclave Sterilization of Pharmaceuticals: Process Control Optimization
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pharmaceutical Autoclave Sterilization Background and Objectives
Autoclave sterilization has been a cornerstone of pharmaceutical manufacturing for over a century, evolving from rudimentary pressure cookers to sophisticated computer-controlled systems. This sterilization method utilizes saturated steam under pressure to eliminate microbial contaminants, ensuring product safety and efficacy. The historical trajectory shows significant advancements in process control, materials science, and regulatory frameworks that have collectively enhanced sterilization reliability and efficiency.
The technology has progressed through several distinct phases, beginning with manual control systems in the early 20th century, advancing to semi-automated controls by mid-century, and culminating in today's fully integrated digital control architectures. Each evolutionary step has addressed specific limitations of previous generations, particularly in areas of temperature uniformity, cycle reproducibility, and process validation.
Current technological trends in pharmaceutical autoclave sterilization focus on energy efficiency, process intensification, and real-time monitoring capabilities. The integration of Industry 4.0 principles—including IoT sensors, machine learning algorithms, and predictive maintenance—represents the cutting edge of development. These innovations aim to minimize cycle times while maximizing sterilization assurance levels, thereby improving both productivity and product quality.
The primary objective of process control optimization in pharmaceutical autoclave sterilization is to establish robust, validated sterilization cycles that consistently deliver sterility assurance levels (SAL) of 10^-6 or better, while minimizing thermal exposure to protect product integrity. Secondary objectives include reducing energy consumption, water usage, and overall operational costs without compromising sterility outcomes.
Technical goals encompass the development of advanced sensing technologies capable of real-time bioburden assessment, adaptive control algorithms that can respond to load variations, and improved thermal distribution systems that eliminate cold spots. Additionally, there is significant interest in creating unified data management platforms that facilitate regulatory compliance through comprehensive batch documentation and trend analysis.
The pharmaceutical industry's increasing focus on personalized medicine and small-batch production presents new challenges for autoclave sterilization, driving research toward more flexible, rapid-cycle technologies. Simultaneously, sustainability imperatives are pushing innovation toward reduced resource consumption and environmental impact, creating tension between traditional validation approaches and emerging green technologies.
The technology has progressed through several distinct phases, beginning with manual control systems in the early 20th century, advancing to semi-automated controls by mid-century, and culminating in today's fully integrated digital control architectures. Each evolutionary step has addressed specific limitations of previous generations, particularly in areas of temperature uniformity, cycle reproducibility, and process validation.
Current technological trends in pharmaceutical autoclave sterilization focus on energy efficiency, process intensification, and real-time monitoring capabilities. The integration of Industry 4.0 principles—including IoT sensors, machine learning algorithms, and predictive maintenance—represents the cutting edge of development. These innovations aim to minimize cycle times while maximizing sterilization assurance levels, thereby improving both productivity and product quality.
The primary objective of process control optimization in pharmaceutical autoclave sterilization is to establish robust, validated sterilization cycles that consistently deliver sterility assurance levels (SAL) of 10^-6 or better, while minimizing thermal exposure to protect product integrity. Secondary objectives include reducing energy consumption, water usage, and overall operational costs without compromising sterility outcomes.
Technical goals encompass the development of advanced sensing technologies capable of real-time bioburden assessment, adaptive control algorithms that can respond to load variations, and improved thermal distribution systems that eliminate cold spots. Additionally, there is significant interest in creating unified data management platforms that facilitate regulatory compliance through comprehensive batch documentation and trend analysis.
The pharmaceutical industry's increasing focus on personalized medicine and small-batch production presents new challenges for autoclave sterilization, driving research toward more flexible, rapid-cycle technologies. Simultaneously, sustainability imperatives are pushing innovation toward reduced resource consumption and environmental impact, creating tension between traditional validation approaches and emerging green technologies.
Market Demand Analysis for Advanced Sterilization Technologies
The global market for advanced sterilization technologies in pharmaceutical manufacturing has witnessed substantial growth, driven by increasing regulatory scrutiny, rising demand for sterile pharmaceutical products, and technological advancements. The autoclave sterilization segment, valued at approximately $1.2 billion in 2022, is projected to grow at a compound annual growth rate of 6.8% through 2028, reflecting the persistent demand for reliable sterilization methods.
Healthcare facilities worldwide are experiencing heightened pressure to improve sterilization processes due to the rising incidence of healthcare-associated infections (HAIs). This concern has catalyzed demand for more sophisticated sterilization technologies with enhanced process control capabilities. The COVID-19 pandemic further accelerated this trend, as pharmaceutical manufacturers faced unprecedented challenges in maintaining sterile production environments while scaling operations.
Regulatory bodies, including the FDA and EMA, have intensified their focus on sterilization validation and process control, creating a robust market pull for advanced monitoring and optimization solutions. The implementation of FDA's Process Analytical Technology (PAT) framework has particularly influenced market dynamics, encouraging pharmaceutical manufacturers to adopt real-time monitoring systems for sterilization processes.
Regional analysis reveals varying market penetration rates for advanced sterilization technologies. North America and Europe currently dominate the market with approximately 65% combined share, attributed to stringent regulatory frameworks and higher technology adoption rates. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and India leading pharmaceutical manufacturing expansion.
Industry surveys indicate that 78% of pharmaceutical manufacturers plan to upgrade their sterilization infrastructure within the next three years, with process control optimization being a primary focus area. This trend is particularly pronounced among biologics and parenteral drug manufacturers, where product sensitivity demands precise sterilization parameters.
The market for sterilization process optimization software has emerged as a high-growth segment, expected to expand at 12.3% annually through 2027. This growth reflects the industry's shift toward digitalization and data-driven decision-making in sterilization processes. Integration capabilities with existing manufacturing execution systems (MES) have become a critical purchasing criterion for pharmaceutical companies investing in sterilization technology.
Cost considerations remain significant market drivers, with energy efficiency improvements and reduced cycle times offering compelling return on investment. Studies demonstrate that optimized autoclave processes can reduce energy consumption by 15-30% while maintaining or improving sterilization efficacy, creating strong economic incentives for technology adoption beyond regulatory compliance.
Healthcare facilities worldwide are experiencing heightened pressure to improve sterilization processes due to the rising incidence of healthcare-associated infections (HAIs). This concern has catalyzed demand for more sophisticated sterilization technologies with enhanced process control capabilities. The COVID-19 pandemic further accelerated this trend, as pharmaceutical manufacturers faced unprecedented challenges in maintaining sterile production environments while scaling operations.
Regulatory bodies, including the FDA and EMA, have intensified their focus on sterilization validation and process control, creating a robust market pull for advanced monitoring and optimization solutions. The implementation of FDA's Process Analytical Technology (PAT) framework has particularly influenced market dynamics, encouraging pharmaceutical manufacturers to adopt real-time monitoring systems for sterilization processes.
Regional analysis reveals varying market penetration rates for advanced sterilization technologies. North America and Europe currently dominate the market with approximately 65% combined share, attributed to stringent regulatory frameworks and higher technology adoption rates. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and India leading pharmaceutical manufacturing expansion.
Industry surveys indicate that 78% of pharmaceutical manufacturers plan to upgrade their sterilization infrastructure within the next three years, with process control optimization being a primary focus area. This trend is particularly pronounced among biologics and parenteral drug manufacturers, where product sensitivity demands precise sterilization parameters.
The market for sterilization process optimization software has emerged as a high-growth segment, expected to expand at 12.3% annually through 2027. This growth reflects the industry's shift toward digitalization and data-driven decision-making in sterilization processes. Integration capabilities with existing manufacturing execution systems (MES) have become a critical purchasing criterion for pharmaceutical companies investing in sterilization technology.
Cost considerations remain significant market drivers, with energy efficiency improvements and reduced cycle times offering compelling return on investment. Studies demonstrate that optimized autoclave processes can reduce energy consumption by 15-30% while maintaining or improving sterilization efficacy, creating strong economic incentives for technology adoption beyond regulatory compliance.
Current Challenges in Autoclave Process Control
Despite significant advancements in autoclave technology, pharmaceutical sterilization processes face several persistent challenges that impact efficiency, reliability, and product quality. The primary challenge remains achieving uniform heat distribution throughout the autoclave chamber. Temperature gradients can form in different areas of the chamber, particularly in larger industrial autoclaves, leading to inconsistent sterilization results. This is especially problematic for dense loads or products with complex geometries where steam penetration may be compromised.
Validation and monitoring systems present another significant hurdle. Current sensor technologies often provide limited spatial resolution, creating blind spots in temperature and pressure monitoring. The placement of biological and chemical indicators may not accurately represent the most challenging sterilization conditions within the load, potentially leading to false assurances of sterility.
Process cycle optimization remains challenging due to the complex interplay between temperature, pressure, and time parameters. Many facilities still rely on conservative, one-size-fits-all cycles rather than tailored approaches for specific product requirements. This results in unnecessarily extended cycle times, increased energy consumption, and potential product degradation from excessive heat exposure.
The integration of automation systems with legacy equipment creates compatibility issues in many pharmaceutical manufacturing environments. Older autoclave systems often lack the necessary interfaces for modern control systems, making real-time monitoring and adaptive control difficult to implement. This technological gap hinders the adoption of Industry 4.0 principles in sterilization processes.
Water quality management presents ongoing challenges, as mineral deposits and contaminants can affect steam quality and subsequently impact sterilization efficacy. Inadequate water treatment systems lead to increased maintenance requirements and potential sterilization failures.
Documentation and compliance with increasingly stringent regulatory requirements create additional process control burdens. The need for comprehensive data collection, storage, and analysis to satisfy regulatory bodies like FDA and EMA demands sophisticated control systems that many facilities have yet to fully implement.
Energy efficiency remains a significant concern, with traditional autoclave processes consuming substantial amounts of steam, water, and electricity. Current control systems often prioritize sterilization assurance over resource optimization, resulting in unnecessarily high operational costs and environmental impact.
Human factors and operator training issues persist, as the complexity of modern autoclave systems requires specialized knowledge that may not be consistently available across all work shifts, potentially leading to procedural variations and errors in process control.
Validation and monitoring systems present another significant hurdle. Current sensor technologies often provide limited spatial resolution, creating blind spots in temperature and pressure monitoring. The placement of biological and chemical indicators may not accurately represent the most challenging sterilization conditions within the load, potentially leading to false assurances of sterility.
Process cycle optimization remains challenging due to the complex interplay between temperature, pressure, and time parameters. Many facilities still rely on conservative, one-size-fits-all cycles rather than tailored approaches for specific product requirements. This results in unnecessarily extended cycle times, increased energy consumption, and potential product degradation from excessive heat exposure.
The integration of automation systems with legacy equipment creates compatibility issues in many pharmaceutical manufacturing environments. Older autoclave systems often lack the necessary interfaces for modern control systems, making real-time monitoring and adaptive control difficult to implement. This technological gap hinders the adoption of Industry 4.0 principles in sterilization processes.
Water quality management presents ongoing challenges, as mineral deposits and contaminants can affect steam quality and subsequently impact sterilization efficacy. Inadequate water treatment systems lead to increased maintenance requirements and potential sterilization failures.
Documentation and compliance with increasingly stringent regulatory requirements create additional process control burdens. The need for comprehensive data collection, storage, and analysis to satisfy regulatory bodies like FDA and EMA demands sophisticated control systems that many facilities have yet to fully implement.
Energy efficiency remains a significant concern, with traditional autoclave processes consuming substantial amounts of steam, water, and electricity. Current control systems often prioritize sterilization assurance over resource optimization, resulting in unnecessarily high operational costs and environmental impact.
Human factors and operator training issues persist, as the complexity of modern autoclave systems requires specialized knowledge that may not be consistently available across all work shifts, potentially leading to procedural variations and errors in process control.
Current Process Control Methodologies and Systems
01 Temperature and pressure monitoring systems
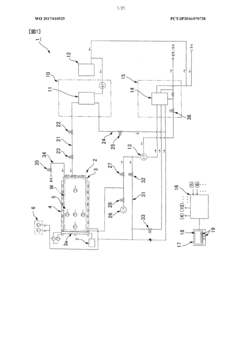
Effective autoclave sterilization requires precise control of temperature and pressure parameters. Advanced monitoring systems are employed to continuously track these critical variables throughout the sterilization cycle. These systems often include sensors, gauges, and digital interfaces that provide real-time data and ensure that sterilization conditions are maintained within specified ranges. Some systems incorporate alarms that activate when parameters deviate from acceptable limits, allowing for immediate corrective action.- Temperature and pressure monitoring systems: Effective autoclave sterilization requires precise control of temperature and pressure parameters. Modern autoclaves incorporate advanced monitoring systems that continuously track these critical variables throughout the sterilization cycle. These systems often include multiple sensors at different locations within the chamber to ensure uniform conditions, digital displays for real-time monitoring, and automated recording capabilities for documentation and validation purposes. Some advanced systems also feature predictive algorithms that can anticipate deviations and make adjustments before parameters fall outside acceptable ranges.
- Biological and chemical indicators for validation: Validation of autoclave sterilization processes often relies on biological and chemical indicators that provide visible confirmation of effective sterilization. Biological indicators contain resistant bacterial spores that are killed only when proper sterilization conditions are achieved. Chemical indicators change color or physical state when exposed to specific temperature and pressure conditions. These indicators are strategically placed within loads, especially in hard-to-sterilize areas, to verify that sterilization parameters have penetrated throughout the entire load. Regular use of these indicators is essential for quality assurance in medical, pharmaceutical, and laboratory settings.
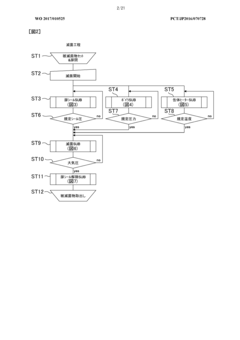
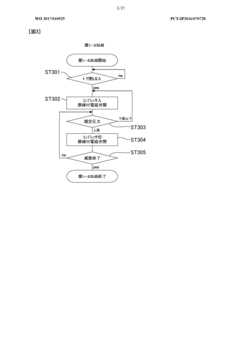
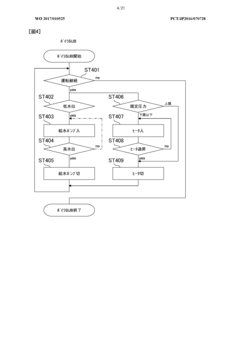
- Automated cycle control and documentation: Modern autoclave sterilization processes employ automated control systems that manage the entire sterilization cycle from start to finish. These systems precisely control the sequence of steps including pre-vacuum phases, heating, sterilization hold time, exhaust, and drying. Automated documentation systems record all critical parameters throughout each cycle, generating detailed reports that serve as evidence of proper sterilization for regulatory compliance. Some advanced systems include barcode tracking for load contents, operator identification, and maintenance records, creating a comprehensive audit trail for each sterilization cycle.
- Steam quality and penetration optimization: The effectiveness of autoclave sterilization depends significantly on steam quality and its ability to penetrate all surfaces of the items being sterilized. Advanced process control systems monitor steam saturation, purity, and distribution throughout the chamber. Pre-vacuum cycles remove air pockets that could prevent steam contact with surfaces. Steam penetration can be enhanced through proper loading techniques, use of appropriate packaging materials, and cycle parameters tailored to specific load types. Some systems incorporate pulsed vacuum phases or steam injection patterns designed to improve penetration into complex instruments or dense loads.
- Fault detection and safety mechanisms: Reliable autoclave sterilization requires robust fault detection systems and safety mechanisms to prevent failed sterilization cycles or equipment damage. These systems continuously monitor for deviations from programmed parameters and can automatically abort cycles when conditions fall outside acceptable ranges. Safety features include pressure relief valves, door interlocks that prevent opening during cycles, and redundant temperature monitoring. Advanced systems incorporate self-diagnostic capabilities that can identify potential issues before they cause cycle failures, along with alarm systems that alert operators to problems requiring intervention.
02 Biological and chemical indicators for validation
Validation of autoclave sterilization processes often relies on biological and chemical indicators. Biological indicators contain resistant bacterial spores that are used to verify that sterilization conditions were sufficient to kill microorganisms. Chemical indicators change color or physical state when exposed to specific sterilization conditions, providing visual confirmation of process parameters. These indicators are strategically placed within loads to ensure that sterilization conditions penetrate throughout the entire chamber and all items being sterilized.Expand Specific Solutions03 Automated control systems and cycle documentation
Modern autoclave sterilization processes utilize automated control systems that manage the entire sterilization cycle. These systems regulate steam injection, temperature maintenance, pressure control, and cycle timing according to pre-programmed parameters. Additionally, they provide comprehensive documentation of each sterilization cycle, recording critical data points throughout the process. This documentation is essential for regulatory compliance and quality assurance, creating verifiable records that sterilization parameters were achieved and maintained for the required duration.Expand Specific Solutions04 Steam quality and distribution optimization
The quality and distribution of steam are crucial factors in effective autoclave sterilization. Control systems monitor steam saturation, purity, and penetration to ensure uniform heat distribution throughout the chamber. Methods for optimizing steam distribution include pre-vacuum phases to remove air pockets, pulsed vacuum systems, and specialized chamber designs. Steam quality is maintained through water treatment systems, steam generators, and filters that remove impurities that could interfere with the sterilization process or contaminate sterilized items.Expand Specific Solutions05 Load configuration and packaging considerations
Proper load configuration and packaging significantly impact autoclave sterilization effectiveness. Control systems may include sensors that detect improper loading patterns or excessive density that could impede steam penetration. Specialized packaging materials are designed to allow steam penetration during sterilization while maintaining sterility afterward. Some advanced systems incorporate load-specific cycle parameters that adjust sterilization conditions based on the specific requirements of different materials and configurations, ensuring effective sterilization while minimizing damage to sensitive items.Expand Specific Solutions
Leading Manufacturers and Technology Providers
Autoclave sterilization in pharmaceuticals is currently in a mature growth phase, with the global market valued at approximately $2.5 billion and projected to expand at a CAGR of 6-8% through 2028. The competitive landscape features established players like Baxter International and F. Hoffmann-La Roche dominating with comprehensive solutions, while specialized equipment manufacturers such as Shinva Medical Instrument and Jiangyin Binjiang Medical Equipment focus on technical innovations. Companies including Olympus Corp. and Canon are advancing process control optimization through automation and IoT integration. The technology has reached high maturity levels with standardized protocols, though innovation continues in energy efficiency and process validation, particularly from Siemens AG and Parker-Hannifin Corp. who are introducing smart monitoring systems for pharmaceutical sterilization processes.
Baxter International, Inc.
Technical Solution: Baxter has developed an advanced autoclave sterilization system for pharmaceuticals featuring real-time monitoring and adaptive process control. Their technology incorporates multiple temperature and pressure sensors throughout the chamber to create thermal mapping profiles that ensure uniform heat distribution. The system employs predictive algorithms that dynamically adjust sterilization parameters based on load density and composition. Baxter's solution includes vapor-phase hydrogen peroxide pre-treatment to reduce bioburden before the main autoclave cycle, significantly improving sterilization efficacy while reducing cycle time by approximately 15%. Their integrated validation system continuously monitors critical parameters (F0 values, temperature distribution, pressure) and automatically adjusts steam injection rates and exhaust valve positions to maintain optimal conditions throughout the cycle.
Strengths: Superior uniformity in heat distribution across varied load configurations; reduced cycle times while maintaining sterility assurance levels; comprehensive data logging for regulatory compliance. Weaknesses: Higher initial capital investment compared to conventional systems; requires specialized training for operators; system complexity can increase maintenance requirements.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva has pioneered a comprehensive autoclave sterilization platform specifically designed for pharmaceutical manufacturing environments. Their system features multi-parameter optimization technology that simultaneously controls temperature, pressure, and humidity with precision tolerances of ±0.5°C and ±0.05 bar. The platform incorporates advanced PID (Proportional-Integral-Derivative) control algorithms that continuously adjust steam input and chamber conditions based on real-time feedback. Shinva's solution includes patented steam distribution technology with multiple injection points to eliminate cold spots and ensure homogeneous conditions throughout the chamber. Their process control system features adaptive cycle programming that automatically optimizes sterilization parameters based on load characteristics, reducing energy consumption by up to 25% compared to fixed-parameter systems. The technology also includes integrated air detection systems to prevent air pockets that could compromise sterilization efficacy.
Strengths: Exceptional precision in maintaining critical parameters; significant energy efficiency improvements; comprehensive validation documentation that meets global regulatory standards. Weaknesses: More complex installation requirements; higher maintenance costs due to sophisticated control systems; requires stable utility infrastructure (steam quality, water, electricity) for optimal performance.
Key Innovations in Autoclave Parameter Optimization
Method of flow-type high-pressure steam sterilization by soft water heat process, and flow-type sterilization device
PatentWO2017010525A1
Innovation
- A flow-through high-pressure steam sterilization method utilizing a soft hydrothermal process, which involves an air removal process, heating and pressurizing, high-pressure steam sterilization with highly saturated steam, and a controlled drying step to minimize condensed water generation and shorten drying time.
Autoclave for terminal sterilization of pharmaceutical preparations and medical products
PatentInactiveJP2010531677A
Innovation
- A method involving ultrahigh pressure (UHP) sterilization, where systems are exposed to pressures above 0.25 MPa and temperatures above 70°C for a sufficient time to achieve sterilization without significant degradation, followed by rapid adiabatic cooling to ambient conditions.
Regulatory Compliance and Validation Requirements
Pharmaceutical autoclave sterilization processes are subject to stringent regulatory frameworks established by global health authorities. The FDA's Current Good Manufacturing Practice (cGMP) regulations, specifically 21 CFR Parts 210 and 211, mandate comprehensive validation of sterilization processes to ensure product safety and efficacy. Similarly, the European Medicines Agency (EMA) enforces GMP guidelines that require thorough documentation and validation of all sterilization parameters.
Process validation for autoclave sterilization typically follows a three-phase approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that equipment is properly installed according to manufacturer specifications. OQ confirms that the autoclave operates within predetermined parameters under various conditions. PQ demonstrates that the process consistently produces sterile products under routine operating conditions.
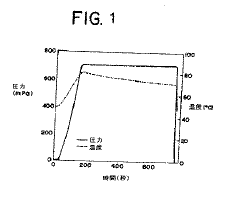
Critical validation requirements include the establishment of scientifically sound acceptance criteria for critical process parameters such as temperature, pressure, and hold time. These parameters must be continuously monitored and documented during each sterilization cycle. The FDA's Process Validation Guidance emphasizes the need for ongoing process verification to ensure continued compliance and product quality.
Validation protocols must include worst-case scenarios and load configurations to demonstrate process robustness. Heat distribution studies using temperature mapping with calibrated thermocouples are essential to identify cold spots within the autoclave chamber. Biological indicators containing resistant microorganisms (typically Geobacillus stearothermophilus spores) serve as the gold standard for validating sterilization effectiveness.
Documentation requirements are extensive and include validation master plans, protocols, reports, and standard operating procedures. All deviations must be thoroughly investigated, and corrective actions implemented and documented. Electronic records must comply with 21 CFR Part 11 requirements for data integrity and security.
Revalidation is necessary following significant changes to equipment, processes, or products. The frequency of routine revalidation should be risk-based and justified through ongoing monitoring data. Regulatory agencies increasingly emphasize Quality by Design (QbD) principles, encouraging manufacturers to build quality into processes rather than relying solely on end-product testing.
International harmonization efforts through organizations like the Pharmaceutical Inspection Co-operation Scheme (PIC/S) and the International Council for Harmonisation (ICH) have established globally recognized standards for validation. Compliance with these standards facilitates regulatory approval across multiple markets and ensures consistent product quality worldwide.
Process validation for autoclave sterilization typically follows a three-phase approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that equipment is properly installed according to manufacturer specifications. OQ confirms that the autoclave operates within predetermined parameters under various conditions. PQ demonstrates that the process consistently produces sterile products under routine operating conditions.
Critical validation requirements include the establishment of scientifically sound acceptance criteria for critical process parameters such as temperature, pressure, and hold time. These parameters must be continuously monitored and documented during each sterilization cycle. The FDA's Process Validation Guidance emphasizes the need for ongoing process verification to ensure continued compliance and product quality.
Validation protocols must include worst-case scenarios and load configurations to demonstrate process robustness. Heat distribution studies using temperature mapping with calibrated thermocouples are essential to identify cold spots within the autoclave chamber. Biological indicators containing resistant microorganisms (typically Geobacillus stearothermophilus spores) serve as the gold standard for validating sterilization effectiveness.
Documentation requirements are extensive and include validation master plans, protocols, reports, and standard operating procedures. All deviations must be thoroughly investigated, and corrective actions implemented and documented. Electronic records must comply with 21 CFR Part 11 requirements for data integrity and security.
Revalidation is necessary following significant changes to equipment, processes, or products. The frequency of routine revalidation should be risk-based and justified through ongoing monitoring data. Regulatory agencies increasingly emphasize Quality by Design (QbD) principles, encouraging manufacturers to build quality into processes rather than relying solely on end-product testing.
International harmonization efforts through organizations like the Pharmaceutical Inspection Co-operation Scheme (PIC/S) and the International Council for Harmonisation (ICH) have established globally recognized standards for validation. Compliance with these standards facilitates regulatory approval across multiple markets and ensures consistent product quality worldwide.
Energy Efficiency and Sustainability Considerations
Autoclave sterilization processes in pharmaceutical manufacturing are significant energy consumers, requiring substantial thermal energy for heating and maintaining high temperatures. Traditional autoclave systems often operate with energy efficiency rates of only 30-40%, representing a critical area for optimization in an industry increasingly focused on sustainability metrics.
Energy consumption in autoclave operations can be substantially reduced through implementation of advanced insulation materials and heat recovery systems. Recent developments in ceramic-based insulation technologies have demonstrated potential energy savings of 15-25% compared to conventional materials, while maintaining required temperature profiles. Heat recovery systems that capture and repurpose steam from completed cycles can further reduce energy requirements by 10-20%, depending on facility configuration and process demands.
Water usage represents another significant sustainability concern in autoclave operations. Standard pharmaceutical sterilization processes may consume 3-5 liters of water per kilogram of product sterilized. Advanced water recycling systems incorporating multi-stage filtration and purification can reduce this consumption by up to 60%, while maintaining compliance with regulatory requirements for water quality in pharmaceutical manufacturing environments.
Carbon footprint reduction has become increasingly important as pharmaceutical companies establish sustainability targets aligned with global climate initiatives. Implementation of optimized process control systems can reduce greenhouse gas emissions associated with autoclave operations by 20-30% through more precise temperature and pressure management, minimizing unnecessary extended sterilization cycles and associated energy expenditure.
Regulatory frameworks increasingly incorporate sustainability considerations, with the FDA and EMA both publishing guidance documents on environmental aspects of pharmaceutical manufacturing. Companies demonstrating improved sustainability metrics in sterilization processes may gain competitive advantages in regulatory approvals and market positioning, particularly in regions with stringent environmental regulations such as the European Union.
Return on investment analyses indicate that energy efficiency improvements in autoclave sterilization typically achieve payback periods of 18-36 months, depending on facility scale and operational patterns. This favorable economic profile, combined with corporate sustainability commitments, has accelerated adoption of energy-efficient technologies across the pharmaceutical manufacturing sector, with implementation rates increasing approximately 15% annually since 2018.
Energy consumption in autoclave operations can be substantially reduced through implementation of advanced insulation materials and heat recovery systems. Recent developments in ceramic-based insulation technologies have demonstrated potential energy savings of 15-25% compared to conventional materials, while maintaining required temperature profiles. Heat recovery systems that capture and repurpose steam from completed cycles can further reduce energy requirements by 10-20%, depending on facility configuration and process demands.
Water usage represents another significant sustainability concern in autoclave operations. Standard pharmaceutical sterilization processes may consume 3-5 liters of water per kilogram of product sterilized. Advanced water recycling systems incorporating multi-stage filtration and purification can reduce this consumption by up to 60%, while maintaining compliance with regulatory requirements for water quality in pharmaceutical manufacturing environments.
Carbon footprint reduction has become increasingly important as pharmaceutical companies establish sustainability targets aligned with global climate initiatives. Implementation of optimized process control systems can reduce greenhouse gas emissions associated with autoclave operations by 20-30% through more precise temperature and pressure management, minimizing unnecessary extended sterilization cycles and associated energy expenditure.
Regulatory frameworks increasingly incorporate sustainability considerations, with the FDA and EMA both publishing guidance documents on environmental aspects of pharmaceutical manufacturing. Companies demonstrating improved sustainability metrics in sterilization processes may gain competitive advantages in regulatory approvals and market positioning, particularly in regions with stringent environmental regulations such as the European Union.
Return on investment analyses indicate that energy efficiency improvements in autoclave sterilization typically achieve payback periods of 18-36 months, depending on facility scale and operational patterns. This favorable economic profile, combined with corporate sustainability commitments, has accelerated adoption of energy-efficient technologies across the pharmaceutical manufacturing sector, with implementation rates increasing approximately 15% annually since 2018.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!