How to Enhance Autoclave Efficiency in Large-Scale Production
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Efficiency Goals
Autoclave technology has evolved significantly since its inception in the early 20th century. Initially developed for sterilization in medical settings, autoclaves have transformed into sophisticated pressure vessels capable of facilitating complex chemical reactions and material processing across various industries. The evolution trajectory shows a clear shift from simple steam-based systems to highly automated, computer-controlled environments with advanced monitoring capabilities. This technological progression has been driven by increasing demands for efficiency, reliability, and scalability in manufacturing processes, particularly in aerospace, composites, and pharmaceutical industries.
The current generation of industrial autoclaves represents a convergence of mechanical engineering, materials science, and digital control systems. Modern units incorporate precision temperature control, uniform pressure distribution mechanisms, and energy recovery systems that were unimaginable in earlier iterations. Despite these advancements, large-scale production environments continue to face significant challenges related to energy consumption, cycle time optimization, and consistent quality outcomes across batch processing.
Industry benchmarks indicate that state-of-the-art autoclave systems operate at approximately 65-75% energy efficiency, with cycle times that have decreased by approximately 30% over the past decade. However, theoretical models suggest potential improvements could push efficiency ratings above 85% while further reducing cycle times by an additional 20-25%. These improvements would translate directly to increased production capacity and reduced operational costs.
The primary efficiency goals for next-generation autoclave technology center around four key dimensions: energy optimization, cycle time reduction, quality consistency, and operational flexibility. Energy optimization focuses on minimizing heat loss, improving insulation systems, and implementing intelligent heating algorithms that adapt to specific material requirements. Cycle time reduction targets include advanced loading/unloading automation, optimized heating and cooling curves, and parallel processing capabilities.
Quality consistency goals involve the development of more sophisticated sensor networks for real-time monitoring and adaptive control systems that can respond to minute variations in processing conditions. Operational flexibility aims to create autoclave systems capable of handling diverse material types and geometries without significant reconfiguration, enabling manufacturers to respond more effectively to changing production demands.
The technological roadmap for achieving these efficiency goals includes the integration of artificial intelligence for predictive maintenance and process optimization, advanced materials for improved thermal performance, and modular design approaches that allow for scalable implementation across different production environments. These developments align with broader industry trends toward smart manufacturing and sustainable production practices.
The current generation of industrial autoclaves represents a convergence of mechanical engineering, materials science, and digital control systems. Modern units incorporate precision temperature control, uniform pressure distribution mechanisms, and energy recovery systems that were unimaginable in earlier iterations. Despite these advancements, large-scale production environments continue to face significant challenges related to energy consumption, cycle time optimization, and consistent quality outcomes across batch processing.
Industry benchmarks indicate that state-of-the-art autoclave systems operate at approximately 65-75% energy efficiency, with cycle times that have decreased by approximately 30% over the past decade. However, theoretical models suggest potential improvements could push efficiency ratings above 85% while further reducing cycle times by an additional 20-25%. These improvements would translate directly to increased production capacity and reduced operational costs.
The primary efficiency goals for next-generation autoclave technology center around four key dimensions: energy optimization, cycle time reduction, quality consistency, and operational flexibility. Energy optimization focuses on minimizing heat loss, improving insulation systems, and implementing intelligent heating algorithms that adapt to specific material requirements. Cycle time reduction targets include advanced loading/unloading automation, optimized heating and cooling curves, and parallel processing capabilities.
Quality consistency goals involve the development of more sophisticated sensor networks for real-time monitoring and adaptive control systems that can respond to minute variations in processing conditions. Operational flexibility aims to create autoclave systems capable of handling diverse material types and geometries without significant reconfiguration, enabling manufacturers to respond more effectively to changing production demands.
The technological roadmap for achieving these efficiency goals includes the integration of artificial intelligence for predictive maintenance and process optimization, advanced materials for improved thermal performance, and modular design approaches that allow for scalable implementation across different production environments. These developments align with broader industry trends toward smart manufacturing and sustainable production practices.
Market Demand Analysis for High-Efficiency Sterilization
The global sterilization market is experiencing robust growth, driven by increasing demand across healthcare, pharmaceutical, food processing, and industrial manufacturing sectors. The market for high-efficiency sterilization equipment, particularly autoclaves, is projected to reach $3.5 billion by 2027, growing at a CAGR of 6.8% from 2022. This growth is primarily fueled by stringent regulatory requirements for product safety and quality assurance in various industries.
In the healthcare and pharmaceutical sectors, the demand for efficient sterilization solutions has intensified due to the global pandemic, highlighting the critical importance of infection control and prevention. Hospitals, clinics, and pharmaceutical manufacturers are increasingly seeking autoclave systems that offer faster cycle times, reduced energy consumption, and enhanced capacity to handle larger batches of medical devices and pharmaceutical products.
The food and beverage industry represents another significant market segment, where high-efficiency sterilization is essential for ensuring product safety and extending shelf life. Consumer preferences for minimally processed foods with extended shelf stability have driven manufacturers to invest in advanced sterilization technologies that preserve nutritional value while ensuring microbial safety.
Market research indicates a growing preference for autoclave systems that incorporate smart technologies, including IoT connectivity, real-time monitoring, and predictive maintenance capabilities. These features not only enhance operational efficiency but also provide valuable data for quality control and regulatory compliance. Approximately 65% of large-scale manufacturers surveyed expressed interest in upgrading to smart autoclave systems within the next three years.
Energy efficiency has emerged as a critical factor influencing purchasing decisions, with manufacturers seeking solutions that reduce operational costs while minimizing environmental impact. The market shows strong demand for autoclaves that incorporate heat recovery systems, improved insulation, and optimized steam generation processes, potentially reducing energy consumption by 20-30% compared to conventional systems.
Regional analysis reveals that North America and Europe currently dominate the high-efficiency sterilization market, accounting for approximately 60% of global demand. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing healthcare expenditure, and growing adoption of international quality standards in manufacturing processes.
Customer feedback indicates that return on investment (ROI) considerations are paramount, with buyers seeking solutions that demonstrate clear cost benefits through improved throughput, reduced cycle times, and lower operational costs. The market increasingly values comprehensive service packages, including installation, training, validation support, and preventive maintenance programs.
In the healthcare and pharmaceutical sectors, the demand for efficient sterilization solutions has intensified due to the global pandemic, highlighting the critical importance of infection control and prevention. Hospitals, clinics, and pharmaceutical manufacturers are increasingly seeking autoclave systems that offer faster cycle times, reduced energy consumption, and enhanced capacity to handle larger batches of medical devices and pharmaceutical products.
The food and beverage industry represents another significant market segment, where high-efficiency sterilization is essential for ensuring product safety and extending shelf life. Consumer preferences for minimally processed foods with extended shelf stability have driven manufacturers to invest in advanced sterilization technologies that preserve nutritional value while ensuring microbial safety.
Market research indicates a growing preference for autoclave systems that incorporate smart technologies, including IoT connectivity, real-time monitoring, and predictive maintenance capabilities. These features not only enhance operational efficiency but also provide valuable data for quality control and regulatory compliance. Approximately 65% of large-scale manufacturers surveyed expressed interest in upgrading to smart autoclave systems within the next three years.
Energy efficiency has emerged as a critical factor influencing purchasing decisions, with manufacturers seeking solutions that reduce operational costs while minimizing environmental impact. The market shows strong demand for autoclaves that incorporate heat recovery systems, improved insulation, and optimized steam generation processes, potentially reducing energy consumption by 20-30% compared to conventional systems.
Regional analysis reveals that North America and Europe currently dominate the high-efficiency sterilization market, accounting for approximately 60% of global demand. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing healthcare expenditure, and growing adoption of international quality standards in manufacturing processes.
Customer feedback indicates that return on investment (ROI) considerations are paramount, with buyers seeking solutions that demonstrate clear cost benefits through improved throughput, reduced cycle times, and lower operational costs. The market increasingly values comprehensive service packages, including installation, training, validation support, and preventive maintenance programs.
Current Autoclave Systems and Technical Limitations
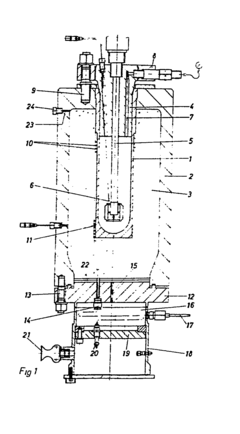
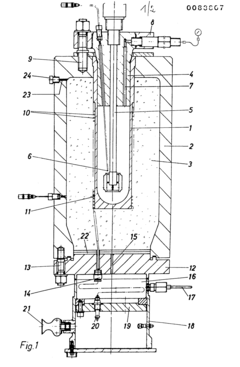
Autoclaves are critical equipment in large-scale production environments across various industries, including aerospace, composites manufacturing, medical device sterilization, and food processing. Current industrial autoclave systems typically consist of pressure vessels designed to withstand high temperatures and pressures, sophisticated control systems, heating mechanisms, vacuum systems, and cooling systems. These components work together to create controlled environments for curing, sterilization, or other chemical processes.
Standard industrial autoclaves range from small laboratory units to massive industrial chambers exceeding 50 meters in length. Most large-scale production autoclaves operate at temperatures between 120-180°C and pressures of 6-12 bar, though specialized applications may require more extreme conditions. Modern systems incorporate computerized control interfaces, programmable cycle parameters, and data logging capabilities for process validation and quality assurance.
Despite technological advancements, current autoclave systems face significant technical limitations that impact production efficiency. Energy consumption remains a primary concern, with large autoclaves requiring substantial power for heating and maintaining temperature uniformity throughout the chamber. This results in high operational costs and considerable carbon footprints, particularly problematic in today's sustainability-focused manufacturing landscape.
Thermal inefficiency represents another major limitation. Temperature gradients within large chambers lead to inconsistent processing conditions, affecting product quality and necessitating longer cycle times to ensure complete processing. Heat loss through vessel walls and door seals further compounds these inefficiencies, despite improvements in insulation technologies.
Cycle time optimization presents ongoing challenges. The inherent physics of heat transfer in large vessels creates bottlenecks in production throughput. Loading and unloading operations, particularly for large or delicate components, introduce additional non-productive time that reduces overall equipment effectiveness.
Control system limitations also persist in many installations. While modern PLCs offer improved capabilities, many systems struggle with real-time adaptive control based on actual part temperatures rather than chamber conditions. This results in conservative processing parameters that extend cycle times unnecessarily.
Maintenance requirements constitute another significant limitation. High-pressure and temperature environments accelerate component wear, particularly for seals, valves, and heating elements. Unplanned downtime for repairs directly impacts production capacity, while preventive maintenance schedules reduce available production time.
Water and resource consumption represents an often-overlooked limitation. Cooling systems, steam generation, and process water requirements contribute to substantial resource usage, creating both cost and environmental sustainability challenges for operators of large autoclave systems.
Standard industrial autoclaves range from small laboratory units to massive industrial chambers exceeding 50 meters in length. Most large-scale production autoclaves operate at temperatures between 120-180°C and pressures of 6-12 bar, though specialized applications may require more extreme conditions. Modern systems incorporate computerized control interfaces, programmable cycle parameters, and data logging capabilities for process validation and quality assurance.
Despite technological advancements, current autoclave systems face significant technical limitations that impact production efficiency. Energy consumption remains a primary concern, with large autoclaves requiring substantial power for heating and maintaining temperature uniformity throughout the chamber. This results in high operational costs and considerable carbon footprints, particularly problematic in today's sustainability-focused manufacturing landscape.
Thermal inefficiency represents another major limitation. Temperature gradients within large chambers lead to inconsistent processing conditions, affecting product quality and necessitating longer cycle times to ensure complete processing. Heat loss through vessel walls and door seals further compounds these inefficiencies, despite improvements in insulation technologies.
Cycle time optimization presents ongoing challenges. The inherent physics of heat transfer in large vessels creates bottlenecks in production throughput. Loading and unloading operations, particularly for large or delicate components, introduce additional non-productive time that reduces overall equipment effectiveness.
Control system limitations also persist in many installations. While modern PLCs offer improved capabilities, many systems struggle with real-time adaptive control based on actual part temperatures rather than chamber conditions. This results in conservative processing parameters that extend cycle times unnecessarily.
Maintenance requirements constitute another significant limitation. High-pressure and temperature environments accelerate component wear, particularly for seals, valves, and heating elements. Unplanned downtime for repairs directly impacts production capacity, while preventive maintenance schedules reduce available production time.
Water and resource consumption represents an often-overlooked limitation. Cooling systems, steam generation, and process water requirements contribute to substantial resource usage, creating both cost and environmental sustainability challenges for operators of large autoclave systems.
Modern Approaches to Autoclave Efficiency Optimization
01 Optimization of autoclave operating parameters
Improving autoclave efficiency through optimization of operating parameters such as temperature, pressure, and cycle time. By carefully controlling these parameters, the sterilization process can be made more effective while reducing energy consumption and processing time. Advanced control systems can monitor and adjust these parameters in real-time to maintain optimal conditions throughout the sterilization cycle.- Optimization of autoclave operating parameters: Optimizing operating parameters such as temperature, pressure, and cycle time can significantly improve autoclave efficiency. Advanced control systems can monitor and adjust these parameters in real-time to ensure optimal sterilization while minimizing energy consumption. Proper calibration and validation of these parameters are essential for maintaining efficiency and ensuring effective sterilization.
- Energy recovery and conservation systems: Implementing energy recovery systems can significantly enhance autoclave efficiency by capturing and reusing heat that would otherwise be wasted. These systems may include heat exchangers, steam recovery mechanisms, and insulation improvements. By reducing energy consumption, these technologies not only improve operational efficiency but also reduce environmental impact and operating costs.
- Advanced monitoring and control technologies: Integration of sensors, IoT devices, and automated control systems enables real-time monitoring of autoclave performance. These technologies allow for predictive maintenance, early detection of inefficiencies, and automated adjustments to optimize sterilization processes. Advanced monitoring systems can track key performance indicators and provide data analytics to continuously improve autoclave efficiency.
- Innovative autoclave design and construction: Novel autoclave designs incorporate features such as improved chamber geometry, enhanced door sealing mechanisms, and optimized steam distribution systems. These design innovations can reduce cycle times, improve temperature uniformity, and minimize energy consumption. Materials with superior thermal properties and corrosion resistance can extend equipment lifespan and maintain efficiency over time.
- Load optimization and preparation techniques: Proper loading techniques and preparation of materials for autoclaving can significantly impact efficiency. This includes optimizing load density, ensuring proper spacing for steam penetration, and using appropriate packaging materials. Pre-vacuum phases and pulsed pressure profiles can improve steam penetration into complex loads, reducing cycle times and energy consumption while ensuring sterilization efficacy.
02 Energy recovery and conservation systems
Implementation of energy recovery and conservation systems to improve autoclave efficiency. These systems capture and reuse heat and steam that would otherwise be wasted, significantly reducing energy consumption. Technologies include heat exchangers, steam recirculation systems, and insulation improvements that minimize heat loss during operation. Such systems can substantially reduce operating costs while maintaining sterilization effectiveness.Expand Specific Solutions03 Advanced loading and chamber design
Innovative chamber designs and loading configurations that optimize space utilization and steam penetration. These designs ensure more uniform heat distribution throughout the load, improving sterilization effectiveness and reducing cycle times. Features may include specialized racks, improved air removal systems, and chamber geometries that facilitate better steam circulation around items being sterilized.Expand Specific Solutions04 Monitoring and validation systems
Implementation of advanced monitoring and validation systems to ensure autoclave efficiency and effectiveness. These systems use sensors and data analytics to track critical parameters throughout the sterilization cycle, providing real-time feedback and documentation. Automated validation processes can verify that sterilization conditions have been met while identifying opportunities for efficiency improvements and preventing failed cycles.Expand Specific Solutions05 Water and resource conservation techniques
Water and resource conservation techniques that reduce the environmental impact of autoclave operations while improving efficiency. These include water recycling systems, condensate recovery, and reduced water consumption designs. By minimizing resource usage without compromising sterilization effectiveness, these techniques lower operational costs and support sustainability goals while maintaining compliance with sterilization standards.Expand Specific Solutions
Leading Manufacturers and Industry Competition Landscape
The autoclave efficiency enhancement market is currently in a growth phase, with increasing demand driven by aerospace, medical, and manufacturing sectors. Major players like Boeing, Airbus, and General Electric are leading innovation in large-scale production applications, focusing on advanced control systems and energy optimization. Mid-tier companies such as Steriflow SAS and SFA Engineering are developing specialized solutions for specific industries. The technology shows varying maturity levels across sectors, with aerospace companies (Boeing, Airbus) demonstrating the most advanced implementations, while companies like Mainstream Engineering and Aerothermal Technology Group are exploring emerging approaches to thermal efficiency and process automation. Market growth is accelerated by sustainability requirements and manufacturing efficiency demands across global industries.
Airbus Operations SAS
Technical Solution: Airbus has pioneered autoclave efficiency improvements through their Composite Manufacturing Excellence initiative, focusing on aerospace-grade component production. Their approach combines optimized loading strategies with advanced thermal management systems that reduce heating and cooling cycles by up to 40%. Airbus has implemented multi-zone temperature control technology that allows different sections of the autoclave to operate at varying temperatures simultaneously, enabling processing of multiple part types in a single cycle. Their proprietary vacuum bagging techniques and materials have demonstrated a 15% improvement in cure quality while reducing preparation time. Airbus has also developed specialized software that simulates curing processes to determine optimal parameters before physical production, reducing trial runs and material waste. Their autoclaves incorporate advanced insulation materials that reduce heat loss by approximately 20% compared to conventional systems.
Strengths: Exceptional precision in temperature and pressure control; extensive experience with large-scale composite structures; proven track record in aerospace-grade quality requirements. Weaknesses: Solutions primarily optimized for aerospace applications; high implementation costs; systems designed for specific composite materials may require adaptation for other industries.
Steriflow SAS
Technical Solution: Steriflow SAS has developed specialized autoclave technology focused on the food and pharmaceutical industries, with innovations in steam management and process control. Their systems feature patented Steam Flow Distribution technology that ensures uniform heat penetration throughout the chamber, reducing processing time by up to 20% while maintaining product quality. Steriflow's autoclaves incorporate advanced PLC control systems with intuitive HMI interfaces that allow operators to monitor and adjust parameters in real-time, with automated recipe management for consistent results across production runs. The company has pioneered water cascade cooling systems that reduce cooling times by approximately 40% compared to conventional air cooling methods. Their autoclaves feature specialized loading systems designed to maximize chamber utilization, increasing batch sizes by up to 25% without expanding equipment footprint. Steriflow has also developed energy-efficient insulation solutions that reduce heat loss by approximately 30% during operation.
Strengths: Specialized expertise in food and pharmaceutical applications; excellent regulatory compliance features; user-friendly control systems requiring minimal operator training. Weaknesses: Less suitable for composite material processing; limited customization options for non-standard applications; solutions primarily optimized for smaller to medium-sized autoclaves.
Key Innovations in Heat Transfer and Pressure Control
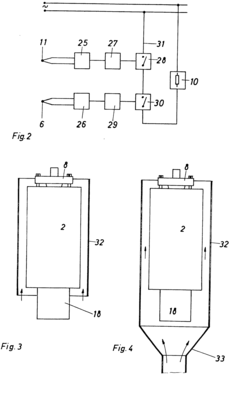
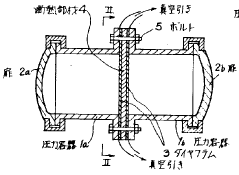
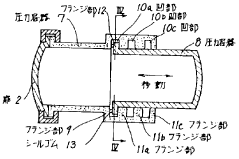
Heatable high-pressure autoclave
PatentInactiveEP0083807A1
Innovation
- A high-pressure autoclave design with a thin-walled reaction vessel and a thick-walled pressure vessel separated by a refractory concrete insulating compound, equipped with a temperature sensor and controller to limit the surface temperature of the heating coil, and an explosion-proof junction box for the heating coil connections, along with optional external cooling using a chimney jacket and cooling fan.
Autoclave for multi-kind and small quantity production
PatentInactiveJP1992135810A
Innovation
- A multi-kind small autoclave with a variable interior pressure vessel volume, allowing adjustable size molding of composite parts, enhanced by sealed diaphragms and heat-insulating members to improve efficiency and reduce energy consumption.
Energy Consumption and Sustainability Considerations
Energy consumption represents a critical factor in autoclave operations, accounting for approximately 60-70% of total operational costs in large-scale production environments. Traditional autoclave systems typically operate at high temperatures (121-134°C) and pressures (15-30 psi), requiring substantial energy inputs from steam generation, heating, and pressurization processes. Recent industry analyses indicate that inefficient autoclave operations can waste up to 25-30% of consumed energy through heat losses, suboptimal loading patterns, and outdated control systems.
Sustainability considerations have become increasingly prominent as manufacturing facilities face stricter environmental regulations and corporate sustainability targets. Carbon footprint assessments of autoclave operations reveal that a standard industrial autoclave can produce between 50-200 tons of CO2 equivalent emissions annually, depending on size, utilization rate, and energy source. Organizations implementing comprehensive energy optimization strategies have demonstrated potential reductions of 15-40% in both energy consumption and associated emissions.
Heat recovery systems represent a significant opportunity for enhancing autoclave efficiency. Advanced heat exchangers can capture and repurpose up to 30% of waste heat from exhaust steam and condensate, redirecting this energy to preheat incoming water or support auxiliary processes. Several case studies from pharmaceutical and aerospace manufacturing sectors document payback periods of 12-24 months for such installations, with ROI exceeding 200% over a five-year operational period.
Alternative energy sources are increasingly viable for powering autoclave operations. Solar thermal systems can supplement conventional heating methods, particularly in regions with high solar irradiance, while biomass-derived steam generation offers carbon-neutral alternatives for facilities with access to appropriate feedstocks. Hybrid systems combining renewable inputs with traditional energy sources have demonstrated reliability comparable to conventional setups while reducing fossil fuel dependency by 20-45%.
Water consumption optimization represents another critical sustainability dimension. Modern autoclave systems with water recycling capabilities can reduce freshwater requirements by 40-60% compared to conventional designs. This approach not only conserves a valuable resource but also reduces the energy associated with water treatment and heating. Implementation of closed-loop water systems further minimizes environmental impact while generating operational cost savings of 5-15% annually.
Sustainability considerations have become increasingly prominent as manufacturing facilities face stricter environmental regulations and corporate sustainability targets. Carbon footprint assessments of autoclave operations reveal that a standard industrial autoclave can produce between 50-200 tons of CO2 equivalent emissions annually, depending on size, utilization rate, and energy source. Organizations implementing comprehensive energy optimization strategies have demonstrated potential reductions of 15-40% in both energy consumption and associated emissions.
Heat recovery systems represent a significant opportunity for enhancing autoclave efficiency. Advanced heat exchangers can capture and repurpose up to 30% of waste heat from exhaust steam and condensate, redirecting this energy to preheat incoming water or support auxiliary processes. Several case studies from pharmaceutical and aerospace manufacturing sectors document payback periods of 12-24 months for such installations, with ROI exceeding 200% over a five-year operational period.
Alternative energy sources are increasingly viable for powering autoclave operations. Solar thermal systems can supplement conventional heating methods, particularly in regions with high solar irradiance, while biomass-derived steam generation offers carbon-neutral alternatives for facilities with access to appropriate feedstocks. Hybrid systems combining renewable inputs with traditional energy sources have demonstrated reliability comparable to conventional setups while reducing fossil fuel dependency by 20-45%.
Water consumption optimization represents another critical sustainability dimension. Modern autoclave systems with water recycling capabilities can reduce freshwater requirements by 40-60% compared to conventional designs. This approach not only conserves a valuable resource but also reduces the energy associated with water treatment and heating. Implementation of closed-loop water systems further minimizes environmental impact while generating operational cost savings of 5-15% annually.
Quality Assurance and Validation Protocols
Quality assurance and validation protocols are critical components in optimizing autoclave efficiency for large-scale production environments. Establishing comprehensive validation procedures ensures that sterilization cycles consistently meet predetermined specifications while maintaining product integrity and safety standards.
The foundation of effective quality assurance begins with the implementation of robust Standard Operating Procedures (SOPs) that detail precise loading configurations, cycle parameters, and acceptance criteria. These protocols must be developed in accordance with international standards such as ISO 17665 for moist heat sterilization and relevant industry-specific regulations including FDA and EMA guidelines.
Validation methodologies for autoclave processes typically follow a three-phase approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that equipment is properly installed according to manufacturer specifications, while OQ confirms that the autoclave operates within established parameters across its intended operating range. PQ demonstrates consistent performance under actual production conditions with real product loads.
Biological indicators containing resistant microorganisms (typically Geobacillus stearothermophilus spores) serve as critical tools for validating sterilization efficacy. These indicators should be strategically placed throughout the load, particularly in hard-to-reach areas and cold spots identified during temperature mapping studies. Chemical indicators provide additional visual confirmation of exposure to sterilization conditions.
Parametric release systems represent an advanced approach to quality assurance, allowing product release based on continuous monitoring of critical process parameters rather than relying solely on biological indicator results. This approach requires sophisticated monitoring systems with calibrated sensors for temperature, pressure, and time measurements at multiple locations within the chamber.
Regular revalidation schedules must be established to account for equipment aging, maintenance activities, and process modifications. Typically, full revalidation is conducted annually, with partial revalidations following significant maintenance events or process changes. This ensures continued process reliability and compliance with regulatory requirements.
Data integrity plays a pivotal role in modern validation protocols. Electronic data capture systems with appropriate security features, audit trails, and backup procedures should be implemented to maintain accurate records of all sterilization cycles. These systems facilitate trend analysis for early detection of process drift and support continuous improvement initiatives.
Risk assessment methodologies such as Failure Mode and Effects Analysis (FMEA) should be integrated into validation protocols to identify potential failure points and establish appropriate mitigation strategies. This proactive approach enhances process robustness and reduces the likelihood of sterilization failures in production environments.
The foundation of effective quality assurance begins with the implementation of robust Standard Operating Procedures (SOPs) that detail precise loading configurations, cycle parameters, and acceptance criteria. These protocols must be developed in accordance with international standards such as ISO 17665 for moist heat sterilization and relevant industry-specific regulations including FDA and EMA guidelines.
Validation methodologies for autoclave processes typically follow a three-phase approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that equipment is properly installed according to manufacturer specifications, while OQ confirms that the autoclave operates within established parameters across its intended operating range. PQ demonstrates consistent performance under actual production conditions with real product loads.
Biological indicators containing resistant microorganisms (typically Geobacillus stearothermophilus spores) serve as critical tools for validating sterilization efficacy. These indicators should be strategically placed throughout the load, particularly in hard-to-reach areas and cold spots identified during temperature mapping studies. Chemical indicators provide additional visual confirmation of exposure to sterilization conditions.
Parametric release systems represent an advanced approach to quality assurance, allowing product release based on continuous monitoring of critical process parameters rather than relying solely on biological indicator results. This approach requires sophisticated monitoring systems with calibrated sensors for temperature, pressure, and time measurements at multiple locations within the chamber.
Regular revalidation schedules must be established to account for equipment aging, maintenance activities, and process modifications. Typically, full revalidation is conducted annually, with partial revalidations following significant maintenance events or process changes. This ensures continued process reliability and compliance with regulatory requirements.
Data integrity plays a pivotal role in modern validation protocols. Electronic data capture systems with appropriate security features, audit trails, and backup procedures should be implemented to maintain accurate records of all sterilization cycles. These systems facilitate trend analysis for early detection of process drift and support continuous improvement initiatives.
Risk assessment methodologies such as Failure Mode and Effects Analysis (FMEA) should be integrated into validation protocols to identify potential failure points and establish appropriate mitigation strategies. This proactive approach enhances process robustness and reduces the likelihood of sterilization failures in production environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!