Autoclave Effectiveness in Hybrid Sterilization Models
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Technology Evolution and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This evolution has been driven by the fundamental principle that saturated steam under pressure provides an efficient and reliable method for eliminating microorganisms through protein denaturation. The initial autoclave designs were simple pressure vessels, but technological advancements have transformed them into sophisticated systems with precise control mechanisms.
The 1950s marked a significant turning point with the introduction of pre-vacuum autoclaves, which enhanced steam penetration by removing air from the chamber before steam introduction. By the 1970s, microprocessor-controlled systems emerged, allowing for automated cycle monitoring and validation. The 1990s saw further refinements with the development of pulsed vacuum systems and advanced sealing technologies that improved efficiency and reliability.
Current autoclave technology incorporates sophisticated monitoring systems, including real-time biological indicators, parametric release protocols, and integrated data management systems that ensure sterilization efficacy while maintaining comprehensive documentation for regulatory compliance. Modern autoclaves also feature energy recovery systems and water conservation mechanisms, addressing sustainability concerns in healthcare and industrial settings.
In the context of hybrid sterilization models, autoclave technology serves as a cornerstone component, often working in conjunction with other modalities such as ethylene oxide, hydrogen peroxide plasma, or radiation-based systems. The primary objective of contemporary autoclave development is to optimize the balance between sterilization efficacy, cycle time reduction, material compatibility, and energy efficiency.
Research objectives in this field now focus on several key areas: developing low-temperature steam sterilization protocols that maintain efficacy while expanding material compatibility; creating intelligent control systems that adapt parameters in real-time based on load characteristics; implementing advanced monitoring technologies that provide absolute sterility assurance; and designing hybrid systems that leverage the strengths of multiple sterilization modalities within a single platform.
The integration of autoclave technology within hybrid sterilization models aims to address the limitations of individual methods while capitalizing on their respective strengths. This approach is particularly relevant for complex medical devices with mixed material compositions, where traditional autoclave processes might damage sensitive components. The ultimate goal is to develop sterilization systems that provide validated sterility assurance across diverse materials while minimizing processing time, energy consumption, and environmental impact.
The 1950s marked a significant turning point with the introduction of pre-vacuum autoclaves, which enhanced steam penetration by removing air from the chamber before steam introduction. By the 1970s, microprocessor-controlled systems emerged, allowing for automated cycle monitoring and validation. The 1990s saw further refinements with the development of pulsed vacuum systems and advanced sealing technologies that improved efficiency and reliability.
Current autoclave technology incorporates sophisticated monitoring systems, including real-time biological indicators, parametric release protocols, and integrated data management systems that ensure sterilization efficacy while maintaining comprehensive documentation for regulatory compliance. Modern autoclaves also feature energy recovery systems and water conservation mechanisms, addressing sustainability concerns in healthcare and industrial settings.
In the context of hybrid sterilization models, autoclave technology serves as a cornerstone component, often working in conjunction with other modalities such as ethylene oxide, hydrogen peroxide plasma, or radiation-based systems. The primary objective of contemporary autoclave development is to optimize the balance between sterilization efficacy, cycle time reduction, material compatibility, and energy efficiency.
Research objectives in this field now focus on several key areas: developing low-temperature steam sterilization protocols that maintain efficacy while expanding material compatibility; creating intelligent control systems that adapt parameters in real-time based on load characteristics; implementing advanced monitoring technologies that provide absolute sterility assurance; and designing hybrid systems that leverage the strengths of multiple sterilization modalities within a single platform.
The integration of autoclave technology within hybrid sterilization models aims to address the limitations of individual methods while capitalizing on their respective strengths. This approach is particularly relevant for complex medical devices with mixed material compositions, where traditional autoclave processes might damage sensitive components. The ultimate goal is to develop sterilization systems that provide validated sterility assurance across diverse materials while minimizing processing time, energy consumption, and environmental impact.
Market Analysis of Hybrid Sterilization Solutions
The global hybrid sterilization solutions market is experiencing robust growth, driven by increasing demand for more efficient, cost-effective, and environmentally friendly sterilization methods across various industries. The market size was valued at approximately $4.2 billion in 2022 and is projected to reach $7.8 billion by 2028, representing a compound annual growth rate (CAGR) of 10.8%. This growth trajectory is particularly evident in healthcare, pharmaceutical manufacturing, food processing, and laboratory settings where autoclave technology plays a crucial role in hybrid sterilization models.
Healthcare facilities represent the largest market segment, accounting for 42% of the total market share. The increasing prevalence of healthcare-associated infections (HAIs) and stringent regulatory requirements for sterilization protocols have significantly boosted demand for advanced hybrid sterilization solutions incorporating autoclave technology. Within this segment, hospitals constitute the primary end-users, followed by ambulatory surgical centers and specialty clinics.
The pharmaceutical industry follows as the second-largest market segment, representing 28% of the market share. The growing emphasis on maintaining sterile manufacturing environments for drug production, coupled with the expansion of biopharmaceutical manufacturing facilities globally, has fueled the adoption of hybrid sterilization approaches that combine autoclave technology with other methods such as radiation or chemical sterilization.
Geographically, North America dominates the market with a 35% share, attributed to advanced healthcare infrastructure, stringent regulatory frameworks, and high adoption rates of innovative sterilization technologies. Europe follows closely with a 30% market share, while the Asia-Pacific region represents the fastest-growing market with a CAGR of 12.5%, driven by expanding healthcare facilities, increasing pharmaceutical manufacturing, and growing awareness about infection control protocols.
Key market trends include the integration of IoT and automation technologies in autoclave systems, enabling real-time monitoring and validation of sterilization processes within hybrid models. Additionally, there is growing demand for energy-efficient autoclave solutions that reduce operational costs and environmental impact when used in combination with other sterilization methods.
Market challenges include the high initial investment required for advanced hybrid sterilization systems, technical complexities in validating combined sterilization approaches, and varying regulatory requirements across different regions. Despite these challenges, the market outlook remains positive, supported by technological advancements and increasing awareness about the benefits of hybrid sterilization approaches that leverage autoclave effectiveness alongside complementary methods.
Healthcare facilities represent the largest market segment, accounting for 42% of the total market share. The increasing prevalence of healthcare-associated infections (HAIs) and stringent regulatory requirements for sterilization protocols have significantly boosted demand for advanced hybrid sterilization solutions incorporating autoclave technology. Within this segment, hospitals constitute the primary end-users, followed by ambulatory surgical centers and specialty clinics.
The pharmaceutical industry follows as the second-largest market segment, representing 28% of the market share. The growing emphasis on maintaining sterile manufacturing environments for drug production, coupled with the expansion of biopharmaceutical manufacturing facilities globally, has fueled the adoption of hybrid sterilization approaches that combine autoclave technology with other methods such as radiation or chemical sterilization.
Geographically, North America dominates the market with a 35% share, attributed to advanced healthcare infrastructure, stringent regulatory frameworks, and high adoption rates of innovative sterilization technologies. Europe follows closely with a 30% market share, while the Asia-Pacific region represents the fastest-growing market with a CAGR of 12.5%, driven by expanding healthcare facilities, increasing pharmaceutical manufacturing, and growing awareness about infection control protocols.
Key market trends include the integration of IoT and automation technologies in autoclave systems, enabling real-time monitoring and validation of sterilization processes within hybrid models. Additionally, there is growing demand for energy-efficient autoclave solutions that reduce operational costs and environmental impact when used in combination with other sterilization methods.
Market challenges include the high initial investment required for advanced hybrid sterilization systems, technical complexities in validating combined sterilization approaches, and varying regulatory requirements across different regions. Despite these challenges, the market outlook remains positive, supported by technological advancements and increasing awareness about the benefits of hybrid sterilization approaches that leverage autoclave effectiveness alongside complementary methods.
Current Autoclave Technology Limitations and Challenges
Despite significant advancements in sterilization technology, current autoclave systems face several critical limitations when integrated into hybrid sterilization models. Traditional autoclaves operate on the principle of saturated steam under pressure, which while effective for many applications, demonstrates reduced efficacy when dealing with complex medical devices featuring narrow lumens, multiple layers, or hydrophobic materials. These physical barriers prevent steam penetration, creating potential sterilization blind spots that compromise patient safety.
Energy consumption represents another significant challenge, with conventional autoclaves requiring substantial power for heating and maintaining high temperatures and pressures. This energy-intensive process not only increases operational costs but also contributes to larger carbon footprints, contradicting modern sustainability initiatives in healthcare facilities. The extended cycle times—typically ranging from 30 minutes to over an hour—further compound these efficiency concerns.
Material compatibility issues persist as a major limitation. High-temperature steam environments can damage heat-sensitive components in modern medical devices, including electronics, adhesives, and certain polymers. This incompatibility has created a technological gap between sterilization requirements and the evolving complexity of medical instrumentation, particularly for hybrid devices combining electronic and traditional surgical components.
Water quality variability across different facilities introduces inconsistency in sterilization outcomes. Mineral deposits from hard water can form on instruments and within the autoclave chamber, potentially interfering with steam contact and reducing sterilization efficacy. These deposits also accelerate equipment deterioration, shortening operational lifespan and increasing maintenance requirements.
Validation and monitoring capabilities present ongoing challenges, particularly in hybrid models where multiple sterilization methods interact. Current autoclave systems often lack real-time monitoring capabilities that can verify sterilization parameters throughout the entire load, creating uncertainty about sterilization efficacy for items positioned in difficult-to-reach areas of the chamber.
The integration of autoclaves into automated workflow systems remains problematic. Many existing units lack standardized communication protocols necessary for seamless integration with tracking systems, inventory management, and quality assurance programs. This interoperability gap hinders the development of truly efficient hybrid sterilization models that could optimize resource allocation and process flow.
Finally, space constraints in modern healthcare facilities create implementation challenges for traditional autoclave systems, which typically require dedicated utility connections, ventilation, and significant floor space. These physical requirements limit flexibility in facility design and can impede the adoption of comprehensive hybrid sterilization approaches that might otherwise improve overall efficiency and effectiveness.
Energy consumption represents another significant challenge, with conventional autoclaves requiring substantial power for heating and maintaining high temperatures and pressures. This energy-intensive process not only increases operational costs but also contributes to larger carbon footprints, contradicting modern sustainability initiatives in healthcare facilities. The extended cycle times—typically ranging from 30 minutes to over an hour—further compound these efficiency concerns.
Material compatibility issues persist as a major limitation. High-temperature steam environments can damage heat-sensitive components in modern medical devices, including electronics, adhesives, and certain polymers. This incompatibility has created a technological gap between sterilization requirements and the evolving complexity of medical instrumentation, particularly for hybrid devices combining electronic and traditional surgical components.
Water quality variability across different facilities introduces inconsistency in sterilization outcomes. Mineral deposits from hard water can form on instruments and within the autoclave chamber, potentially interfering with steam contact and reducing sterilization efficacy. These deposits also accelerate equipment deterioration, shortening operational lifespan and increasing maintenance requirements.
Validation and monitoring capabilities present ongoing challenges, particularly in hybrid models where multiple sterilization methods interact. Current autoclave systems often lack real-time monitoring capabilities that can verify sterilization parameters throughout the entire load, creating uncertainty about sterilization efficacy for items positioned in difficult-to-reach areas of the chamber.
The integration of autoclaves into automated workflow systems remains problematic. Many existing units lack standardized communication protocols necessary for seamless integration with tracking systems, inventory management, and quality assurance programs. This interoperability gap hinders the development of truly efficient hybrid sterilization models that could optimize resource allocation and process flow.
Finally, space constraints in modern healthcare facilities create implementation challenges for traditional autoclave systems, which typically require dedicated utility connections, ventilation, and significant floor space. These physical requirements limit flexibility in facility design and can impede the adoption of comprehensive hybrid sterilization approaches that might otherwise improve overall efficiency and effectiveness.
Contemporary Hybrid Sterilization Implementation Approaches
01 Sterilization parameters and effectiveness monitoring
Autoclave effectiveness depends on proper sterilization parameters including temperature, pressure, and exposure time. Monitoring systems can verify these parameters are maintained throughout the sterilization cycle. Various indicators and monitoring devices can be used to confirm that sterilization conditions have been achieved, ensuring the autoclave effectively eliminates microorganisms and pathogens.- Sterilization parameters and effectiveness monitoring: Autoclave effectiveness depends on proper control and monitoring of key sterilization parameters including temperature, pressure, and exposure time. Modern autoclaves incorporate sensors and control systems to maintain optimal conditions throughout the sterilization cycle. Effectiveness can be verified through biological indicators, chemical indicators, and parametric monitoring systems that ensure all critical parameters are met for complete sterilization.
- Design improvements for enhanced efficiency: Innovations in autoclave design have significantly improved sterilization effectiveness. These include optimized chamber geometry, improved steam distribution systems, better door sealing mechanisms, and advanced heating elements. Such design enhancements ensure more uniform temperature distribution, better steam penetration, and reduced cold spots, resulting in more reliable sterilization outcomes while potentially reducing cycle times and energy consumption.
- Load configuration and packaging considerations: The arrangement and packaging of items within an autoclave significantly impacts sterilization effectiveness. Proper spacing between items allows for adequate steam penetration, while appropriate packaging materials must permit steam to reach all surfaces while maintaining sterility after processing. Overloading can create steam shadows and prevent proper sterilization, while certain materials may require specific positioning to ensure complete exposure to sterilizing conditions.
- Validation protocols and quality assurance: Standardized validation protocols are essential for confirming autoclave effectiveness. These include installation qualification, operational qualification, and performance qualification procedures. Regular testing using biological and chemical indicators verifies that sterilization parameters are consistently achieved. Documentation of cycle parameters, maintenance records, and validation results provides evidence of ongoing autoclave effectiveness and compliance with regulatory requirements.
- Advanced technologies for improved sterilization: Emerging technologies have enhanced autoclave effectiveness through innovations such as pulsed vacuum systems, superheated steam, rapid cooling mechanisms, and computerized monitoring. These advancements improve steam penetration into complex instruments, reduce cycle times, minimize material damage, and provide detailed digital records of sterilization parameters. Some systems incorporate RFID tracking, automated loading systems, and remote monitoring capabilities for improved process control.
02 Autoclave design and construction features
The effectiveness of autoclaves is influenced by their design and construction features. Key aspects include chamber design, door sealing mechanisms, steam distribution systems, and materials of construction. Advanced designs incorporate features that enhance steam penetration, ensure uniform temperature distribution, and maintain pressure stability, all of which contribute to improved sterilization efficacy.Expand Specific Solutions03 Load configuration and packaging considerations
The arrangement and packaging of items within an autoclave significantly impacts sterilization effectiveness. Proper spacing between items allows for adequate steam penetration, while appropriate packaging materials permit steam to reach all surfaces while maintaining sterility after processing. Overloading can create cold spots where sterilization may be incomplete, reducing overall effectiveness.Expand Specific Solutions04 Validation and testing protocols
Standardized validation and testing protocols are essential for confirming autoclave effectiveness. These include biological indicators containing resistant bacterial spores, chemical indicators that change appearance when exposed to sterilization conditions, and physical monitoring of cycle parameters. Regular validation testing ensures continued effectiveness and compliance with regulatory requirements for sterilization processes.Expand Specific Solutions05 Advanced technologies for improved autoclave performance
Innovations in autoclave technology have enhanced sterilization effectiveness. These include pulsed vacuum systems for improved air removal, microprocessor controls for precise cycle management, rapid cooling mechanisms, and energy-efficient designs. Some advanced autoclaves incorporate specialized steam generation systems, real-time monitoring capabilities, and automated documentation features that ensure consistent sterilization results.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Sterilization
The hybrid sterilization technology market is currently in a growth phase, characterized by increasing adoption across healthcare and industrial sectors. The global market size for sterilization equipment is expanding rapidly, projected to reach significant valuation due to heightened focus on infection control and safety standards. Technologically, autoclave effectiveness in hybrid models shows varying maturity levels among key players. Companies like Nakanishi Inc. and W&H Sterilization SRL demonstrate advanced capabilities in medical sterilization, while Fresenius SE & Co. KGaA and LTE Scientific Ltd. lead in innovative hybrid approaches. Educational institutions including China Agricultural University and Shinshu University contribute valuable research advancing sterilization science. The competitive landscape features specialized medical equipment manufacturers (Surgical Design, Inc., S.M.I. Medical S.R.L.) alongside diversified healthcare conglomerates (Covidien, Hospira), creating a dynamic environment for technological advancement and market expansion.
China Agricultural University
Technical Solution: China Agricultural University has developed innovative hybrid sterilization models that combine traditional autoclave technology with novel pre-treatment methods specifically optimized for agricultural and food safety applications. Their research team has pioneered a dual-phase approach that first subjects materials to controlled ultraviolet radiation exposure, followed by optimized autoclave cycles at reduced temperatures (110-115°C). This hybrid methodology has demonstrated particular efficacy for heat-sensitive agricultural products and soil samples where traditional high-temperature autoclaving would damage valuable biological components. Their system incorporates precision humidity control throughout the sterilization process, maintaining optimal moisture levels that enhance steam penetration while preventing excessive drying. The university's research has documented that this combined approach achieves complete inactivation of resistant fungal spores and bacterial endospores while preserving up to 85% of beneficial soil microbiota structure compared to conventional autoclave methods. Their latest models incorporate real-time microbial detection systems that can verify sterilization efficacy without traditional biological indicators, significantly reducing validation time.
Strengths: Exceptional preservation of sample integrity for research applications; reduced energy consumption through lower operating temperatures; particularly effective for agricultural materials with complex matrices. Weaknesses: Longer total processing time compared to high-temperature methods; requires more sophisticated monitoring equipment; limited scalability for industrial applications.
W&H Sterilization SRL
Technical Solution: W&H Sterilization has developed sophisticated hybrid sterilization technology centered around their Lisa and Lina autoclave series. Their approach combines traditional steam sterilization with patented Eco Dry+ technology that adapts drying times to the specific load mass, significantly reducing cycle duration while ensuring complete sterilization. The company's hybrid models incorporate a multi-stage vacuum system that achieves deeper air removal from complex instruments, particularly those with lumens and cavities that traditional autoclaves struggle to sterilize effectively. Their systems feature dual heating elements strategically positioned to eliminate cold spots and ensure uniform temperature distribution throughout the chamber. W&H's proprietary Helix Test system provides enhanced process validation for hollow instruments, while their integrated water quality monitoring prevents mineral buildup that could compromise sterilization efficacy. The company's research indicates their hybrid approach achieves sterilization assurance levels exceeding 10^-6 (one-in-a-million failure rate) while reducing energy consumption by approximately 20% compared to conventional systems with equivalent capacity.
Strengths: Exceptional performance with complex hollow instruments; adaptive cycle technology reduces resource consumption; comprehensive traceability systems for regulatory compliance. Weaknesses: Premium pricing positions these systems at the higher end of the market; requires regular maintenance to maintain optimal performance; proprietary consumables increase operational costs.
Critical Patents and Innovations in Autoclave Technology
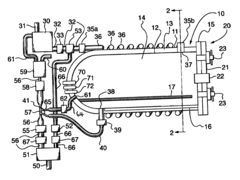
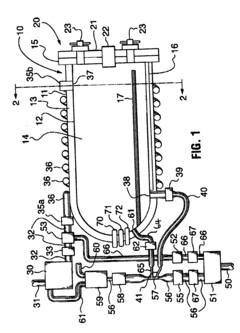
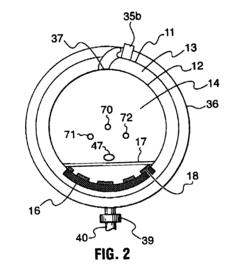
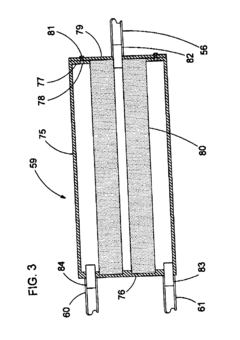
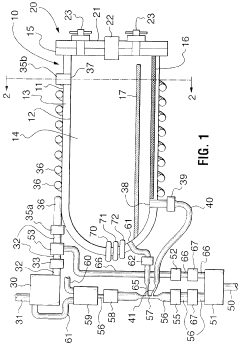
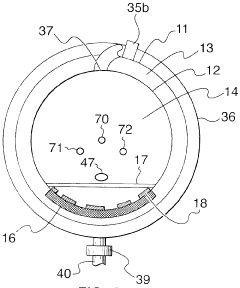
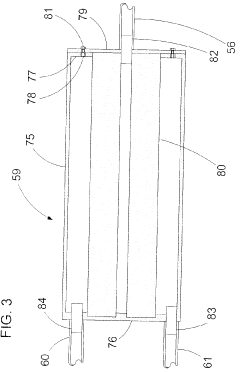
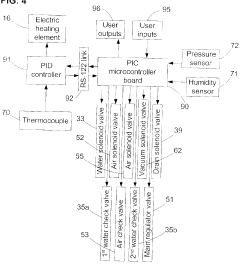
Fixed vacuum-insulated saturated steam autoclave
PatentInactiveUS20060057021A1
Innovation
- A double-walled vacuum-sealed vessel with a self-contained water supply and heat-conductive metal tubing, combined with a PID temperature controller and PIC microprocessor, allows for precise temperature control and rapid steam generation and removal, using concurrent positive and negative air pressures to ensure thorough sterilization and efficient operation.
Fixed vacuum-insulated saturated steam autoclave
PatentInactiveCA2559406A1
Innovation
- A double-walled vacuum-sealed vessel with a self-contained water supply and airflow system, using positive and negative air pressures to rapidly generate and remove steam, combined with a PID temperature controller and PIC microprocessor for precise temperature control, ensures a consistent sterilization environment within a portable, insulated chamber.
Energy Efficiency and Sustainability Considerations
The energy consumption of autoclave sterilization processes represents a significant operational cost and environmental concern in healthcare facilities, pharmaceutical manufacturing, and food processing industries. Traditional autoclave systems typically consume between 1,200-3,000 kWh per cycle, depending on chamber size and cycle parameters. This substantial energy footprint has prompted increased scrutiny of autoclave efficiency within hybrid sterilization models.
Recent advancements in autoclave design have yielded promising improvements in energy efficiency. Vacuum-assisted steam penetration systems reduce cycle times by 15-30%, directly translating to proportional energy savings. Additionally, heat recovery systems that capture and repurpose waste heat from exhaust steam can improve overall thermal efficiency by up to 25%, according to studies conducted by the American Society for Healthcare Engineering.
Water consumption represents another critical sustainability consideration. Conventional autoclaves may use 50-150 gallons of water per cycle for steam generation and cooling. Closed-loop water recirculation systems have demonstrated potential water savings of 80-90% compared to traditional designs, significantly reducing both resource consumption and wastewater treatment requirements.
The integration of autoclaves within hybrid sterilization models presents unique opportunities for optimization. By strategically scheduling autoclave operations during off-peak electricity periods and balancing workloads between autoclave and alternative sterilization methods, facilities can reduce peak energy demands by 30-40%. This load-balancing approach not only reduces operational costs but also minimizes strain on local energy infrastructure.
Carbon footprint analysis reveals that autoclave operations contribute significantly to healthcare facility emissions. A medium-sized hospital typically generates 1,000-1,500 metric tons of CO2 annually from sterilization processes alone. Implementation of high-efficiency autoclaves within optimized hybrid systems can reduce these emissions by 20-35%, supporting organizational sustainability goals and regulatory compliance.
Lifecycle assessment methodologies increasingly factor into equipment procurement decisions. Modern autoclave systems designed with sustainability principles demonstrate 15-20% lower lifetime environmental impact compared to conventional models when accounting for manufacturing, operation, and end-of-life considerations. Key sustainability features include recyclable components, reduced use of rare earth elements, and modular design facilitating repair and component replacement.
The economic case for energy-efficient autoclaves continues to strengthen as energy costs rise. While high-efficiency models typically command a 15-30% price premium, return on investment analyses demonstrate payback periods of 2-4 years through operational savings, with particularly favorable economics in regions with high utility costs or carbon pricing mechanisms.
Recent advancements in autoclave design have yielded promising improvements in energy efficiency. Vacuum-assisted steam penetration systems reduce cycle times by 15-30%, directly translating to proportional energy savings. Additionally, heat recovery systems that capture and repurpose waste heat from exhaust steam can improve overall thermal efficiency by up to 25%, according to studies conducted by the American Society for Healthcare Engineering.
Water consumption represents another critical sustainability consideration. Conventional autoclaves may use 50-150 gallons of water per cycle for steam generation and cooling. Closed-loop water recirculation systems have demonstrated potential water savings of 80-90% compared to traditional designs, significantly reducing both resource consumption and wastewater treatment requirements.
The integration of autoclaves within hybrid sterilization models presents unique opportunities for optimization. By strategically scheduling autoclave operations during off-peak electricity periods and balancing workloads between autoclave and alternative sterilization methods, facilities can reduce peak energy demands by 30-40%. This load-balancing approach not only reduces operational costs but also minimizes strain on local energy infrastructure.
Carbon footprint analysis reveals that autoclave operations contribute significantly to healthcare facility emissions. A medium-sized hospital typically generates 1,000-1,500 metric tons of CO2 annually from sterilization processes alone. Implementation of high-efficiency autoclaves within optimized hybrid systems can reduce these emissions by 20-35%, supporting organizational sustainability goals and regulatory compliance.
Lifecycle assessment methodologies increasingly factor into equipment procurement decisions. Modern autoclave systems designed with sustainability principles demonstrate 15-20% lower lifetime environmental impact compared to conventional models when accounting for manufacturing, operation, and end-of-life considerations. Key sustainability features include recyclable components, reduced use of rare earth elements, and modular design facilitating repair and component replacement.
The economic case for energy-efficient autoclaves continues to strengthen as energy costs rise. While high-efficiency models typically command a 15-30% price premium, return on investment analyses demonstrate payback periods of 2-4 years through operational savings, with particularly favorable economics in regions with high utility costs or carbon pricing mechanisms.
Validation Protocols and Quality Assurance Standards
Validation protocols for autoclave sterilization in hybrid models require systematic approaches that ensure consistent and reliable outcomes. These protocols typically follow international standards such as ISO 17665 for moist heat sterilization and must be tailored to the specific requirements of hybrid sterilization environments where multiple methods may be employed simultaneously or sequentially. The validation process generally encompasses installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ), each serving distinct purposes in establishing autoclave effectiveness.
Installation qualification verifies that the autoclave equipment is properly installed according to manufacturer specifications, with all necessary utilities and safety features functioning correctly. This includes verification of calibration for critical instruments such as temperature probes, pressure sensors, and timing devices that directly impact sterilization efficacy in hybrid models.
Operational qualification focuses on demonstrating that the autoclave operates within predetermined parameters across its intended operating range. For hybrid sterilization models, this becomes particularly complex as the interaction between different sterilization methods must be accounted for. Testing must verify that the autoclave can consistently achieve and maintain the required temperature, pressure, and exposure time combinations across various load configurations.
Performance qualification represents the most critical validation component, as it confirms that the sterilization process consistently delivers the expected sterility assurance level (SAL) under actual operating conditions. This typically involves biological indicator testing using resistant microorganisms such as Geobacillus stearothermophilus spores, which serve as surrogates for potential contaminants.
Quality assurance standards for autoclave validation in hybrid models must address both process and documentation requirements. Process standards include regular calibration of monitoring equipment, preventive maintenance schedules, and established procedures for routine testing. Documentation standards require comprehensive record-keeping of all validation activities, including test results, deviations, corrective actions, and revalidation schedules.
Parametric release, an approach gaining acceptance in modern sterilization validation, relies on monitoring and controlling critical process parameters rather than end-product testing alone. This approach is particularly valuable in hybrid sterilization models where multiple variables interact. By establishing the correlation between process parameters and sterilization effectiveness, organizations can implement real-time monitoring systems that provide immediate feedback on process efficacy.
Risk-based approaches to validation have become increasingly important, particularly in pharmaceutical and medical device industries. These approaches focus validation efforts on aspects of the sterilization process most likely to impact product quality and patient safety. For hybrid sterilization models, risk assessment must consider potential failure modes at the interface between different sterilization methods and establish appropriate mitigation strategies.
Installation qualification verifies that the autoclave equipment is properly installed according to manufacturer specifications, with all necessary utilities and safety features functioning correctly. This includes verification of calibration for critical instruments such as temperature probes, pressure sensors, and timing devices that directly impact sterilization efficacy in hybrid models.
Operational qualification focuses on demonstrating that the autoclave operates within predetermined parameters across its intended operating range. For hybrid sterilization models, this becomes particularly complex as the interaction between different sterilization methods must be accounted for. Testing must verify that the autoclave can consistently achieve and maintain the required temperature, pressure, and exposure time combinations across various load configurations.
Performance qualification represents the most critical validation component, as it confirms that the sterilization process consistently delivers the expected sterility assurance level (SAL) under actual operating conditions. This typically involves biological indicator testing using resistant microorganisms such as Geobacillus stearothermophilus spores, which serve as surrogates for potential contaminants.
Quality assurance standards for autoclave validation in hybrid models must address both process and documentation requirements. Process standards include regular calibration of monitoring equipment, preventive maintenance schedules, and established procedures for routine testing. Documentation standards require comprehensive record-keeping of all validation activities, including test results, deviations, corrective actions, and revalidation schedules.
Parametric release, an approach gaining acceptance in modern sterilization validation, relies on monitoring and controlling critical process parameters rather than end-product testing alone. This approach is particularly valuable in hybrid sterilization models where multiple variables interact. By establishing the correlation between process parameters and sterilization effectiveness, organizations can implement real-time monitoring systems that provide immediate feedback on process efficacy.
Risk-based approaches to validation have become increasingly important, particularly in pharmaceutical and medical device industries. These approaches focus validation efforts on aspects of the sterilization process most likely to impact product quality and patient safety. For hybrid sterilization models, risk assessment must consider potential failure modes at the interface between different sterilization methods and establish appropriate mitigation strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!