How to Optimize Autoclave Sterilization for Bioreactors
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioreactor Sterilization Background and Objectives
Bioreactor sterilization has evolved significantly since the early days of industrial biotechnology in the 1940s. Initially, simple heat-based methods were employed, but as bioreactor technology advanced, so did the need for more sophisticated sterilization approaches. The autoclave sterilization method, which utilizes pressurized steam, emerged as the gold standard due to its reliability and effectiveness in eliminating microbial contaminants. This technique has been continuously refined over decades to meet the increasing demands of biopharmaceutical production, food processing, and other bioprocessing applications.
The evolution of bioreactor sterilization technology has been driven by several factors, including the need for higher production efficiency, stricter regulatory requirements, and the growing complexity of bioreactor designs. Modern bioreactors range from small-scale laboratory units to massive industrial systems exceeding 20,000 liters, each presenting unique sterilization challenges. The industry has witnessed a shift from batch sterilization to more continuous and integrated approaches, reflecting broader trends in bioprocessing.
Current technological trends in bioreactor sterilization focus on energy efficiency, process automation, validation improvements, and integration with Industry 4.0 principles. Advanced monitoring systems using IoT sensors now allow for real-time tracking of critical sterilization parameters, while machine learning algorithms are beginning to optimize sterilization cycles based on historical performance data. These innovations aim to reduce cycle times while maintaining or improving sterilization efficacy.
The primary objective of optimizing autoclave sterilization for bioreactors is to achieve complete microbial inactivation while minimizing negative impacts on the bioreactor system and reducing operational costs. Specifically, this involves developing protocols that ensure sterility assurance levels (SAL) of 10^-6 or better, while minimizing energy consumption, reducing cycle times, and preventing damage to sensitive bioreactor components such as sensors, seals, and agitation systems.
Additional technical goals include improving heat distribution uniformity throughout complex bioreactor geometries, enhancing validation methodologies to ensure regulatory compliance, and developing predictive models for sterilization cycle optimization. There is also growing interest in developing more sustainable sterilization approaches that reduce water usage and overall environmental impact, aligning with broader industry sustainability initiatives.
The optimization of autoclave sterilization represents a critical enabling technology for the continued advancement of bioprocessing. As the biopharmaceutical industry moves toward more complex production systems for advanced therapies, including cell and gene therapies, the demands on sterilization technology will only increase, driving further innovation in this fundamental but often overlooked aspect of bioprocessing.
The evolution of bioreactor sterilization technology has been driven by several factors, including the need for higher production efficiency, stricter regulatory requirements, and the growing complexity of bioreactor designs. Modern bioreactors range from small-scale laboratory units to massive industrial systems exceeding 20,000 liters, each presenting unique sterilization challenges. The industry has witnessed a shift from batch sterilization to more continuous and integrated approaches, reflecting broader trends in bioprocessing.
Current technological trends in bioreactor sterilization focus on energy efficiency, process automation, validation improvements, and integration with Industry 4.0 principles. Advanced monitoring systems using IoT sensors now allow for real-time tracking of critical sterilization parameters, while machine learning algorithms are beginning to optimize sterilization cycles based on historical performance data. These innovations aim to reduce cycle times while maintaining or improving sterilization efficacy.
The primary objective of optimizing autoclave sterilization for bioreactors is to achieve complete microbial inactivation while minimizing negative impacts on the bioreactor system and reducing operational costs. Specifically, this involves developing protocols that ensure sterility assurance levels (SAL) of 10^-6 or better, while minimizing energy consumption, reducing cycle times, and preventing damage to sensitive bioreactor components such as sensors, seals, and agitation systems.
Additional technical goals include improving heat distribution uniformity throughout complex bioreactor geometries, enhancing validation methodologies to ensure regulatory compliance, and developing predictive models for sterilization cycle optimization. There is also growing interest in developing more sustainable sterilization approaches that reduce water usage and overall environmental impact, aligning with broader industry sustainability initiatives.
The optimization of autoclave sterilization represents a critical enabling technology for the continued advancement of bioprocessing. As the biopharmaceutical industry moves toward more complex production systems for advanced therapies, including cell and gene therapies, the demands on sterilization technology will only increase, driving further innovation in this fundamental but often overlooked aspect of bioprocessing.
Market Demand Analysis for Advanced Sterilization Technologies
The global market for advanced sterilization technologies in biopharmaceutical manufacturing is experiencing robust growth, driven primarily by the expanding biopharmaceutical sector and increasing demand for sterile production environments. Current market valuations indicate that the bioreactor sterilization segment alone represents a significant portion of the broader industrial sterilization market, which is projected to reach $7.5 billion by 2026, growing at a CAGR of 6.8%.
Pharmaceutical and biotechnology companies are increasingly demanding more efficient, reliable, and cost-effective sterilization solutions for bioreactors due to the rising production of biologics, vaccines, and cell-based therapies. The COVID-19 pandemic has further accelerated this trend, with manufacturers scaling up production capacities and requiring advanced sterilization technologies that can maintain sterility while accommodating higher throughput.
Energy efficiency has emerged as a critical market demand factor, with companies actively seeking autoclave technologies that reduce steam consumption and overall energy usage. This shift is driven both by sustainability initiatives and cost-reduction strategies, as traditional autoclave processes can account for up to 30% of energy consumption in biopharmaceutical facilities.
Regulatory compliance continues to shape market demands, with stringent requirements from FDA, EMA, and other global regulatory bodies necessitating validated sterilization processes with comprehensive documentation. This has created a market preference for sterilization technologies with integrated monitoring systems, automated cycle recording, and validation capabilities.
The contract manufacturing organization (CMO) segment represents the fastest-growing market for advanced sterilization technologies, with these organizations requiring flexible, scalable solutions that can accommodate diverse bioreactor configurations and sterilization protocols. This has led to increased demand for modular sterilization systems that can be adapted to different production scenarios.
Regional analysis reveals that North America currently dominates the market for advanced bioreactor sterilization technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to expanding biopharmaceutical manufacturing capabilities in China, India, and Singapore.
End-user feedback indicates growing interest in sterilization technologies that reduce cycle times without compromising sterility assurance levels. Market research shows that technologies capable of reducing traditional autoclave cycles by 25-30% while maintaining or improving sterilization efficacy command premium pricing and rapid market adoption.
Pharmaceutical and biotechnology companies are increasingly demanding more efficient, reliable, and cost-effective sterilization solutions for bioreactors due to the rising production of biologics, vaccines, and cell-based therapies. The COVID-19 pandemic has further accelerated this trend, with manufacturers scaling up production capacities and requiring advanced sterilization technologies that can maintain sterility while accommodating higher throughput.
Energy efficiency has emerged as a critical market demand factor, with companies actively seeking autoclave technologies that reduce steam consumption and overall energy usage. This shift is driven both by sustainability initiatives and cost-reduction strategies, as traditional autoclave processes can account for up to 30% of energy consumption in biopharmaceutical facilities.
Regulatory compliance continues to shape market demands, with stringent requirements from FDA, EMA, and other global regulatory bodies necessitating validated sterilization processes with comprehensive documentation. This has created a market preference for sterilization technologies with integrated monitoring systems, automated cycle recording, and validation capabilities.
The contract manufacturing organization (CMO) segment represents the fastest-growing market for advanced sterilization technologies, with these organizations requiring flexible, scalable solutions that can accommodate diverse bioreactor configurations and sterilization protocols. This has led to increased demand for modular sterilization systems that can be adapted to different production scenarios.
Regional analysis reveals that North America currently dominates the market for advanced bioreactor sterilization technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to expanding biopharmaceutical manufacturing capabilities in China, India, and Singapore.
End-user feedback indicates growing interest in sterilization technologies that reduce cycle times without compromising sterility assurance levels. Market research shows that technologies capable of reducing traditional autoclave cycles by 25-30% while maintaining or improving sterilization efficacy command premium pricing and rapid market adoption.
Current Autoclave Technology Challenges for Bioreactors
Despite significant advancements in bioreactor technology, autoclave sterilization remains a critical bottleneck in bioprocessing operations. Current autoclave systems face several technical challenges when sterilizing bioreactors, particularly as bioreactor sizes increase to meet industrial production demands. The primary limitation is the uneven heat distribution during sterilization cycles, resulting in "cold spots" where sterilization may be incomplete, posing contamination risks to entire batches.
Energy efficiency represents another significant challenge, with traditional autoclave systems consuming excessive steam and electricity. This not only increases operational costs but also extends sterilization cycle times, reducing overall production throughput. For facilities operating multiple bioreactors, these inefficiencies compound into substantial resource wastage and production delays.
Validation and monitoring systems in current autoclave technology often lack real-time feedback capabilities. Most systems rely on post-process biological indicators or chemical integrators that provide results only after sterilization is complete, making it impossible to adjust parameters during the sterilization process to address potential issues.
Material compatibility issues persist as modern bioreactors incorporate increasingly sophisticated sensors, control systems, and specialized materials. These components may degrade under repeated exposure to high-temperature steam cycles, leading to premature equipment failure and increased maintenance costs. Particularly vulnerable are electronic components, gaskets, and certain polymer-based parts essential for bioreactor functionality.
Scale-up challenges become evident when transitioning from laboratory to industrial-scale operations. Sterilization protocols developed for small bioreactors often fail to translate effectively to larger systems due to differences in heat transfer dynamics and load configurations. This scaling discrepancy necessitates extensive revalidation and often results in overly conservative sterilization parameters that waste resources.
Automation limitations further complicate efficient operation, with many systems lacking sophisticated control algorithms that could optimize cycle parameters based on load characteristics. The integration between autoclave systems and bioreactor control platforms remains rudimentary in many facilities, preventing truly integrated bioprocessing workflows.
Water consumption presents an additional environmental and economic concern, with traditional autoclave systems requiring significant quantities of purified water for steam generation and cooling processes. This resource intensity conflicts with growing sustainability initiatives in bioprocessing industries.
These technical challenges collectively create significant barriers to efficient bioreactor operation, particularly in continuous manufacturing environments where sterilization downtime directly impacts production economics and product availability.
Energy efficiency represents another significant challenge, with traditional autoclave systems consuming excessive steam and electricity. This not only increases operational costs but also extends sterilization cycle times, reducing overall production throughput. For facilities operating multiple bioreactors, these inefficiencies compound into substantial resource wastage and production delays.
Validation and monitoring systems in current autoclave technology often lack real-time feedback capabilities. Most systems rely on post-process biological indicators or chemical integrators that provide results only after sterilization is complete, making it impossible to adjust parameters during the sterilization process to address potential issues.
Material compatibility issues persist as modern bioreactors incorporate increasingly sophisticated sensors, control systems, and specialized materials. These components may degrade under repeated exposure to high-temperature steam cycles, leading to premature equipment failure and increased maintenance costs. Particularly vulnerable are electronic components, gaskets, and certain polymer-based parts essential for bioreactor functionality.
Scale-up challenges become evident when transitioning from laboratory to industrial-scale operations. Sterilization protocols developed for small bioreactors often fail to translate effectively to larger systems due to differences in heat transfer dynamics and load configurations. This scaling discrepancy necessitates extensive revalidation and often results in overly conservative sterilization parameters that waste resources.
Automation limitations further complicate efficient operation, with many systems lacking sophisticated control algorithms that could optimize cycle parameters based on load characteristics. The integration between autoclave systems and bioreactor control platforms remains rudimentary in many facilities, preventing truly integrated bioprocessing workflows.
Water consumption presents an additional environmental and economic concern, with traditional autoclave systems requiring significant quantities of purified water for steam generation and cooling processes. This resource intensity conflicts with growing sustainability initiatives in bioprocessing industries.
These technical challenges collectively create significant barriers to efficient bioreactor operation, particularly in continuous manufacturing environments where sterilization downtime directly impacts production economics and product availability.
Current Autoclave Optimization Approaches
01 Temperature and pressure control systems
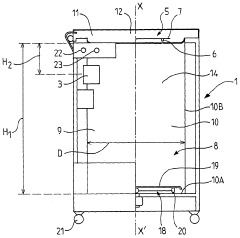
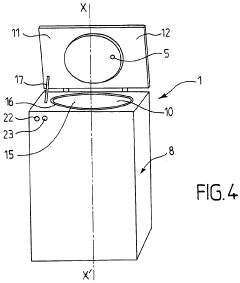
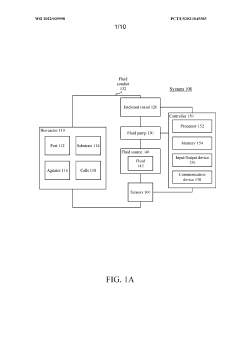
Advanced control systems for optimizing autoclave sterilization processes focus on precise temperature and pressure regulation. These systems incorporate sensors and monitoring equipment to maintain optimal sterilization conditions throughout the cycle. By ensuring accurate temperature distribution and pressure levels, these control mechanisms enhance sterilization efficacy while preventing damage to sensitive materials. Some implementations include automated adjustment capabilities that respond to real-time data, improving consistency and reliability of the sterilization process.- Temperature and pressure control systems: Advanced temperature and pressure control systems are crucial for optimizing autoclave sterilization processes. These systems ensure precise monitoring and regulation of sterilization parameters, maintaining optimal conditions throughout the cycle. Improved control mechanisms can include digital sensors, automated pressure relief valves, and computerized monitoring systems that help achieve consistent sterilization results while preventing damage to sensitive materials.
- Cycle time optimization techniques: Optimization of autoclave cycle times involves balancing sterilization efficacy with operational efficiency. This includes developing algorithms for determining the minimum required exposure time based on load characteristics, implementing rapid heating and cooling phases, and utilizing pre-vacuum cycles to remove air pockets. These techniques ensure complete sterilization while minimizing processing time, energy consumption, and potential damage to heat-sensitive items.
- Load configuration and packaging innovations: The arrangement and packaging of items within an autoclave significantly impact sterilization effectiveness. Innovations in this area include specialized trays and containers that optimize steam penetration, materials that allow for better heat transfer, and load configuration guidelines that prevent the formation of cold spots. Proper spacing between items and appropriate packaging materials ensure uniform sterilization throughout the entire load.
- Water quality and steam generation improvements: The quality of water used for steam generation directly affects autoclave performance and equipment longevity. Improvements include water purification systems to remove minerals and contaminants, steam quality monitoring devices, and advanced steam generators that produce consistent, high-quality steam. These enhancements prevent scaling, corrosion, and the formation of deposits that could compromise sterilization effectiveness or damage instruments.
- Validation and monitoring technologies: Advanced validation and monitoring technologies ensure sterilization processes meet required standards. These include biological and chemical indicators that verify sterilization effectiveness, real-time monitoring systems that track critical parameters throughout the cycle, and data logging capabilities for compliance documentation. Modern autoclaves may incorporate wireless sensors, RFID technology for load tracking, and automated documentation systems that improve process reliability and regulatory compliance.
02 Cycle time optimization techniques
Methods for reducing autoclave sterilization cycle times while maintaining effectiveness involve optimized heating and cooling phases. These techniques include rapid heat transfer systems, improved steam distribution mechanisms, and algorithmic approaches to determine the minimum required exposure time for specific loads. By analyzing load characteristics and adjusting cycle parameters accordingly, these methods achieve complete sterilization with reduced energy consumption and increased throughput. Some approaches incorporate pre-vacuum phases to remove air pockets that could otherwise impede steam penetration.Expand Specific Solutions03 Load configuration and packaging innovations
Innovations in load arrangement and packaging materials significantly impact autoclave sterilization efficiency. Specialized trays, containers, and wrapping materials are designed to optimize steam penetration while maintaining sterility post-processing. These solutions include configurations that prevent condensation pooling, facilitate uniform heat distribution, and accommodate various instrument types. Some designs incorporate indicators that verify sterilization conditions were achieved throughout the load, while others feature materials specifically engineered to withstand repeated sterilization cycles without degradation.Expand Specific Solutions04 Water quality and steam generation improvements
Enhanced water treatment and steam generation systems improve autoclave performance and extend equipment lifespan. These innovations focus on water purification methods that reduce mineral content, preventing scale formation and corrosion in steam generators and chamber surfaces. Advanced steam generation technologies ensure consistent steam quality with appropriate moisture content for optimal sterilization. Some systems incorporate water recycling capabilities to reduce resource consumption, while others feature rapid steam generation mechanisms that contribute to shorter cycle times.Expand Specific Solutions05 Validation and monitoring technologies
Advanced monitoring and validation technologies ensure autoclave sterilization processes meet required standards. These systems incorporate biological indicators, chemical integrators, and electronic monitoring devices that verify all critical parameters are maintained throughout the sterilization cycle. Real-time data collection allows for process verification and documentation for regulatory compliance. Some implementations include remote monitoring capabilities and automated record-keeping systems that generate detailed reports of each sterilization cycle, facilitating quality assurance and troubleshooting when deviations occur.Expand Specific Solutions
Leading Manufacturers and Technology Providers
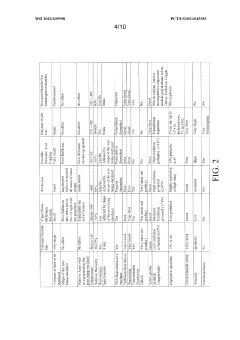
The autoclave sterilization optimization for bioreactors market is currently in a growth phase, with increasing demand driven by expanding biopharmaceutical manufacturing and cellular agriculture sectors. The global market size is estimated to exceed $2 billion, growing at 8-10% annually as bioreactor applications diversify. Technologically, the field shows varying maturity levels with established players like Stryker Corp. and Covidien offering advanced solutions, while innovative companies such as UPSIDE Foods and ICell Shanghai are pushing boundaries in specialized applications. Academic institutions including China Agricultural University and University of Nottingham contribute significant research advancements. Companies like Cytiva BioProcess and EMD Millipore lead in integrating digital monitoring systems with traditional sterilization processes, while Straumann and Nakanishi focus on specialized sterilization protocols for sensitive biological materials.
Covidien Pte Ltd.
Technical Solution: Covidien (now part of Medtronic) has developed specialized autoclave sterilization technologies for bioreactors that focus on steam penetration optimization and load configuration. Their approach incorporates advanced steam distribution systems that ensure uniform steam penetration throughout complex bioreactor geometries, addressing a primary challenge in achieving effective sterilization. Covidien's technology includes specialized pulsed vacuum cycles that enhance steam penetration into difficult-to-reach areas of bioreactors, such as valves, ports, and complex internal structures. Their research has demonstrated that optimized pulsed vacuum protocols can reduce total cycle times by up to 35% compared to traditional gravity displacement methods while improving sterilization efficacy. The company has also developed specialized load configuration guidelines and accessories that optimize steam flow patterns within autoclaves containing bioreactor components. Covidien's sterilization monitoring systems incorporate both biological and chemical indicators specifically validated for bioreactor applications, providing enhanced assurance of sterilization effectiveness. Additionally, they have pioneered energy-efficient autoclave designs that reduce steam consumption by approximately 20% through improved insulation and steam recycling technologies.
Strengths: Specialized focus on steam penetration addresses one of the most critical challenges in bioreactor sterilization. Validated monitoring systems provide enhanced regulatory compliance assurance. Energy efficiency improvements offer significant operational cost savings. Weaknesses: Optimal results require adherence to specific load configuration guidelines, which may limit flexibility in some operations. Implementation may require modification of existing autoclave equipment or procedures.
EMD Millipore Corp.
Technical Solution: EMD Millipore has pioneered an integrated approach to bioreactor sterilization optimization that combines advanced material science with process engineering. Their Mobius® single-use bioreactor systems eliminate traditional autoclave sterilization requirements altogether, providing pre-sterilized, ready-to-use alternatives for many bioprocessing applications. For traditional stainless steel bioreactors requiring autoclave sterilization, EMD Millipore has developed specialized steam-in-place (SIP) protocols that incorporate precise temperature mapping and F0 value calculations to ensure sterilization efficacy while minimizing cycle duration. Their approach includes proprietary valve designs that improve steam penetration and condensate removal, addressing key challenges in autoclave sterilization. EMD Millipore's sterilization optimization platform incorporates computational fluid dynamics modeling to predict heat transfer patterns within complex bioreactor geometries, allowing for customized sterilization protocols based on specific vessel configurations. The company has also developed specialized cleaning validation protocols that work in conjunction with sterilization processes to ensure both cleanliness and sterility are achieved efficiently.
Strengths: Comprehensive approach addressing both single-use and traditional bioreactor sterilization needs. Advanced modeling capabilities enable customized protocol development for specific equipment configurations. Integration with cleaning validation provides a holistic solution. Weaknesses: Single-use systems, while eliminating autoclave requirements, introduce environmental sustainability concerns. Implementation of advanced modeling requires specialized expertise not always available in smaller operations.
Key Innovations in Bioreactor Sterilization
Sterilisation device and corresponding method
PatentWO2010128217A1
Innovation
- A sterilization device using wave emission, specifically S-band microwaves, that employs a temperature variation protocol involving a first rise, a decrease, and a second rise to effectively sterilize products without the need for high pressure and temperature, allowing for automatic control and safe operation.
Systems, devices, and methods for sterilizing bioreactors and culture media
PatentWO2022039998A1
Innovation
- The use of a sterilant like chlorine dioxide gas, hydrogen peroxide vapor, or peracetic acid vapor is circulated through a bioreactor and enclosed vessel containing cell culture media for a dwell time sufficient to sterilize the system, allowing for simultaneous sterilization of the bioreactor, vessel, and media without the need for high-pressure designs, and enabling the use of lighter, more cost-effective materials.
Validation and Regulatory Compliance
Validation and regulatory compliance represent critical components in the optimization of autoclave sterilization processes for bioreactors. The biopharmaceutical industry operates under stringent regulatory frameworks established by agencies such as the FDA, EMA, and WHO, which mandate comprehensive validation protocols to ensure product safety and efficacy.
Validation of autoclave sterilization processes typically follows a three-phase approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that equipment is properly installed according to manufacturer specifications, while OQ confirms that the autoclave operates within predetermined parameters. PQ, the most critical phase, demonstrates that the sterilization process consistently achieves the required sterility assurance level (SAL) under actual production conditions.
Regulatory compliance for autoclave sterilization is primarily governed by standards such as ISO 17665 for moist heat sterilization and USP <1211> for sterilization and sterility assurance. These standards establish minimum requirements for validation, routine control, and monitoring of sterilization processes. Additionally, Good Manufacturing Practice (GMP) guidelines provide frameworks for quality management systems that ensure consistent production and control according to quality standards.
Biological indicators (BIs) containing resistant bacterial spores (typically Geobacillus stearothermophilus) serve as critical tools for validating autoclave cycles. These indicators are strategically placed at the most challenging locations within bioreactors during validation studies to verify sterilization efficacy. Chemical indicators and parametric monitoring systems provide supplementary data but cannot replace the definitive results obtained from BIs.
Documentation requirements for validation include detailed protocols, acceptance criteria, and comprehensive reports that demonstrate the achievement of SAL targets. Modern regulatory approaches increasingly emphasize process analytical technology (PAT) and quality by design (QbD) principles, which involve continuous monitoring and real-time adjustments to ensure consistent sterilization outcomes.
Risk assessment methodologies, such as Failure Mode and Effects Analysis (FMEA), have become integral to validation strategies. These approaches help identify potential failure points in sterilization processes and establish appropriate control measures. The implementation of automated data management systems has significantly enhanced compliance capabilities by ensuring data integrity, facilitating audit trails, and enabling trend analysis for process optimization.
Revalidation requirements present another regulatory consideration, with periodic reassessment necessary following equipment modifications, process changes, or according to predetermined schedules. This ensures continued compliance and process effectiveness throughout the lifecycle of bioreactor operations.
Validation of autoclave sterilization processes typically follows a three-phase approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that equipment is properly installed according to manufacturer specifications, while OQ confirms that the autoclave operates within predetermined parameters. PQ, the most critical phase, demonstrates that the sterilization process consistently achieves the required sterility assurance level (SAL) under actual production conditions.
Regulatory compliance for autoclave sterilization is primarily governed by standards such as ISO 17665 for moist heat sterilization and USP <1211> for sterilization and sterility assurance. These standards establish minimum requirements for validation, routine control, and monitoring of sterilization processes. Additionally, Good Manufacturing Practice (GMP) guidelines provide frameworks for quality management systems that ensure consistent production and control according to quality standards.
Biological indicators (BIs) containing resistant bacterial spores (typically Geobacillus stearothermophilus) serve as critical tools for validating autoclave cycles. These indicators are strategically placed at the most challenging locations within bioreactors during validation studies to verify sterilization efficacy. Chemical indicators and parametric monitoring systems provide supplementary data but cannot replace the definitive results obtained from BIs.
Documentation requirements for validation include detailed protocols, acceptance criteria, and comprehensive reports that demonstrate the achievement of SAL targets. Modern regulatory approaches increasingly emphasize process analytical technology (PAT) and quality by design (QbD) principles, which involve continuous monitoring and real-time adjustments to ensure consistent sterilization outcomes.
Risk assessment methodologies, such as Failure Mode and Effects Analysis (FMEA), have become integral to validation strategies. These approaches help identify potential failure points in sterilization processes and establish appropriate control measures. The implementation of automated data management systems has significantly enhanced compliance capabilities by ensuring data integrity, facilitating audit trails, and enabling trend analysis for process optimization.
Revalidation requirements present another regulatory consideration, with periodic reassessment necessary following equipment modifications, process changes, or according to predetermined schedules. This ensures continued compliance and process effectiveness throughout the lifecycle of bioreactor operations.
Energy Efficiency and Sustainability Considerations
Autoclave sterilization for bioreactors represents a significant energy consumption point in bioprocessing operations. Traditional autoclave processes typically operate at high temperatures (121-134°C) and pressures (15-30 psi) for extended periods, consuming substantial amounts of energy. Recent industry analyses indicate that sterilization can account for up to 30% of the total energy consumption in bioprocessing facilities, highlighting the urgent need for optimization from both economic and environmental perspectives.
Energy efficiency improvements in autoclave sterilization can be achieved through several strategic approaches. Load optimization represents a primary opportunity, where properly configured loading patterns can reduce cycle times by up to 25%. This involves strategic placement of bioreactor components to maximize steam penetration and heat transfer efficiency. Additionally, implementing heat recovery systems can recapture up to 40% of thermal energy from exhaust steam, which can then be redirected to preheat incoming water or support other facility operations.
Advanced control systems offer another avenue for energy conservation. Implementing precise temperature and pressure monitoring with adaptive control algorithms can prevent overprocessing while maintaining sterilization efficacy. Studies demonstrate that PID (Proportional-Integral-Derivative) controllers with machine learning capabilities can reduce energy consumption by 15-20% compared to traditional time-based systems, by dynamically adjusting sterilization parameters based on real-time conditions.
Water conservation represents a critical sustainability consideration, as conventional autoclave processes consume significant volumes of purified water. Implementing water recycling systems can reduce consumption by up to 60%, while vacuum systems that minimize condensate generation can further reduce water requirements. Some facilities have successfully implemented closed-loop water systems that capture, treat, and reuse autoclave water, significantly reducing both water consumption and wastewater generation.
Carbon footprint reduction strategies include transitioning to renewable energy sources for autoclave operations. Facilities utilizing solar thermal systems for water preheating have reported energy savings of 30-45% in regions with favorable solar conditions. Additionally, electrification of steam generation using renewable electricity rather than fossil fuel-based boilers can substantially reduce greenhouse gas emissions, though this requires significant infrastructure investment.
Life cycle assessment (LCA) studies indicate that optimizing autoclave sterilization can reduce the environmental impact of biopharmaceutical manufacturing by 10-15%. This includes considerations beyond operational efficiency, such as equipment longevity, maintenance requirements, and end-of-life disposal. Manufacturers are increasingly adopting sustainable design principles that emphasize modular components with extended service lives and recyclable materials, further enhancing the sustainability profile of bioreactor sterilization processes.
Energy efficiency improvements in autoclave sterilization can be achieved through several strategic approaches. Load optimization represents a primary opportunity, where properly configured loading patterns can reduce cycle times by up to 25%. This involves strategic placement of bioreactor components to maximize steam penetration and heat transfer efficiency. Additionally, implementing heat recovery systems can recapture up to 40% of thermal energy from exhaust steam, which can then be redirected to preheat incoming water or support other facility operations.
Advanced control systems offer another avenue for energy conservation. Implementing precise temperature and pressure monitoring with adaptive control algorithms can prevent overprocessing while maintaining sterilization efficacy. Studies demonstrate that PID (Proportional-Integral-Derivative) controllers with machine learning capabilities can reduce energy consumption by 15-20% compared to traditional time-based systems, by dynamically adjusting sterilization parameters based on real-time conditions.
Water conservation represents a critical sustainability consideration, as conventional autoclave processes consume significant volumes of purified water. Implementing water recycling systems can reduce consumption by up to 60%, while vacuum systems that minimize condensate generation can further reduce water requirements. Some facilities have successfully implemented closed-loop water systems that capture, treat, and reuse autoclave water, significantly reducing both water consumption and wastewater generation.
Carbon footprint reduction strategies include transitioning to renewable energy sources for autoclave operations. Facilities utilizing solar thermal systems for water preheating have reported energy savings of 30-45% in regions with favorable solar conditions. Additionally, electrification of steam generation using renewable electricity rather than fossil fuel-based boilers can substantially reduce greenhouse gas emissions, though this requires significant infrastructure investment.
Life cycle assessment (LCA) studies indicate that optimizing autoclave sterilization can reduce the environmental impact of biopharmaceutical manufacturing by 10-15%. This includes considerations beyond operational efficiency, such as equipment longevity, maintenance requirements, and end-of-life disposal. Manufacturers are increasingly adopting sustainable design principles that emphasize modular components with extended service lives and recyclable materials, further enhancing the sustainability profile of bioreactor sterilization processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!