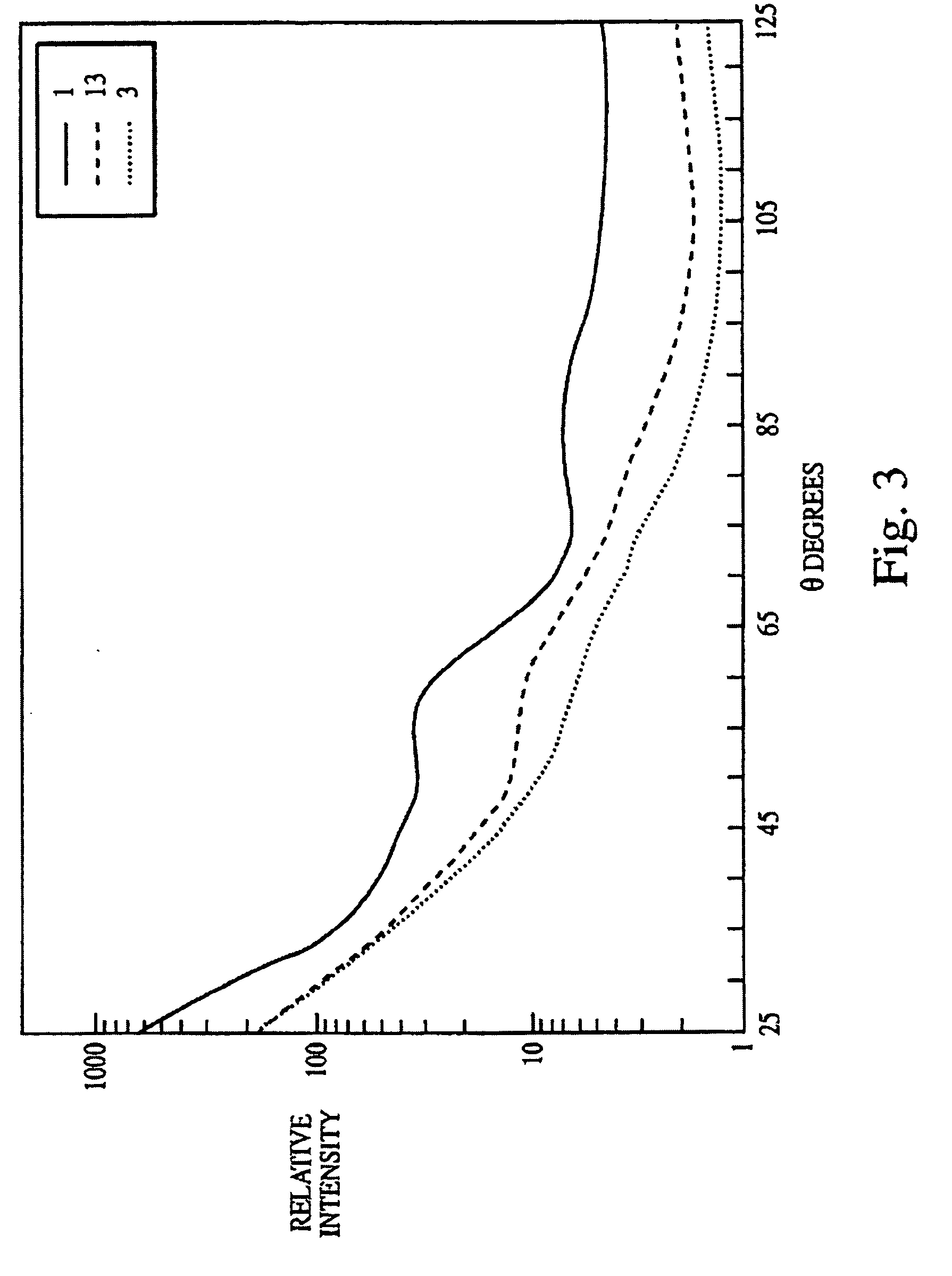
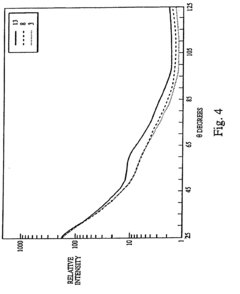
Measure Bioindicator Effectiveness in Autoclave Sterility Assurance
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Technology Background and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This technology leverages the principle of using saturated steam under pressure to eliminate microorganisms through protein denaturation and coagulation. Over the decades, autoclave technology has progressed from simple pressure cookers to sophisticated computerized systems with precise control mechanisms, validation protocols, and monitoring capabilities.
The evolution of autoclave technology has been driven by increasing demands in healthcare, pharmaceutical manufacturing, and laboratory settings where sterility assurance is critical. Modern autoclaves incorporate advanced features such as vacuum systems for air removal, rapid cooling mechanisms, and integrated process monitoring, representing significant improvements over earlier generations of equipment.
Bioindicators have emerged as a crucial component in sterility assurance, serving as biological validation tools that verify an autoclave's ability to achieve complete sterilization. These indicators contain highly resistant bacterial spores, typically Geobacillus stearothermophilus, which are more difficult to kill than most pathogens, thus providing a stringent test of sterilization efficacy.
The current technological trajectory is focused on enhancing the reliability, efficiency, and verifiability of autoclave sterilization processes. This includes the development of more sensitive and rapid-response bioindicators that can provide sterility assurance results in shorter timeframes compared to traditional methods that require 24-48 hours of incubation.
The primary objective of measuring bioindicator effectiveness in autoclave sterility assurance is to establish standardized, reliable methods for validating sterilization processes across various industries and applications. This involves developing protocols that accurately assess the kill rate of biological indicators under different sterilization parameters, including temperature, pressure, and exposure time.
Additional objectives include reducing the time required for sterility verification, minimizing false positive and negative results, and creating more environmentally sustainable bioindicator systems. There is also a growing emphasis on developing real-time monitoring capabilities that can provide immediate feedback on sterilization efficacy, potentially revolutionizing quality control in sterile processing.
As regulatory requirements become increasingly stringent worldwide, the technology must also evolve to meet international standards such as ISO 11138 for biological indicators and ISO 17665 for moist heat sterilization validation, ensuring global harmonization of sterility assurance practices.
The evolution of autoclave technology has been driven by increasing demands in healthcare, pharmaceutical manufacturing, and laboratory settings where sterility assurance is critical. Modern autoclaves incorporate advanced features such as vacuum systems for air removal, rapid cooling mechanisms, and integrated process monitoring, representing significant improvements over earlier generations of equipment.
Bioindicators have emerged as a crucial component in sterility assurance, serving as biological validation tools that verify an autoclave's ability to achieve complete sterilization. These indicators contain highly resistant bacterial spores, typically Geobacillus stearothermophilus, which are more difficult to kill than most pathogens, thus providing a stringent test of sterilization efficacy.
The current technological trajectory is focused on enhancing the reliability, efficiency, and verifiability of autoclave sterilization processes. This includes the development of more sensitive and rapid-response bioindicators that can provide sterility assurance results in shorter timeframes compared to traditional methods that require 24-48 hours of incubation.
The primary objective of measuring bioindicator effectiveness in autoclave sterility assurance is to establish standardized, reliable methods for validating sterilization processes across various industries and applications. This involves developing protocols that accurately assess the kill rate of biological indicators under different sterilization parameters, including temperature, pressure, and exposure time.
Additional objectives include reducing the time required for sterility verification, minimizing false positive and negative results, and creating more environmentally sustainable bioindicator systems. There is also a growing emphasis on developing real-time monitoring capabilities that can provide immediate feedback on sterilization efficacy, potentially revolutionizing quality control in sterile processing.
As regulatory requirements become increasingly stringent worldwide, the technology must also evolve to meet international standards such as ISO 11138 for biological indicators and ISO 17665 for moist heat sterilization validation, ensuring global harmonization of sterility assurance practices.
Market Demand Analysis for Sterility Assurance Solutions
The global market for sterility assurance solutions has experienced significant growth in recent years, driven primarily by heightened awareness of infection control and increasing regulatory requirements across healthcare, pharmaceutical, and food processing industries. The autoclave sterility assurance market specifically is projected to reach $2.3 billion by 2027, growing at a CAGR of 6.8% from 2022, reflecting the critical importance of reliable sterilization validation methods.
Healthcare facilities represent the largest market segment, accounting for approximately 42% of the total demand. This is attributed to the rising number of surgical procedures worldwide and the corresponding need to prevent healthcare-associated infections (HAIs). According to the World Health Organization, HAIs affect hundreds of millions of patients globally each year, creating an urgent demand for effective sterility assurance systems.
The pharmaceutical and biotechnology sectors collectively constitute the fastest-growing segment, with an estimated growth rate of 7.5% annually. This acceleration is driven by stringent regulatory frameworks such as FDA's 21 CFR Part 11 and EU GMP Annex 1, which mandate comprehensive documentation and validation of sterilization processes. The COVID-19 pandemic has further amplified this trend, as vaccine production facilities and medical device manufacturers have had to scale up operations while maintaining rigorous sterility standards.
Regional analysis reveals North America as the dominant market (34% share), followed by Europe (28%) and Asia-Pacific (22%). However, emerging economies in Asia and Latin America are displaying the highest growth rates, fueled by expanding healthcare infrastructure and increasing adoption of international quality standards in manufacturing facilities.
Customer surveys indicate a growing preference for integrated sterility assurance systems that combine traditional biological indicators with rapid readout technologies. Approximately 68% of end-users express willingness to invest in advanced bioindicator systems that can reduce validation time from the traditional 24-48 hours to under 3 hours, even at premium pricing.
Market challenges include cost sensitivity among smaller healthcare facilities and the technical complexity of implementing new validation methodologies. Additionally, there is increasing demand for environmentally sustainable sterilization methods that reduce chemical waste while maintaining efficacy.
Industry forecasts suggest that innovations in bioindicator technology, particularly those focusing on rapid detection methods and increased sensitivity to sterilization parameters, will capture significant market share in the coming years. The integration of digital monitoring capabilities and data analytics for real-time process verification represents another high-growth opportunity, with an estimated 75% of large healthcare and pharmaceutical facilities planning to implement such systems within the next five years.
Healthcare facilities represent the largest market segment, accounting for approximately 42% of the total demand. This is attributed to the rising number of surgical procedures worldwide and the corresponding need to prevent healthcare-associated infections (HAIs). According to the World Health Organization, HAIs affect hundreds of millions of patients globally each year, creating an urgent demand for effective sterility assurance systems.
The pharmaceutical and biotechnology sectors collectively constitute the fastest-growing segment, with an estimated growth rate of 7.5% annually. This acceleration is driven by stringent regulatory frameworks such as FDA's 21 CFR Part 11 and EU GMP Annex 1, which mandate comprehensive documentation and validation of sterilization processes. The COVID-19 pandemic has further amplified this trend, as vaccine production facilities and medical device manufacturers have had to scale up operations while maintaining rigorous sterility standards.
Regional analysis reveals North America as the dominant market (34% share), followed by Europe (28%) and Asia-Pacific (22%). However, emerging economies in Asia and Latin America are displaying the highest growth rates, fueled by expanding healthcare infrastructure and increasing adoption of international quality standards in manufacturing facilities.
Customer surveys indicate a growing preference for integrated sterility assurance systems that combine traditional biological indicators with rapid readout technologies. Approximately 68% of end-users express willingness to invest in advanced bioindicator systems that can reduce validation time from the traditional 24-48 hours to under 3 hours, even at premium pricing.
Market challenges include cost sensitivity among smaller healthcare facilities and the technical complexity of implementing new validation methodologies. Additionally, there is increasing demand for environmentally sustainable sterilization methods that reduce chemical waste while maintaining efficacy.
Industry forecasts suggest that innovations in bioindicator technology, particularly those focusing on rapid detection methods and increased sensitivity to sterilization parameters, will capture significant market share in the coming years. The integration of digital monitoring capabilities and data analytics for real-time process verification represents another high-growth opportunity, with an estimated 75% of large healthcare and pharmaceutical facilities planning to implement such systems within the next five years.
Current Bioindicator Technologies and Challenges
Bioindicators serve as critical tools in autoclave sterility assurance, providing visible confirmation of sterilization effectiveness. Currently, the industry primarily relies on biological indicators containing bacterial spores, particularly Geobacillus stearothermophilus, which are highly resistant to heat sterilization. These standardized indicators are designed to be more difficult to kill than most microbial contaminants, ensuring a robust safety margin in sterilization processes.
Traditional bioindicator systems involve self-contained vials with bacterial spores and growth media, which change color when viable spores grow after unsuccessful sterilization. However, these systems face significant challenges, including lengthy incubation periods of 24-48 hours before results are available, delaying the release of sterilized materials and potentially impacting healthcare delivery timelines.
Sensitivity and specificity issues also plague current technologies. False negatives can lead to the release of non-sterile materials, posing serious patient safety risks, while false positives result in unnecessary reprocessing, increasing operational costs and resource utilization. The variability in manufacturing processes can lead to inconsistent spore populations or resistance characteristics between batches, complicating validation efforts.
Environmental factors present additional challenges, as temperature fluctuations, humidity levels, and positioning within the autoclave chamber can significantly impact bioindicator performance. This spatial variability makes it difficult to ensure uniform sterilization throughout the load, particularly in complex medical devices with lumens or crevices.
Recent technological advancements have introduced rapid readout biological indicators (RRBIs) that utilize enzyme activity detection rather than spore growth, reducing result times to 1-3 hours. However, these systems often require specialized equipment and training, limiting their accessibility for smaller facilities with budget constraints.
Integration challenges exist between bioindicator systems and autoclave monitoring infrastructure. Many facilities still rely on manual documentation and interpretation of results, increasing the risk of human error and complicating compliance with regulatory requirements from organizations like the FDA and ISO.
Sustainability concerns are emerging as traditional single-use bioindicators generate significant waste. The industry is exploring more environmentally friendly alternatives, but these must maintain the same reliability and performance standards as established technologies.
As healthcare facilities increasingly adopt lean principles and just-in-time inventory management, the pressure to reduce turnaround times for sterility assurance testing continues to grow, driving demand for faster, more reliable bioindicator technologies that can maintain patient safety while improving operational efficiency.
Traditional bioindicator systems involve self-contained vials with bacterial spores and growth media, which change color when viable spores grow after unsuccessful sterilization. However, these systems face significant challenges, including lengthy incubation periods of 24-48 hours before results are available, delaying the release of sterilized materials and potentially impacting healthcare delivery timelines.
Sensitivity and specificity issues also plague current technologies. False negatives can lead to the release of non-sterile materials, posing serious patient safety risks, while false positives result in unnecessary reprocessing, increasing operational costs and resource utilization. The variability in manufacturing processes can lead to inconsistent spore populations or resistance characteristics between batches, complicating validation efforts.
Environmental factors present additional challenges, as temperature fluctuations, humidity levels, and positioning within the autoclave chamber can significantly impact bioindicator performance. This spatial variability makes it difficult to ensure uniform sterilization throughout the load, particularly in complex medical devices with lumens or crevices.
Recent technological advancements have introduced rapid readout biological indicators (RRBIs) that utilize enzyme activity detection rather than spore growth, reducing result times to 1-3 hours. However, these systems often require specialized equipment and training, limiting their accessibility for smaller facilities with budget constraints.
Integration challenges exist between bioindicator systems and autoclave monitoring infrastructure. Many facilities still rely on manual documentation and interpretation of results, increasing the risk of human error and complicating compliance with regulatory requirements from organizations like the FDA and ISO.
Sustainability concerns are emerging as traditional single-use bioindicators generate significant waste. The industry is exploring more environmentally friendly alternatives, but these must maintain the same reliability and performance standards as established technologies.
As healthcare facilities increasingly adopt lean principles and just-in-time inventory management, the pressure to reduce turnaround times for sterility assurance testing continues to grow, driving demand for faster, more reliable bioindicator technologies that can maintain patient safety while improving operational efficiency.
Current Bioindicator Validation Methodologies
01 Aquatic organisms as bioindicators for environmental monitoring
Aquatic organisms serve as effective bioindicators for monitoring water quality and environmental health. These organisms, including certain fish species, invertebrates, and algae, show measurable responses to changes in water conditions, pollutants, and ecosystem disturbances. Their physiological responses, population dynamics, and community structure changes provide valuable data for environmental assessment and early warning systems for contamination events.- Aquatic organisms as bioindicators for environmental monitoring: Aquatic organisms can serve as effective bioindicators for monitoring water quality and environmental health. These organisms respond to changes in their environment, making them valuable tools for detecting pollution, toxicity, and ecological disturbances. Their physiological responses, population dynamics, and community structure changes can provide early warning signs of environmental degradation before significant damage occurs.
- Biomarkers for health assessment and disease detection: Biological indicators or biomarkers can be used to assess human health conditions and detect diseases at early stages. These biomarkers include specific molecules, genetic markers, or cellular responses that indicate normal or abnormal biological processes. The effectiveness of these bioindicators depends on their sensitivity, specificity, and reliability in reflecting the targeted health condition or disease state.
- Soil and plant bioindicators for ecosystem health: Soil microorganisms and plant species can function as bioindicators for assessing soil quality and ecosystem health. Changes in microbial diversity, enzyme activities, and plant community composition can reflect soil contamination, nutrient availability, and overall ecosystem functioning. These bioindicators are particularly valuable for monitoring agricultural lands, forest ecosystems, and areas affected by industrial activities.
- Digital and technological systems for bioindicator monitoring: Advanced technological systems have been developed to enhance the effectiveness of bioindicator monitoring. These include digital platforms, sensor networks, and automated data collection systems that can continuously track biological responses and environmental parameters. Such technologies improve the accuracy, efficiency, and real-time capabilities of bioindicator-based environmental and health monitoring programs.
- Performance metrics and validation methods for bioindicators: The effectiveness of bioindicators can be evaluated through various performance metrics and validation methods. These include statistical analyses of sensitivity, specificity, predictive value, and reliability under different environmental conditions. Standardized protocols for bioindicator selection, data collection, and interpretation are essential for ensuring the scientific validity and practical utility of bioindicator-based monitoring programs.
02 Plant-based bioindicators for soil and air quality assessment
Plants function as reliable bioindicators for monitoring soil contamination and air pollution. Certain plant species exhibit visible symptoms such as leaf discoloration, growth abnormalities, or biochemical changes when exposed to specific pollutants. These plant responses can be measured and analyzed to assess environmental quality, detect the presence of toxins, and evaluate the effectiveness of remediation efforts in contaminated areas.Expand Specific Solutions03 Microbial bioindicators for ecosystem health assessment
Microorganisms serve as sensitive bioindicators for assessing ecosystem health and environmental changes. Bacterial and fungal communities respond rapidly to environmental stressors, making them valuable early warning systems. Changes in microbial diversity, abundance, and activity can indicate soil degradation, pollution levels, and ecosystem disturbances before effects become visible in larger organisms. These bioindicators provide cost-effective methods for environmental monitoring and remediation assessment.Expand Specific Solutions04 Bioindicator effectiveness measurement systems and methodologies
Advanced systems and methodologies have been developed to measure and evaluate the effectiveness of bioindicators in environmental monitoring. These include standardized protocols for sample collection, data analysis algorithms, and integrated monitoring platforms that combine bioindicator data with physical and chemical measurements. Such systems enable more accurate interpretation of bioindicator responses, improve reliability of environmental assessments, and facilitate the comparison of results across different studies and locations.Expand Specific Solutions05 Digital platforms for bioindicator data management and analysis
Digital platforms and software solutions have been developed specifically for bioindicator data management, analysis, and interpretation. These systems incorporate machine learning algorithms to process complex bioindicator datasets, identify patterns, and generate predictive models for environmental changes. Cloud-based platforms enable real-time monitoring, data sharing among researchers, and integration with other environmental monitoring systems, enhancing the utility and accessibility of bioindicator information for environmental management and policy decisions.Expand Specific Solutions
Key Industry Players in Sterilization Monitoring
The bioindicator effectiveness market for autoclave sterility assurance is in a mature growth phase, with an estimated global market size of $300-400 million annually. The competitive landscape features established players like 3M Innovative Properties, STERIS, and Crosstex International dominating with comprehensive product portfolios, alongside emerging specialists such as Verrix LLC and Terragene LLC focusing on innovative technologies. Technical maturity varies across solution types, with traditional biological indicators well-established while newer electronic and smart holographic indicators (developed by companies like Smart Holograms Ltd) represent cutting-edge advancements. Regional competition is intensifying with Chinese manufacturers like Shinva Medical Instrument and Hengshui Nuodun Biotechnology expanding global presence through cost-effective alternatives to premium Western solutions.
Verrix LLC
Technical Solution: Verrix has developed an innovative optical detection platform for biological indicators that uses advanced spectroscopy to detect metabolic activity of surviving spores within minutes after sterilization. Their technology employs proprietary fluorescent markers that bind specifically to viable bacterial spores, enabling detection at extremely low concentrations. The Verrix system incorporates machine learning algorithms that analyze spectral signatures to determine sterilization efficacy with greater precision than traditional methods. Their platform features a portable, user-friendly reader device that can be used at the point of sterilization, eliminating the need for separate incubation facilities. Verrix's indicators are designed with internal calibration controls that verify proper functioning of both the indicator and reader system, reducing false results. The technology also includes wireless connectivity for automatic documentation and integration with hospital information systems.
Strengths: Extremely rapid detection capability (minutes versus hours) significantly improves workflow efficiency. The portable reader system enables point-of-use verification without specialized laboratory facilities. Weaknesses: Relatively new technology with less extensive validation history compared to established methods. Higher initial investment costs for implementation of the proprietary reader system and indicators.
TERRAGENE LLC
Technical Solution: TERRAGENE has pioneered Super Rapid biological indicators for steam sterilization processes with results available in just 20 minutes, representing a significant advancement over conventional methods. Their technology employs genetically optimized Geobacillus stearothermophilus spores with enhanced resistance properties specifically calibrated for different autoclave parameters. TERRAGENE's system includes dual-readout indicators that combine colorimetric and fluorescent detection methods, providing both visual confirmation and quantitative analysis of sterilization efficacy. Their BIs incorporate RFID technology for automated tracking and documentation, minimizing human error in the verification process. TERRAGENE has also developed specialized indicators for specific load configurations and medical device types, addressing the unique challenges of complex instruments with narrow lumens or porous materials.
Strengths: Ultra-rapid results enable near real-time release of sterilized items, significantly improving workflow efficiency. The dual-readout system provides redundant verification mechanisms, enhancing reliability. Weaknesses: Specialized indicators for different load types increase inventory management complexity. The system requires more sophisticated equipment and maintenance compared to traditional biological indicators.
Critical Patents and Research in Bioindicator Technology
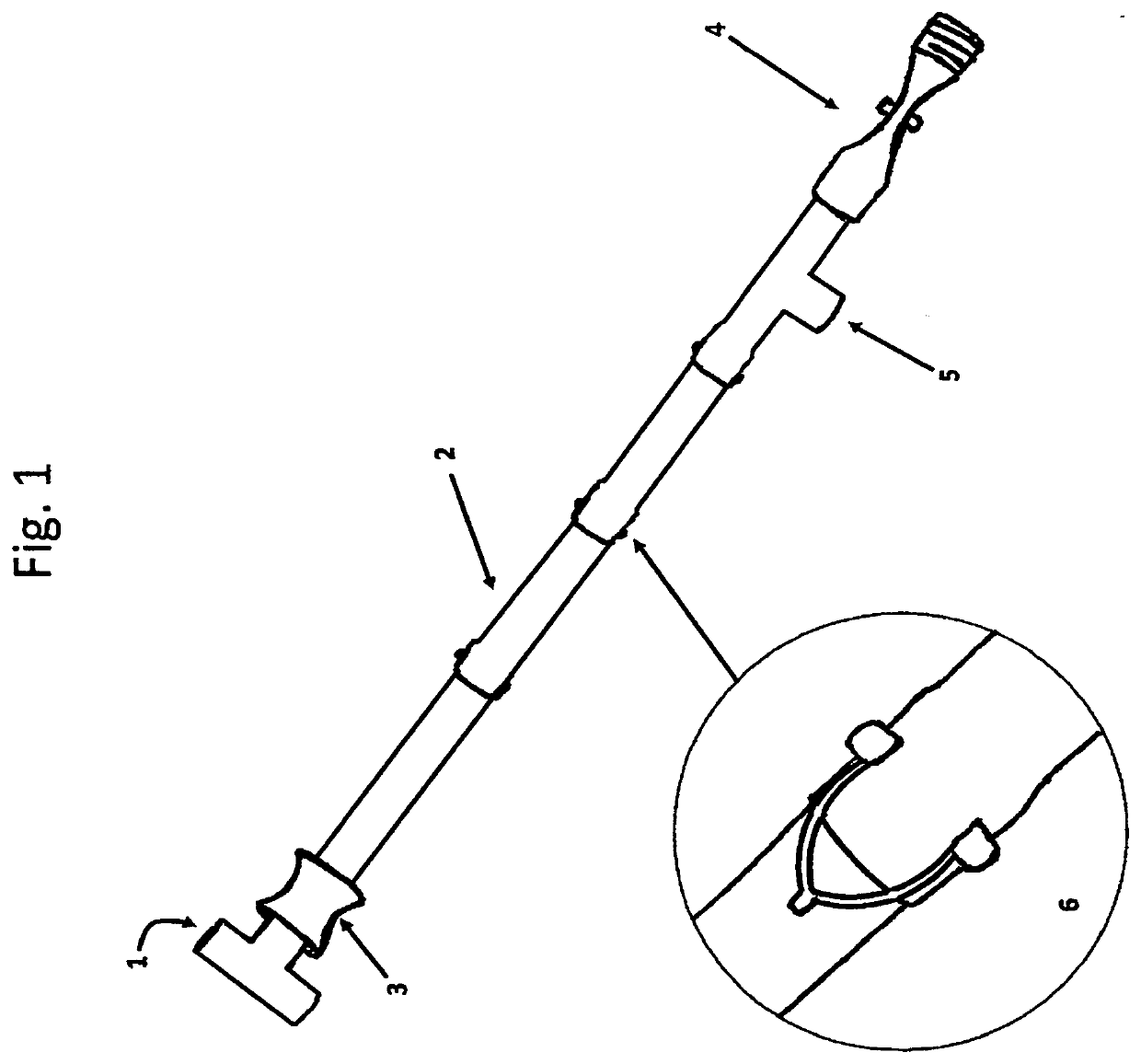
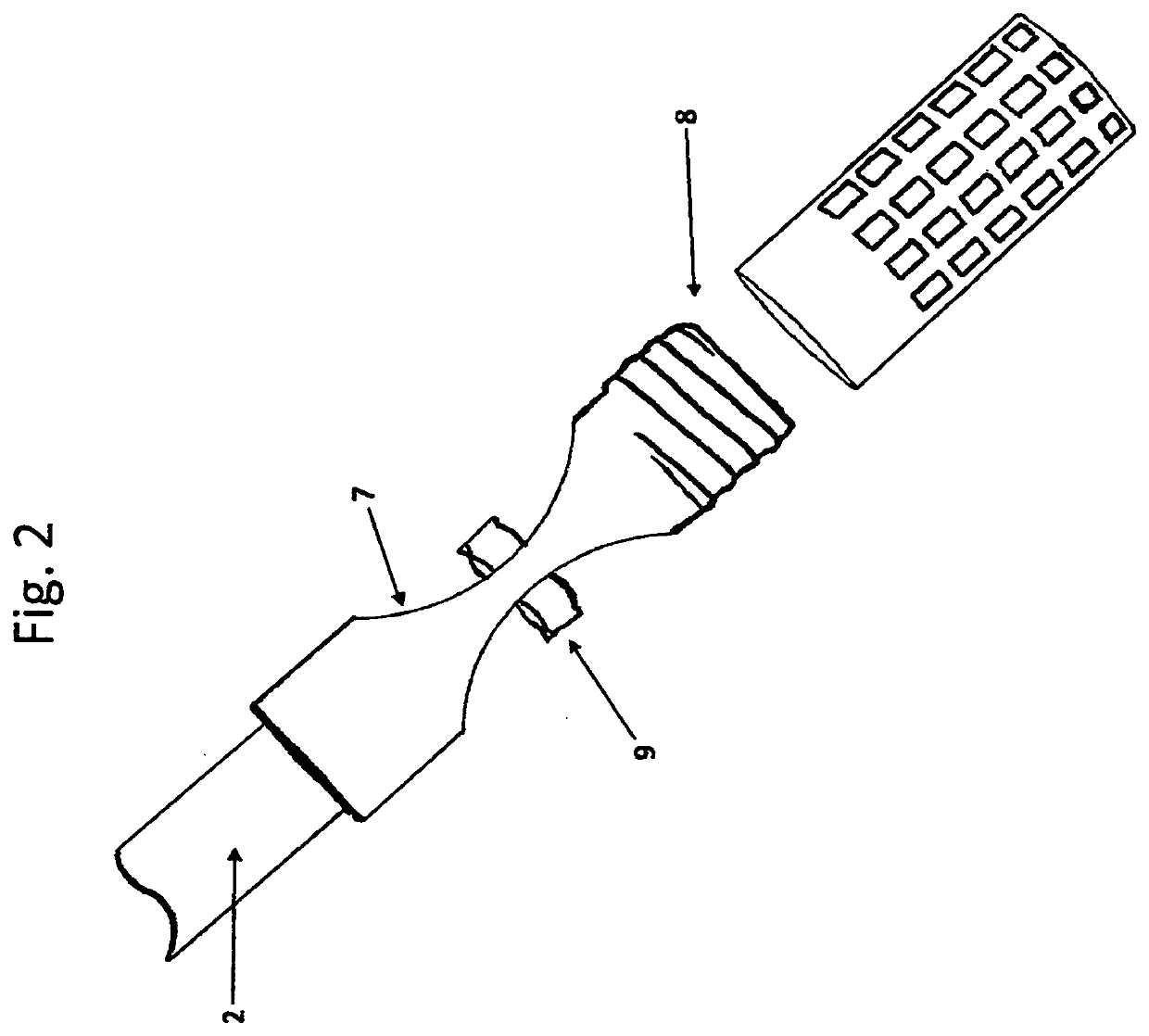
Autoclave Biological Indicator Retrieval Device with Steam and Pressure Pass-through Tube
PatentInactiveUS20200237945A1
Innovation
- A device with a copper pass-through tube and composite biological indicator holder is placed in the autoclave bag, allowing steam penetration and safe positioning of indicators near the center, with easy retrieval after sterilization, using a T-shaped handle and adjustable sections to accommodate various bag sizes and types of indicators.
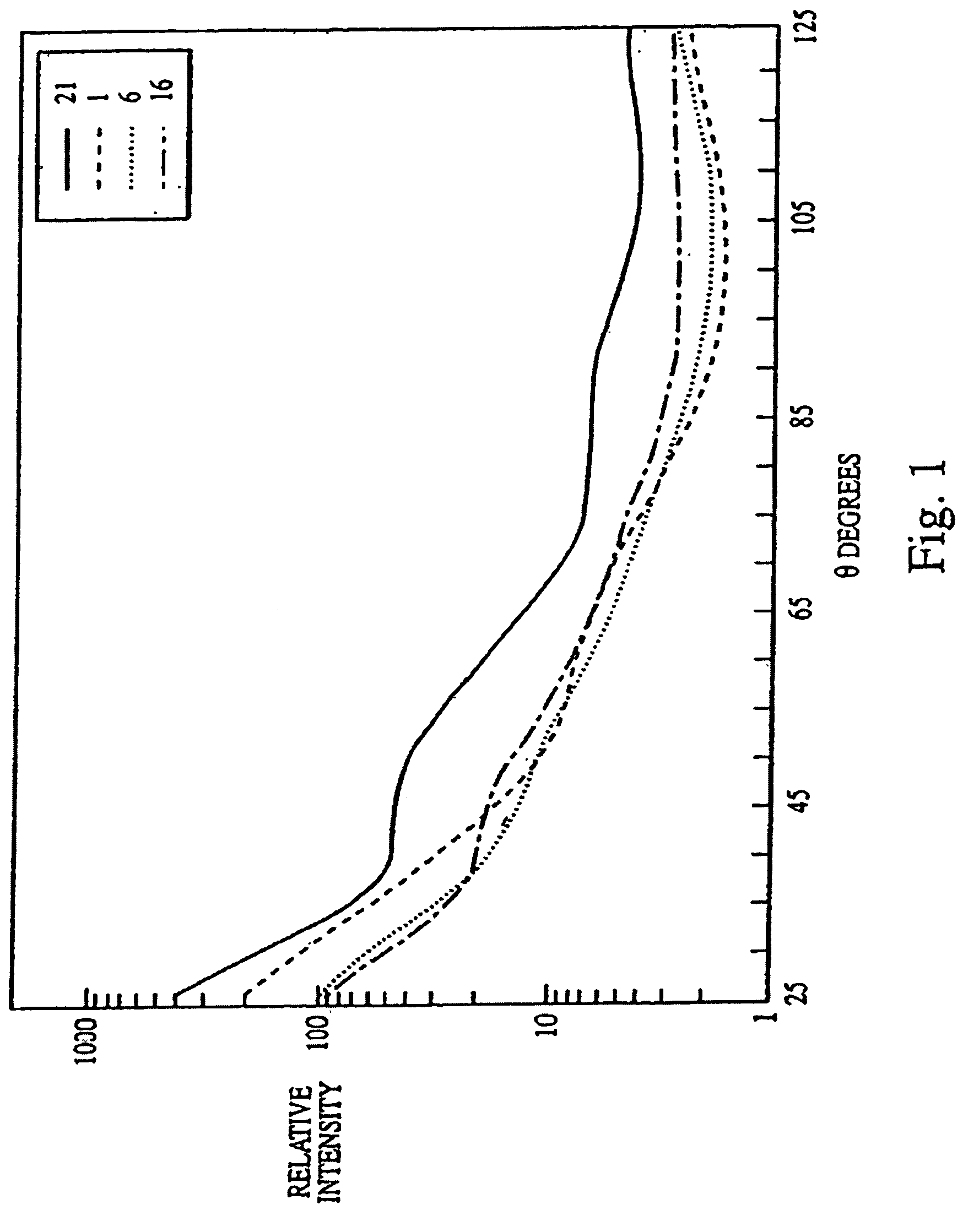
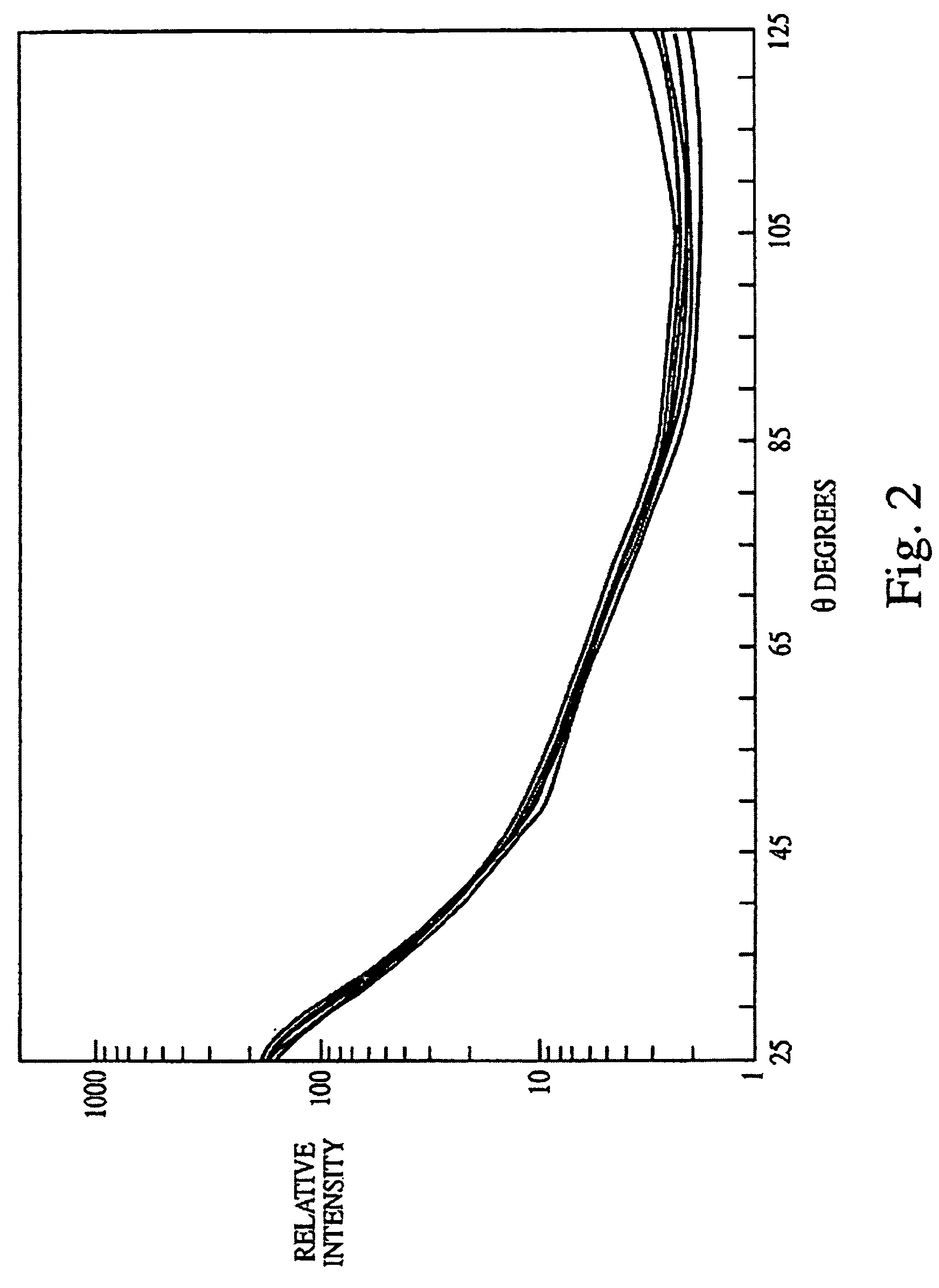
Biological indicator system to detect effectiveness of sterilization
PatentInactiveUS7326562B2
Innovation
- A system utilizing a biological indicator, a solid support, and a multiangle light scattering instrument to rapidly assess the viability of spores after sterilization, allowing for immediate detection of sterilization effectiveness without the need for extensive culture methods or lengthy incubation.
Regulatory Standards for Autoclave Validation
Regulatory standards for autoclave validation represent a critical framework that ensures sterilization processes meet established safety and efficacy requirements. The global landscape of autoclave validation is governed by several key regulatory bodies, including the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and the International Organization for Standardization (ISO) worldwide.
The FDA's guidance document on steam sterilization validation (21 CFR Part 820) outlines comprehensive requirements for medical device manufacturers, emphasizing the importance of biological indicators as primary validation tools. These regulations mandate specific performance parameters, including the requirement that biological indicators demonstrate a minimum 6-log reduction in microbial population during validation cycles.
European standards, particularly EN ISO 17665, provide detailed specifications for the development, validation, and routine control of moist heat sterilization processes. This standard specifically addresses the use of bioindicators, requiring documentation of their resistance characteristics and performance under various operating conditions.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed ST79, a comprehensive guide to steam sterilization and sterility assurance in healthcare facilities. This standard details protocols for bioindicator placement, incubation procedures, and interpretation of results, serving as a benchmark for healthcare institutions globally.
ISO 11138 series standards specifically address biological indicators for sterilization processes, with ISO 11138-3 focusing on biological indicators for moist heat sterilization processes. These standards define critical parameters such as D-value (decimal reduction time), Z-value (temperature coefficient), and survival/kill time calculations that form the foundation for bioindicator effectiveness measurement.
Regulatory compliance requires documented evidence of validation through three essential phases: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). Bioindicators play a crucial role in the PQ phase, where they must demonstrate consistent inactivation under worst-case scenarios.
Recent regulatory trends show increasing emphasis on parametric release methodologies, where physical parameters are monitored in real-time rather than relying solely on biological indicators. However, bioindicators remain the gold standard for initial validation and periodic revalidation processes, with regulatory bodies requiring specific documentation of their effectiveness.
Compliance with these standards necessitates rigorous testing protocols, comprehensive documentation systems, and regular revalidation schedules. Failure to meet these regulatory requirements can result in significant consequences, including product recalls, facility shutdowns, and legal liabilities.
The FDA's guidance document on steam sterilization validation (21 CFR Part 820) outlines comprehensive requirements for medical device manufacturers, emphasizing the importance of biological indicators as primary validation tools. These regulations mandate specific performance parameters, including the requirement that biological indicators demonstrate a minimum 6-log reduction in microbial population during validation cycles.
European standards, particularly EN ISO 17665, provide detailed specifications for the development, validation, and routine control of moist heat sterilization processes. This standard specifically addresses the use of bioindicators, requiring documentation of their resistance characteristics and performance under various operating conditions.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed ST79, a comprehensive guide to steam sterilization and sterility assurance in healthcare facilities. This standard details protocols for bioindicator placement, incubation procedures, and interpretation of results, serving as a benchmark for healthcare institutions globally.
ISO 11138 series standards specifically address biological indicators for sterilization processes, with ISO 11138-3 focusing on biological indicators for moist heat sterilization processes. These standards define critical parameters such as D-value (decimal reduction time), Z-value (temperature coefficient), and survival/kill time calculations that form the foundation for bioindicator effectiveness measurement.
Regulatory compliance requires documented evidence of validation through three essential phases: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). Bioindicators play a crucial role in the PQ phase, where they must demonstrate consistent inactivation under worst-case scenarios.
Recent regulatory trends show increasing emphasis on parametric release methodologies, where physical parameters are monitored in real-time rather than relying solely on biological indicators. However, bioindicators remain the gold standard for initial validation and periodic revalidation processes, with regulatory bodies requiring specific documentation of their effectiveness.
Compliance with these standards necessitates rigorous testing protocols, comprehensive documentation systems, and regular revalidation schedules. Failure to meet these regulatory requirements can result in significant consequences, including product recalls, facility shutdowns, and legal liabilities.
Risk Assessment in Sterilization Process Monitoring
Risk assessment in sterilization process monitoring represents a critical component of quality assurance systems in healthcare facilities, pharmaceutical manufacturing, and laboratory environments. When evaluating bioindicator effectiveness in autoclave sterility assurance, a comprehensive risk assessment framework must be established to identify potential failure points and implement appropriate mitigation strategies.
The primary risk factors in autoclave sterilization processes include temperature variations, steam penetration inconsistencies, loading patterns, and biological indicator placement. These variables can significantly impact the reliability of sterility assurance measurements and must be systematically evaluated through structured risk assessment methodologies such as Failure Mode and Effects Analysis (FMEA) or Hazard Analysis and Critical Control Points (HACCP).
Statistical process control techniques provide valuable tools for quantifying risks associated with bioindicator performance. By establishing control limits and monitoring trends in biological indicator results, facilities can detect subtle shifts in autoclave performance before sterility failures occur. This proactive approach enables risk-based decision making regarding maintenance schedules, validation frequencies, and process improvements.
Documentation systems play a crucial role in risk management for sterilization processes. Comprehensive records of biological indicator results, physical parameter measurements, and equipment maintenance create an audit trail that supports risk assessment activities. Modern electronic monitoring systems can enhance this capability by providing real-time alerts when process parameters deviate from established specifications.
Personnel training represents another significant risk factor in sterilization monitoring programs. Staff must understand the proper handling, incubation, and interpretation of biological indicators to ensure accurate results. Risk assessment should include evaluation of training effectiveness and competency verification to minimize human error in the monitoring process.
Regulatory compliance adds another dimension to risk assessment in sterilization monitoring. Different jurisdictions may impose varying requirements for biological indicator testing frequency, documentation, and validation protocols. Organizations must incorporate these regulatory expectations into their risk assessment frameworks to avoid compliance-related risks while ensuring patient safety and product sterility.
Cost-benefit analysis should inform risk mitigation strategies in sterilization monitoring programs. While more frequent biological indicator testing may reduce certain risks, it also increases operational costs. A balanced approach based on scientific evidence and risk assessment can optimize resource allocation while maintaining appropriate sterility assurance levels.
The primary risk factors in autoclave sterilization processes include temperature variations, steam penetration inconsistencies, loading patterns, and biological indicator placement. These variables can significantly impact the reliability of sterility assurance measurements and must be systematically evaluated through structured risk assessment methodologies such as Failure Mode and Effects Analysis (FMEA) or Hazard Analysis and Critical Control Points (HACCP).
Statistical process control techniques provide valuable tools for quantifying risks associated with bioindicator performance. By establishing control limits and monitoring trends in biological indicator results, facilities can detect subtle shifts in autoclave performance before sterility failures occur. This proactive approach enables risk-based decision making regarding maintenance schedules, validation frequencies, and process improvements.
Documentation systems play a crucial role in risk management for sterilization processes. Comprehensive records of biological indicator results, physical parameter measurements, and equipment maintenance create an audit trail that supports risk assessment activities. Modern electronic monitoring systems can enhance this capability by providing real-time alerts when process parameters deviate from established specifications.
Personnel training represents another significant risk factor in sterilization monitoring programs. Staff must understand the proper handling, incubation, and interpretation of biological indicators to ensure accurate results. Risk assessment should include evaluation of training effectiveness and competency verification to minimize human error in the monitoring process.
Regulatory compliance adds another dimension to risk assessment in sterilization monitoring. Different jurisdictions may impose varying requirements for biological indicator testing frequency, documentation, and validation protocols. Organizations must incorporate these regulatory expectations into their risk assessment frameworks to avoid compliance-related risks while ensuring patient safety and product sterility.
Cost-benefit analysis should inform risk mitigation strategies in sterilization monitoring programs. While more frequent biological indicator testing may reduce certain risks, it also increases operational costs. A balanced approach based on scientific evidence and risk assessment can optimize resource allocation while maintaining appropriate sterility assurance levels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!