Autoclave Cycle Optimization for Reduced Turnaround Time
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Optimization Goals
Autoclaves have evolved significantly since their inception in the early 20th century, transitioning from simple pressure vessels to sophisticated systems incorporating advanced materials, precise control mechanisms, and integrated monitoring capabilities. The initial autoclaves were primarily designed for sterilization in medical settings, with limited control parameters and manual operation requirements. By the mid-20th century, industrial applications expanded dramatically, particularly in aerospace and composite manufacturing, where autoclaves became essential for curing advanced materials under controlled temperature and pressure conditions.
The evolution trajectory shows a clear shift from batch processing with fixed cycles to more flexible, adaptive systems capable of real-time adjustments. Modern autoclaves incorporate multiple heating zones, variable pressure capabilities, and programmable logic controllers that enable precise manipulation of process parameters. This technological progression has been driven by increasing demands for higher throughput, improved energy efficiency, and enhanced product quality across various industries.
Despite these advancements, turnaround time remains a critical challenge in autoclave operations. The complete cycle—including loading, heating, curing/processing, cooling, and unloading—often represents a significant bottleneck in production workflows. Current industry benchmarks indicate that a standard composite curing cycle can range from 6 to 24 hours, with substantial variations depending on material thickness, complexity, and quality requirements.
The primary optimization goals for autoclave cycle reduction focus on several key areas. First, minimizing heat-up and cool-down phases through improved thermal management systems and advanced insulation materials. Second, optimizing cure cycles through better understanding of material behavior and reaction kinetics, potentially enabling accelerated curing without compromising quality. Third, implementing predictive modeling and simulation tools to pre-emptively identify optimal process parameters rather than relying on empirical testing.
Energy efficiency represents another crucial optimization target, as traditional autoclave operations consume substantial power during heating and pressure maintenance phases. Reducing cycle times directly correlates with lower energy consumption, making this a dual-benefit optimization area with both operational and sustainability advantages.
The ultimate goal of autoclave cycle optimization extends beyond mere time reduction to encompass a holistic improvement in operational efficiency. This includes minimizing variability between cycles, reducing defect rates, extending equipment lifespan through more balanced thermal loading, and creating more predictable production schedules that facilitate better resource allocation and planning throughout the manufacturing process.
The evolution trajectory shows a clear shift from batch processing with fixed cycles to more flexible, adaptive systems capable of real-time adjustments. Modern autoclaves incorporate multiple heating zones, variable pressure capabilities, and programmable logic controllers that enable precise manipulation of process parameters. This technological progression has been driven by increasing demands for higher throughput, improved energy efficiency, and enhanced product quality across various industries.
Despite these advancements, turnaround time remains a critical challenge in autoclave operations. The complete cycle—including loading, heating, curing/processing, cooling, and unloading—often represents a significant bottleneck in production workflows. Current industry benchmarks indicate that a standard composite curing cycle can range from 6 to 24 hours, with substantial variations depending on material thickness, complexity, and quality requirements.
The primary optimization goals for autoclave cycle reduction focus on several key areas. First, minimizing heat-up and cool-down phases through improved thermal management systems and advanced insulation materials. Second, optimizing cure cycles through better understanding of material behavior and reaction kinetics, potentially enabling accelerated curing without compromising quality. Third, implementing predictive modeling and simulation tools to pre-emptively identify optimal process parameters rather than relying on empirical testing.
Energy efficiency represents another crucial optimization target, as traditional autoclave operations consume substantial power during heating and pressure maintenance phases. Reducing cycle times directly correlates with lower energy consumption, making this a dual-benefit optimization area with both operational and sustainability advantages.
The ultimate goal of autoclave cycle optimization extends beyond mere time reduction to encompass a holistic improvement in operational efficiency. This includes minimizing variability between cycles, reducing defect rates, extending equipment lifespan through more balanced thermal loading, and creating more predictable production schedules that facilitate better resource allocation and planning throughout the manufacturing process.
Market Demand for Rapid Sterilization Solutions
The global market for rapid sterilization solutions has experienced significant growth in recent years, driven primarily by healthcare facilities seeking to optimize operational efficiency while maintaining rigorous sterilization standards. The demand for reduced autoclave cycle times stems from multiple interconnected factors across various sectors, with healthcare institutions representing the largest market segment.
Healthcare facilities worldwide face increasing pressure to process more surgical instruments and medical devices within shorter timeframes due to rising surgical volumes and cost containment initiatives. According to market research, hospitals typically process between 30-50 sterilization cycles daily, with traditional autoclave cycles requiring 45-60 minutes per load. This creates substantial operational bottlenecks, particularly in high-volume surgical centers.
The economic implications of extended sterilization turnaround times are considerable. Hospitals must maintain larger instrument inventories to compensate for items in the sterilization pipeline, representing significant capital investment. Each additional hour of autoclave processing time translates to approximately 2-3 fewer surgical procedures per operating room daily, directly impacting revenue generation and patient throughput.
Ambulatory surgical centers represent the fastest-growing segment in this market, with their business models particularly sensitive to operational efficiency metrics. These facilities typically operate with smaller instrument inventories than hospitals, making rapid sterilization cycles essential to their workflow and profitability.
Pharmaceutical and biotechnology manufacturing facilities constitute another significant market segment, where production downtime for equipment sterilization directly impacts manufacturing schedules and product availability. In these settings, even marginal improvements in autoclave cycle times can yield substantial productivity gains across production lines.
Regulatory trends have also influenced market demand, with healthcare accreditation bodies increasingly emphasizing both sterilization efficacy and operational efficiency metrics in their evaluation criteria. This dual focus has prompted healthcare administrators to prioritize investments in sterilization optimization technologies.
Geographically, North America currently represents the largest market for autoclave cycle optimization solutions, followed by Europe and Asia-Pacific. However, emerging economies are showing accelerated growth rates as their healthcare infrastructure expands and modernizes, creating new market opportunities for advanced sterilization technologies.
The COVID-19 pandemic has further intensified market demand, as healthcare facilities worldwide have faced unprecedented pressure to process more equipment through their central sterile processing departments while managing staffing constraints and heightened infection control requirements.
Healthcare facilities worldwide face increasing pressure to process more surgical instruments and medical devices within shorter timeframes due to rising surgical volumes and cost containment initiatives. According to market research, hospitals typically process between 30-50 sterilization cycles daily, with traditional autoclave cycles requiring 45-60 minutes per load. This creates substantial operational bottlenecks, particularly in high-volume surgical centers.
The economic implications of extended sterilization turnaround times are considerable. Hospitals must maintain larger instrument inventories to compensate for items in the sterilization pipeline, representing significant capital investment. Each additional hour of autoclave processing time translates to approximately 2-3 fewer surgical procedures per operating room daily, directly impacting revenue generation and patient throughput.
Ambulatory surgical centers represent the fastest-growing segment in this market, with their business models particularly sensitive to operational efficiency metrics. These facilities typically operate with smaller instrument inventories than hospitals, making rapid sterilization cycles essential to their workflow and profitability.
Pharmaceutical and biotechnology manufacturing facilities constitute another significant market segment, where production downtime for equipment sterilization directly impacts manufacturing schedules and product availability. In these settings, even marginal improvements in autoclave cycle times can yield substantial productivity gains across production lines.
Regulatory trends have also influenced market demand, with healthcare accreditation bodies increasingly emphasizing both sterilization efficacy and operational efficiency metrics in their evaluation criteria. This dual focus has prompted healthcare administrators to prioritize investments in sterilization optimization technologies.
Geographically, North America currently represents the largest market for autoclave cycle optimization solutions, followed by Europe and Asia-Pacific. However, emerging economies are showing accelerated growth rates as their healthcare infrastructure expands and modernizes, creating new market opportunities for advanced sterilization technologies.
The COVID-19 pandemic has further intensified market demand, as healthcare facilities worldwide have faced unprecedented pressure to process more equipment through their central sterile processing departments while managing staffing constraints and heightened infection control requirements.
Current Autoclave Cycle Limitations and Challenges
Autoclave processing represents a critical manufacturing step in various industries, particularly in aerospace, medical device manufacturing, and composite materials production. Despite its widespread use, current autoclave cycles face significant limitations that impact operational efficiency and production throughput. The conventional autoclave process typically involves lengthy heating and cooling phases, with total cycle times often exceeding 8-12 hours for complex composite parts, creating substantial production bottlenecks.
One primary challenge is the thermal inertia inherent in autoclave systems. The massive thermal mass of both the autoclave vessel and the tooling requires extended time to reach target temperatures and subsequently cool down. This physical limitation creates significant inefficiencies, particularly when processing temperature-sensitive materials that require precise thermal profiles. Temperature gradients within the autoclave chamber further complicate matters, leading to non-uniform curing and potential quality inconsistencies.
Pressure application and control systems present additional challenges. Current autoclave technology often employs relatively slow pressurization and depressurization rates to maintain part quality and prevent defects. The mechanical constraints of pressure vessels and safety considerations limit the speed at which pressure changes can be implemented, extending overall cycle duration. Moreover, the integration of pressure and temperature control systems lacks sophisticated coordination algorithms that could potentially optimize these parameters simultaneously.
Energy consumption represents another critical limitation. Traditional autoclave cycles are notoriously energy-intensive, with significant thermal losses occurring throughout the process. The inefficient use of energy not only increases operational costs but also contradicts growing sustainability imperatives across manufacturing industries. Current systems typically lack advanced energy recovery mechanisms that could capture and repurpose waste heat.
Process monitoring and control technologies present further constraints. Many existing autoclave systems rely on limited sensor arrays that provide insufficient real-time data about actual part conditions during processing. This data deficiency prevents the implementation of truly adaptive control strategies that could dynamically adjust cycle parameters based on actual part state rather than predetermined time-temperature-pressure profiles.
Workflow integration challenges also contribute to extended turnaround times. The manual handling requirements for part preparation, loading, unloading, and post-processing create significant non-value-added time in the overall manufacturing sequence. These operational inefficiencies compound the technical limitations of the autoclave cycle itself, further extending the effective turnaround time from raw material to finished product.
One primary challenge is the thermal inertia inherent in autoclave systems. The massive thermal mass of both the autoclave vessel and the tooling requires extended time to reach target temperatures and subsequently cool down. This physical limitation creates significant inefficiencies, particularly when processing temperature-sensitive materials that require precise thermal profiles. Temperature gradients within the autoclave chamber further complicate matters, leading to non-uniform curing and potential quality inconsistencies.
Pressure application and control systems present additional challenges. Current autoclave technology often employs relatively slow pressurization and depressurization rates to maintain part quality and prevent defects. The mechanical constraints of pressure vessels and safety considerations limit the speed at which pressure changes can be implemented, extending overall cycle duration. Moreover, the integration of pressure and temperature control systems lacks sophisticated coordination algorithms that could potentially optimize these parameters simultaneously.
Energy consumption represents another critical limitation. Traditional autoclave cycles are notoriously energy-intensive, with significant thermal losses occurring throughout the process. The inefficient use of energy not only increases operational costs but also contradicts growing sustainability imperatives across manufacturing industries. Current systems typically lack advanced energy recovery mechanisms that could capture and repurpose waste heat.
Process monitoring and control technologies present further constraints. Many existing autoclave systems rely on limited sensor arrays that provide insufficient real-time data about actual part conditions during processing. This data deficiency prevents the implementation of truly adaptive control strategies that could dynamically adjust cycle parameters based on actual part state rather than predetermined time-temperature-pressure profiles.
Workflow integration challenges also contribute to extended turnaround times. The manual handling requirements for part preparation, loading, unloading, and post-processing create significant non-value-added time in the overall manufacturing sequence. These operational inefficiencies compound the technical limitations of the autoclave cycle itself, further extending the effective turnaround time from raw material to finished product.
Current Approaches to Autoclave Cycle Time Reduction
01 Monitoring and optimization of autoclave cycles
Systems and methods for monitoring and optimizing autoclave cycles to reduce turnaround time. These include real-time monitoring of sterilization parameters, automated cycle selection based on load characteristics, and predictive analytics to optimize cycle duration while ensuring sterilization efficacy. Such systems can identify inefficiencies in the sterilization process and suggest improvements to reduce overall cycle time.- Monitoring and optimization of autoclave cycles: Systems and methods for monitoring and optimizing autoclave cycles to reduce turnaround time. These include real-time monitoring of sterilization parameters, automated cycle selection based on load characteristics, and predictive analytics to optimize cycle duration while ensuring sterilization efficacy. Advanced sensors and control systems allow for precise management of temperature, pressure, and time parameters to minimize the overall processing time.
- Scheduling and workflow management for autoclave operations: Software solutions and methodologies for scheduling and managing autoclave operations to minimize turnaround time. These systems provide optimized scheduling of sterilization batches, prioritization of urgent loads, and coordination of pre and post-sterilization activities. Workflow management tools track the entire sterilization process from instrument collection to redistribution, identifying bottlenecks and streamlining operations to reduce overall turnaround time.
- Rapid cooling technologies for autoclaves: Innovations in cooling systems designed to reduce the cooling phase of the autoclave cycle, which is often a significant contributor to overall turnaround time. These technologies include forced air cooling, water spray systems, and heat exchangers that accelerate the temperature reduction after the sterilization phase. By reducing cooling time while maintaining sterilization integrity, these systems significantly decrease the total turnaround time for autoclave operations.
- Automated loading and unloading systems: Mechanical systems and robotic solutions for automating the loading and unloading of autoclaves to reduce handling time. These systems include conveyor mechanisms, robotic arms, and automated transfer carts that minimize manual intervention and streamline the movement of items to and from the autoclave. By reducing the time required for loading and unloading operations, these systems contribute to shorter overall turnaround times.
- Maintenance optimization for autoclave efficiency: Strategies and systems for optimizing autoclave maintenance to minimize downtime and ensure consistent performance. These include predictive maintenance algorithms, real-time monitoring of autoclave components, and scheduled preventive maintenance during low-demand periods. By reducing unexpected failures and optimizing maintenance schedules, these approaches help maintain optimal autoclave performance and minimize turnaround times over the long term.
02 Scheduling and workflow management for autoclave operations
Software solutions and methods for scheduling and managing autoclave operations to minimize turnaround time. These include automated scheduling systems that optimize the sequence of sterilization batches, workflow management tools that track the status of items through the sterilization process, and resource allocation algorithms that ensure efficient use of autoclave equipment and personnel.Expand Specific Solutions03 Rapid cooling and loading/unloading systems
Mechanical innovations designed to reduce the cooling phase and loading/unloading time of autoclaves. These include advanced cooling systems that accelerate the post-sterilization temperature reduction, automated loading and unloading mechanisms that minimize handling time, and specialized cart systems that allow for quick transfer of materials in and out of the autoclave chamber.Expand Specific Solutions04 Process automation and control systems
Automated control systems that optimize the entire autoclave process to reduce turnaround time. These include programmable logic controllers that precisely manage sterilization parameters, integrated sensors that provide real-time feedback on cycle progress, and adaptive control algorithms that adjust cycle parameters based on load characteristics to minimize overall processing time.Expand Specific Solutions05 Maintenance optimization and failure prevention
Strategies and systems for optimizing autoclave maintenance schedules and preventing failures that could extend turnaround time. These include predictive maintenance algorithms that identify potential issues before they cause downtime, automated diagnostic systems that quickly pinpoint problems, and maintenance scheduling tools that minimize the impact on operational availability of autoclave equipment.Expand Specific Solutions
Leading Autoclave Manufacturers and Industry Landscape
The autoclave cycle optimization market is currently in a growth phase, driven by increasing demand for efficiency in industries requiring sterilization processes. The global market size is estimated to reach $2.5 billion by 2025, with a CAGR of 6.8%. From a technological maturity perspective, established players like Boeing, General Electric, and Shinva Medical Instrument are leading with advanced solutions that integrate AI and IoT for real-time monitoring and adjustment of autoclave parameters. Academic institutions such as China Agricultural University and Huazhong University of Science & Technology are contributing significant research in thermal process optimization. Meanwhile, specialized companies like Aerothermal Group and ILLIG are developing niche solutions focused on specific industry applications, creating a competitive landscape that balances innovation from research institutions with practical implementation from industrial leaders.
The Boeing Co.
Technical Solution: Boeing has developed advanced autoclave cycle optimization systems specifically for aerospace composite manufacturing. Their approach combines machine learning algorithms with thermal modeling to predict optimal cure cycles based on part geometry and material properties. Boeing's Autoclave Management System (AMS) uses real-time monitoring with multiple temperature and pressure sensors throughout the autoclave chamber to create dynamic cure profiles. The system employs predictive modeling that adjusts parameters during the cycle based on actual part temperature response rather than following fixed time-based recipes. Boeing has implemented adaptive control algorithms that can reduce cure cycles by up to 25% while maintaining or improving part quality through precise resin viscosity management during critical transition phases.
Strengths: Highly specialized for aerospace-grade composites with exceptional quality control; integration with digital twin technology for virtual testing before physical implementation. Weaknesses: Systems are primarily designed for large-scale aerospace applications and may be cost-prohibitive for smaller operations; requires significant computational resources and specialized expertise.
General Electric Company
Technical Solution: GE has pioneered autoclave optimization technology through their Digital Industrial approach, combining IoT sensors, advanced analytics, and machine learning. Their Smart Autoclave System incorporates hundreds of sensors monitoring temperature, pressure, vacuum integrity, and part response in real-time. GE's proprietary algorithms analyze historical cure data alongside current process parameters to continuously optimize cycles. The system features adaptive heating and cooling control that precisely manages temperature ramp rates and dwell times based on actual part response rather than predetermined schedules. GE has implemented digital twin modeling that simulates cure cycles before execution, predicting outcomes and identifying optimization opportunities. Their technology has demonstrated cycle time reductions of 15-30% while maintaining strict aerospace quality requirements through precise control of critical cure parameters.
Strengths: Comprehensive integration with broader manufacturing systems; extensive database of historical cure cycles enabling continuous improvement; sophisticated predictive maintenance capabilities. Weaknesses: High implementation costs; requires significant infrastructure changes to existing autoclave systems; complex system requires specialized training.
Key Innovations in Rapid Sterilization Processes
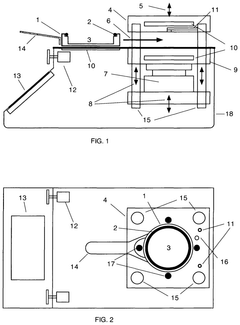
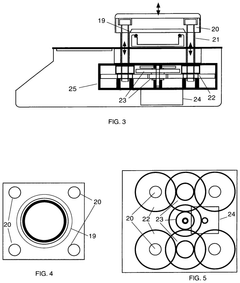
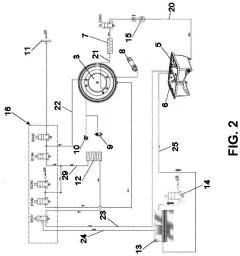
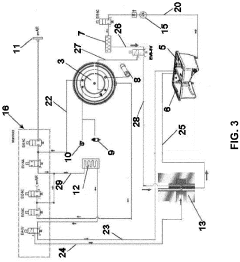
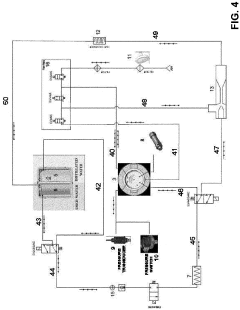
Pressure vessel and closure system for improved pressure processing
PatentActiveUS12121884B2
Innovation
- A pressure vessel design with a lid that slides onto a surface, allowing horizontal movement and vertical actuation using low surface area rods, eliminating the need for bolts and providing a sealed closure system that can accommodate different sizes without vertical lid movement, and featuring actuator mechanisms sealed from the clean room environment.
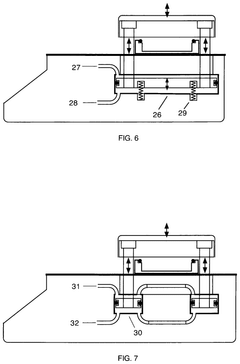
autoclave
PatentActiveEP4190365A1
Innovation
- Replacing the vacuum pump with a Venturi ejector that utilizes the Venturi effect to generate suction for removing residual air and water steam from the sterilization chamber, either actuated by compressed air or steam generated within the autoclave, thereby eliminating the need for a vacuum pump and reducing costs.
Energy Efficiency Considerations in Cycle Optimization
Energy efficiency has emerged as a critical factor in autoclave cycle optimization, directly impacting both operational costs and environmental sustainability. The traditional autoclave processes consume substantial amounts of energy, primarily in the form of steam generation and heating, which accounts for approximately 60-70% of the total operational costs. When optimizing autoclave cycles for reduced turnaround time, energy consumption patterns must be carefully analyzed to identify potential efficiency improvements without compromising sterilization efficacy.
Recent advancements in thermal insulation technologies have demonstrated potential energy savings of 15-25% in autoclave operations. These innovations include multi-layered composite insulation materials and vacuum-sealed barriers that significantly reduce heat loss during the sterilization process. Additionally, heat recovery systems that capture and reuse thermal energy from exhaust steam can further reduce energy requirements by up to 30%, creating a more sustainable operational profile while simultaneously decreasing cycle times.
The relationship between pressure ramp rates and energy consumption presents another optimization opportunity. Research indicates that carefully controlled pressure increase profiles can reduce energy usage by 10-18% compared to traditional rapid pressurization methods. This approach not only conserves energy but also extends equipment lifespan by reducing thermal stress on autoclave components, thereby decreasing maintenance requirements and associated downtime.
Water consumption represents another significant aspect of energy efficiency in autoclave operations. Advanced water recirculation systems can reduce water usage by up to 40%, simultaneously decreasing the energy required for water heating. The implementation of precise water level controls and optimized steam generation algorithms further contributes to energy conservation while maintaining effective sterilization parameters.
Digital twin modeling and real-time monitoring systems have revolutionized energy management in autoclave operations. These technologies enable predictive energy optimization by simulating various operational scenarios and identifying the most efficient process parameters. Studies show that facilities implementing such systems have achieved energy reductions of 20-35% while simultaneously decreasing cycle times by 15-25%, demonstrating the synergistic relationship between energy efficiency and turnaround time optimization.
The integration of renewable energy sources into autoclave operations represents an emerging trend with significant potential. Solar thermal systems and biomass-powered steam generation can offset traditional energy sources, reducing both operational costs and carbon footprint. While initial implementation costs remain high, the long-term benefits include enhanced energy security and compliance with increasingly stringent environmental regulations, positioning energy-efficient autoclave operations as a competitive advantage in various industries.
Recent advancements in thermal insulation technologies have demonstrated potential energy savings of 15-25% in autoclave operations. These innovations include multi-layered composite insulation materials and vacuum-sealed barriers that significantly reduce heat loss during the sterilization process. Additionally, heat recovery systems that capture and reuse thermal energy from exhaust steam can further reduce energy requirements by up to 30%, creating a more sustainable operational profile while simultaneously decreasing cycle times.
The relationship between pressure ramp rates and energy consumption presents another optimization opportunity. Research indicates that carefully controlled pressure increase profiles can reduce energy usage by 10-18% compared to traditional rapid pressurization methods. This approach not only conserves energy but also extends equipment lifespan by reducing thermal stress on autoclave components, thereby decreasing maintenance requirements and associated downtime.
Water consumption represents another significant aspect of energy efficiency in autoclave operations. Advanced water recirculation systems can reduce water usage by up to 40%, simultaneously decreasing the energy required for water heating. The implementation of precise water level controls and optimized steam generation algorithms further contributes to energy conservation while maintaining effective sterilization parameters.
Digital twin modeling and real-time monitoring systems have revolutionized energy management in autoclave operations. These technologies enable predictive energy optimization by simulating various operational scenarios and identifying the most efficient process parameters. Studies show that facilities implementing such systems have achieved energy reductions of 20-35% while simultaneously decreasing cycle times by 15-25%, demonstrating the synergistic relationship between energy efficiency and turnaround time optimization.
The integration of renewable energy sources into autoclave operations represents an emerging trend with significant potential. Solar thermal systems and biomass-powered steam generation can offset traditional energy sources, reducing both operational costs and carbon footprint. While initial implementation costs remain high, the long-term benefits include enhanced energy security and compliance with increasingly stringent environmental regulations, positioning energy-efficient autoclave operations as a competitive advantage in various industries.
Validation Standards for Accelerated Sterilization Protocols
The validation of accelerated sterilization protocols requires adherence to stringent standards to ensure both efficacy and safety. Current regulatory frameworks, including those established by the FDA, ISO, and AAMI, provide comprehensive guidelines for validating traditional autoclave cycles but offer limited specific guidance for accelerated protocols. This regulatory gap necessitates the development of specialized validation methodologies that can accommodate shorter cycle times while maintaining sterilization assurance levels.
Key validation parameters for accelerated sterilization protocols include biological indicators with appropriate resistance characteristics, chemical indicators capable of responding accurately to shortened exposure times, and physical monitoring systems with enhanced precision for critical parameters such as temperature, pressure, and humidity. These validation tools must demonstrate reliability under the intensified conditions typical of accelerated cycles.
The validation process for accelerated protocols typically follows a three-phase approach: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). For accelerated cycles, these phases require modifications to account for the reduced exposure times and potentially higher operating parameters. The IQ phase must verify that equipment can consistently achieve and maintain the more demanding conditions, while OQ protocols need to establish tighter control limits for critical parameters.
Performance qualification for accelerated cycles demands more extensive biological challenge testing, often requiring increased sample sizes and more stringent acceptance criteria. The bioburden recovery studies must be particularly thorough, as the margin for error decreases with shortened cycle times. Additionally, validation protocols should include worst-case scenario testing that specifically addresses the unique challenges of accelerated cycles, such as steam penetration under compressed timeframes.
Documentation requirements for accelerated protocol validation are more extensive than for conventional cycles. Detailed records must demonstrate the scientific rationale for parameter selection, comprehensive risk assessments addressing potential failure modes specific to accelerated cycles, and robust statistical analyses supporting the validation conclusions. Revalidation frequencies may need to be increased, particularly during initial implementation phases, to ensure continued compliance with sterility assurance levels.
Emerging standards specifically addressing accelerated sterilization are being developed by industry consortia and standards organizations. These include proposed modifications to existing standards such as ISO 17665 and AAMI ST79, with additional requirements for equipment qualification, process monitoring, and cycle development when implementing reduced turnaround times. Organizations implementing accelerated protocols should actively monitor these evolving standards to ensure ongoing compliance.
Key validation parameters for accelerated sterilization protocols include biological indicators with appropriate resistance characteristics, chemical indicators capable of responding accurately to shortened exposure times, and physical monitoring systems with enhanced precision for critical parameters such as temperature, pressure, and humidity. These validation tools must demonstrate reliability under the intensified conditions typical of accelerated cycles.
The validation process for accelerated protocols typically follows a three-phase approach: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). For accelerated cycles, these phases require modifications to account for the reduced exposure times and potentially higher operating parameters. The IQ phase must verify that equipment can consistently achieve and maintain the more demanding conditions, while OQ protocols need to establish tighter control limits for critical parameters.
Performance qualification for accelerated cycles demands more extensive biological challenge testing, often requiring increased sample sizes and more stringent acceptance criteria. The bioburden recovery studies must be particularly thorough, as the margin for error decreases with shortened cycle times. Additionally, validation protocols should include worst-case scenario testing that specifically addresses the unique challenges of accelerated cycles, such as steam penetration under compressed timeframes.
Documentation requirements for accelerated protocol validation are more extensive than for conventional cycles. Detailed records must demonstrate the scientific rationale for parameter selection, comprehensive risk assessments addressing potential failure modes specific to accelerated cycles, and robust statistical analyses supporting the validation conclusions. Revalidation frequencies may need to be increased, particularly during initial implementation phases, to ensure continued compliance with sterility assurance levels.
Emerging standards specifically addressing accelerated sterilization are being developed by industry consortia and standards organizations. These include proposed modifications to existing standards such as ISO 17665 and AAMI ST79, with additional requirements for equipment qualification, process monitoring, and cycle development when implementing reduced turnaround times. Organizations implementing accelerated protocols should actively monitor these evolving standards to ensure ongoing compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!