Autoclave Load Study: Effects on Sterilization Consistency
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Background and Objectives
Autoclave sterilization has been a cornerstone of contamination control in healthcare, laboratory, and industrial settings for over a century. Developed in the late 19th century, this technology leverages the power of pressurized steam to achieve thermal destruction of microorganisms. The fundamental principle relies on exposing materials to saturated steam at temperatures typically ranging from 121°C to 134°C under pressure, which effectively denatures proteins and disrupts cellular structures of microorganisms, rendering them non-viable.
The evolution of autoclave technology has progressed from basic pressure cooker designs to sophisticated computer-controlled systems with precise parameter monitoring capabilities. This progression reflects the increasing demands for sterilization reliability across various sectors, including medical device manufacturing, pharmaceutical production, and clinical applications where sterility assurance is critical to patient safety and product integrity.
Current technological trends in autoclave sterilization focus on energy efficiency, process optimization, load-specific cycle development, and enhanced validation methodologies. The integration of Industry 4.0 principles has introduced smart monitoring systems, predictive maintenance capabilities, and data-driven process control, significantly improving operational reliability and documentation compliance with regulatory standards.
The primary objective of this technical research is to investigate the relationship between autoclave load configurations and sterilization consistency. This investigation aims to identify critical parameters that influence steam penetration, heat distribution, and ultimately sterilization efficacy across different load compositions, densities, and arrangements. Understanding these relationships is essential for developing robust sterilization protocols that can accommodate diverse material types and packaging configurations while maintaining sterility assurance levels.
Secondary objectives include quantifying the impact of load heterogeneity on cycle parameters, establishing mathematical models for predicting steam penetration in complex loads, and developing optimization algorithms for load arrangement that maximize sterilization efficiency while minimizing cycle duration and utility consumption. These objectives align with industry demands for increased throughput without compromising sterility assurance.
The significance of this research extends beyond operational efficiency to address regulatory compliance challenges, as health authorities worldwide increasingly require scientific evidence supporting sterilization process validation. By establishing a comprehensive understanding of load-dependent sterilization dynamics, this research aims to provide a scientific foundation for risk-based approaches to sterilization validation, potentially reducing validation costs while enhancing sterility assurance levels across diverse applications.
The evolution of autoclave technology has progressed from basic pressure cooker designs to sophisticated computer-controlled systems with precise parameter monitoring capabilities. This progression reflects the increasing demands for sterilization reliability across various sectors, including medical device manufacturing, pharmaceutical production, and clinical applications where sterility assurance is critical to patient safety and product integrity.
Current technological trends in autoclave sterilization focus on energy efficiency, process optimization, load-specific cycle development, and enhanced validation methodologies. The integration of Industry 4.0 principles has introduced smart monitoring systems, predictive maintenance capabilities, and data-driven process control, significantly improving operational reliability and documentation compliance with regulatory standards.
The primary objective of this technical research is to investigate the relationship between autoclave load configurations and sterilization consistency. This investigation aims to identify critical parameters that influence steam penetration, heat distribution, and ultimately sterilization efficacy across different load compositions, densities, and arrangements. Understanding these relationships is essential for developing robust sterilization protocols that can accommodate diverse material types and packaging configurations while maintaining sterility assurance levels.
Secondary objectives include quantifying the impact of load heterogeneity on cycle parameters, establishing mathematical models for predicting steam penetration in complex loads, and developing optimization algorithms for load arrangement that maximize sterilization efficiency while minimizing cycle duration and utility consumption. These objectives align with industry demands for increased throughput without compromising sterility assurance.
The significance of this research extends beyond operational efficiency to address regulatory compliance challenges, as health authorities worldwide increasingly require scientific evidence supporting sterilization process validation. By establishing a comprehensive understanding of load-dependent sterilization dynamics, this research aims to provide a scientific foundation for risk-based approaches to sterilization validation, potentially reducing validation costs while enhancing sterility assurance levels across diverse applications.
Market Demand Analysis for Consistent Sterilization Solutions
The global market for sterilization solutions has experienced significant growth in recent years, driven primarily by increasing healthcare expenditures, rising surgical procedures, and growing awareness about infection control. The sterilization equipment market was valued at approximately $6.1 billion in 2020 and is projected to reach $9.1 billion by 2025, growing at a CAGR of 8.3%. Within this broader market, autoclave sterilization remains a dominant segment, particularly in healthcare facilities, pharmaceutical manufacturing, and research laboratories.
Healthcare facilities worldwide are facing mounting pressure to ensure consistent sterilization outcomes due to stringent regulatory requirements and the rising costs associated with healthcare-acquired infections (HAIs). According to the Centers for Disease Control and Prevention (CDC), HAIs affect about 1 in 31 hospital patients in the United States alone, resulting in nearly 100,000 deaths annually and adding an estimated $28-45 billion to healthcare costs.
The demand for consistent sterilization solutions is particularly acute in surgical settings, where the consequences of inadequate sterilization can be severe. With over 300 million surgical procedures performed globally each year, the market for reliable autoclave sterilization processes continues to expand. Hospitals and ambulatory surgical centers are increasingly seeking solutions that can provide validated sterilization outcomes with minimal variability across different load configurations.
Pharmaceutical and biotechnology companies represent another significant market segment, driven by stringent GMP requirements and the need for contamination control in manufacturing processes. The global pharmaceutical manufacturing market, valued at $405 billion, demands highly consistent sterilization processes to ensure product safety and regulatory compliance.
Regional analysis reveals that North America currently holds the largest market share for advanced sterilization solutions, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate, with a projected CAGR of 10.2% through 2025, due to expanding healthcare infrastructure and increasing adoption of international sterilization standards.
End-users are increasingly demanding sterilization solutions that offer not only consistency but also efficiency, reduced cycle times, and comprehensive documentation capabilities. The market shows a clear trend toward integrated systems that provide real-time monitoring, automated load configuration optimization, and predictive analytics to ensure sterilization consistency across various load types and densities.
Industry surveys indicate that approximately 78% of healthcare facilities consider sterilization consistency a "critical" or "very important" factor in their purchasing decisions for new autoclave equipment, highlighting the significant market opportunity for innovations that address variability in sterilization outcomes.
Healthcare facilities worldwide are facing mounting pressure to ensure consistent sterilization outcomes due to stringent regulatory requirements and the rising costs associated with healthcare-acquired infections (HAIs). According to the Centers for Disease Control and Prevention (CDC), HAIs affect about 1 in 31 hospital patients in the United States alone, resulting in nearly 100,000 deaths annually and adding an estimated $28-45 billion to healthcare costs.
The demand for consistent sterilization solutions is particularly acute in surgical settings, where the consequences of inadequate sterilization can be severe. With over 300 million surgical procedures performed globally each year, the market for reliable autoclave sterilization processes continues to expand. Hospitals and ambulatory surgical centers are increasingly seeking solutions that can provide validated sterilization outcomes with minimal variability across different load configurations.
Pharmaceutical and biotechnology companies represent another significant market segment, driven by stringent GMP requirements and the need for contamination control in manufacturing processes. The global pharmaceutical manufacturing market, valued at $405 billion, demands highly consistent sterilization processes to ensure product safety and regulatory compliance.
Regional analysis reveals that North America currently holds the largest market share for advanced sterilization solutions, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate, with a projected CAGR of 10.2% through 2025, due to expanding healthcare infrastructure and increasing adoption of international sterilization standards.
End-users are increasingly demanding sterilization solutions that offer not only consistency but also efficiency, reduced cycle times, and comprehensive documentation capabilities. The market shows a clear trend toward integrated systems that provide real-time monitoring, automated load configuration optimization, and predictive analytics to ensure sterilization consistency across various load types and densities.
Industry surveys indicate that approximately 78% of healthcare facilities consider sterilization consistency a "critical" or "very important" factor in their purchasing decisions for new autoclave equipment, highlighting the significant market opportunity for innovations that address variability in sterilization outcomes.
Current Challenges in Autoclave Load Configuration
Despite significant advancements in autoclave technology, several persistent challenges in load configuration continue to impact sterilization consistency and efficiency. The primary challenge stems from the complex relationship between load density and steam penetration. When instruments and materials are packed too densely or improperly arranged, steam cannot effectively reach all surfaces, creating "cold spots" where sterilization may be incomplete. This issue is particularly pronounced in healthcare settings where diverse instrument sets with varying geometries must be processed simultaneously.
Material composition heterogeneity presents another significant obstacle. Different materials exhibit varying thermal conductivity properties and steam absorption rates. For instance, porous materials like textiles require longer exposure times compared to solid metal instruments. When these diverse materials are combined in a single load, achieving uniform sterilization conditions becomes exceedingly difficult, often necessitating compromise settings that may be suboptimal for certain items.
Load positioning inconsistencies further complicate sterilization processes. Research indicates that items placed at different locations within the autoclave chamber experience varying temperature profiles and steam exposure. Central positions typically reach target temperatures more slowly than peripheral locations, while items placed near the chamber door may experience temperature fluctuations due to heat loss. These spatial variations can lead to inconsistent sterilization outcomes even when following standardized protocols.
The challenge of proper packaging and wrapping methods significantly impacts steam penetration. Overly tight wrapping or inappropriate packaging materials can create barriers to steam penetration, while inadequate wrapping may fail to maintain sterility post-processing. Studies have shown that up to 15% of sterilization failures can be attributed to packaging issues, highlighting the critical nature of this challenge.
Validation and monitoring limitations constitute another major hurdle. Current biological indicators and chemical integrators provide only limited spatial information about sterilization conditions within complex loads. These tools typically monitor conditions at specific points rather than comprehensively throughout the load, potentially missing problematic areas where sterilization parameters are not met.
Workflow pressures in clinical environments often lead to rushed load configuration practices. Time constraints frequently result in overloaded chambers or improper arrangement of items as staff attempt to maximize throughput. This operational reality directly conflicts with best practices for optimal sterilization, creating a persistent tension between efficiency demands and quality assurance requirements.
Material composition heterogeneity presents another significant obstacle. Different materials exhibit varying thermal conductivity properties and steam absorption rates. For instance, porous materials like textiles require longer exposure times compared to solid metal instruments. When these diverse materials are combined in a single load, achieving uniform sterilization conditions becomes exceedingly difficult, often necessitating compromise settings that may be suboptimal for certain items.
Load positioning inconsistencies further complicate sterilization processes. Research indicates that items placed at different locations within the autoclave chamber experience varying temperature profiles and steam exposure. Central positions typically reach target temperatures more slowly than peripheral locations, while items placed near the chamber door may experience temperature fluctuations due to heat loss. These spatial variations can lead to inconsistent sterilization outcomes even when following standardized protocols.
The challenge of proper packaging and wrapping methods significantly impacts steam penetration. Overly tight wrapping or inappropriate packaging materials can create barriers to steam penetration, while inadequate wrapping may fail to maintain sterility post-processing. Studies have shown that up to 15% of sterilization failures can be attributed to packaging issues, highlighting the critical nature of this challenge.
Validation and monitoring limitations constitute another major hurdle. Current biological indicators and chemical integrators provide only limited spatial information about sterilization conditions within complex loads. These tools typically monitor conditions at specific points rather than comprehensively throughout the load, potentially missing problematic areas where sterilization parameters are not met.
Workflow pressures in clinical environments often lead to rushed load configuration practices. Time constraints frequently result in overloaded chambers or improper arrangement of items as staff attempt to maximize throughput. This operational reality directly conflicts with best practices for optimal sterilization, creating a persistent tension between efficiency demands and quality assurance requirements.
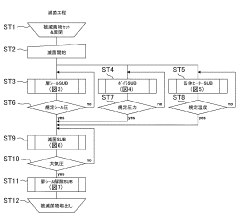
Current Load Study Methodologies and Protocols
01 Temperature and pressure monitoring systems
Advanced monitoring systems are essential for maintaining consistent autoclave sterilization. These systems continuously track temperature and pressure parameters throughout the sterilization cycle, ensuring that critical thresholds are maintained. Real-time monitoring allows for immediate detection of deviations from set parameters, triggering alerts when conditions fall outside acceptable ranges. This technology helps maintain sterilization consistency by providing accurate data for validation and documentation of the process.- Temperature and pressure monitoring systems: Advanced monitoring systems are crucial for maintaining consistent autoclave sterilization. These systems continuously track temperature and pressure parameters throughout the sterilization cycle, ensuring that critical thresholds are maintained. Real-time monitoring allows for immediate detection of deviations from set parameters, triggering alerts when conditions fall outside acceptable ranges. This technology helps validate that sterilization conditions are consistently achieved across all areas of the autoclave chamber.
- Steam quality and distribution optimization: Effective steam quality and distribution are essential for consistent autoclave sterilization. Innovations in steam generation systems and chamber design ensure uniform steam penetration throughout the load. Advanced steam distribution mechanisms prevent the formation of air pockets that could compromise sterilization. These technologies include specialized steam generators, improved chamber geometry, and flow directors that optimize steam circulation patterns to achieve consistent sterilization conditions across the entire load.
- Validation and testing protocols: Standardized validation and testing protocols are implemented to verify autoclave sterilization consistency. These include biological indicators, chemical indicators, and physical parameter monitoring to confirm that sterilization conditions have been achieved. Regular testing regimens help identify potential issues before they affect sterilization outcomes. Automated documentation systems record cycle parameters and test results, providing traceability and evidence of consistent sterilization performance over time.
- Load configuration and packaging innovations: Proper load configuration and specialized packaging materials significantly impact sterilization consistency. Innovations in loading systems ensure optimal spacing between items, allowing for adequate steam penetration. Advanced packaging materials are designed to permit steam penetration while maintaining sterility post-processing. Standardized loading patterns and container systems help eliminate variables that could lead to inconsistent sterilization results across different cycle runs.
- Cycle customization and automation: Automated control systems allow for customized sterilization cycles based on load type and requirements. These systems precisely control cycle parameters including temperature ramp rates, hold times, and pressure changes to ensure consistent results across different load types. Pre-programmed cycle recipes eliminate human error and ensure reproducibility. Advanced algorithms can adjust parameters in real-time based on feedback from monitoring systems, compensating for variables that might otherwise lead to inconsistent sterilization outcomes.
02 Validation protocols and quality control
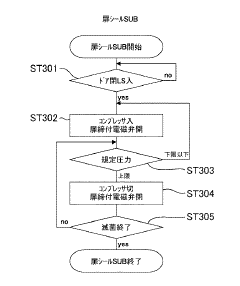
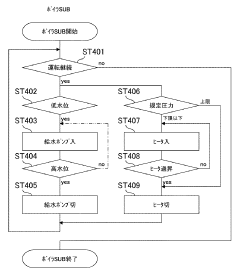
Standardized validation protocols are crucial for ensuring autoclave sterilization consistency. These protocols include biological indicators, chemical indicators, and physical monitors that verify sterilization effectiveness. Regular testing and documentation of sterilization cycles help maintain quality control standards. Validation procedures may include pre-vacuum tests, Bowie-Dick tests, and load configuration verification to ensure steam penetration and consistent sterilization across all items in the autoclave.Expand Specific Solutions03 Steam distribution and penetration optimization
Effective steam distribution is fundamental to consistent autoclave sterilization. Innovations in chamber design and steam injection systems ensure uniform steam penetration throughout the load. Pulsed vacuum systems remove air pockets that could prevent steam contact with surfaces. Proper load configuration and spacing guidelines maximize steam flow between items. These technologies collectively ensure that all surfaces reach the required temperature for the specified time, eliminating cold spots that could compromise sterilization.Expand Specific Solutions04 Automated cycle control and documentation
Automated control systems enhance sterilization consistency by precisely managing cycle parameters. These systems regulate temperature, pressure, and time according to predefined protocols for different load types. Automated documentation features record cycle data, creating audit trails that verify sterilization parameters were maintained throughout the process. Integration with electronic record systems allows for trend analysis and early detection of equipment performance issues that could affect consistency.Expand Specific Solutions05 Load configuration and packaging materials
Proper load configuration and appropriate packaging materials significantly impact sterilization consistency. Specialized packaging allows steam penetration while maintaining sterility after processing. Guidelines for item arrangement prevent overloading and ensure adequate steam circulation. Compatible packaging materials withstand autoclave conditions without compromising the sterilization process or product integrity. Standardized loading patterns and material selection contribute to reproducible sterilization results across multiple cycles.Expand Specific Solutions
Key Industry Players in Sterilization Equipment
The autoclave load study market is currently in a growth phase, with increasing demand for sterilization consistency across healthcare and industrial sectors. The market size is expanding due to stringent regulatory requirements and growing awareness of infection control. Technologically, the field shows varying maturity levels, with established players like Fedegari Autoclavi SpA and W&H Sterilization SRL offering advanced solutions, while newer entrants like LTE Scientific Ltd. focus on innovation. Companies such as Stryker Corp. and Olympus Corp. are integrating autoclave technology into broader medical device ecosystems, while specialized firms like Solstice Medical LLC are developing monitoring systems to enhance sterilization reliability. The competitive landscape features both large medical technology conglomerates and specialized sterilization equipment manufacturers competing to address consistency challenges through automation, validation protocols, and load optimization technologies.
Stryker Corp.
Technical Solution: Stryker has developed the AMSCO® Evolution® sterilization platform specifically addressing load study challenges in medical device sterilization. Their system incorporates Dynamic Phase Monitoring technology that continuously evaluates steam penetration and temperature distribution throughout the load using a network of precision sensors. The platform features adaptive algorithm technology that automatically modifies cycle parameters based on real-time feedback from load sensors, ensuring sterilization efficacy regardless of load composition or configuration. Stryker's ProConnect™ monitoring system provides comprehensive data visualization and analysis tools that identify sterilization consistency patterns across thousands of cycles, enabling predictive maintenance and process optimization. Their load-specific cycle development process utilizes thermal imaging and computational modeling to identify potential cold spots before physical validation.
Strengths: Exceptional reliability with redundant safety systems; comprehensive data management and reporting capabilities; proven performance with complex medical device loads. Weaknesses: Significant capital investment required; proprietary consumables increase operational costs; complex installation requirements including specialized utility connections.
W&H Sterilization SRL
Technical Solution: W&H has pioneered the Lisa autoclave series featuring their patented Eco Dry+ technology specifically designed to address load study challenges. Their system adapts sterilization cycle duration based on the actual load mass, optimizing both sterilization efficacy and resource efficiency. The company's Advanced Air Removal System creates precisely controlled vacuum pulses tailored to specific load characteristics, ensuring complete air evacuation even from complex instrument configurations. Their Real-Time Monitoring System employs multiple temperature and pressure sensors throughout the chamber and within test loads to continuously validate sterilization parameters. W&H's proprietary algorithm analyzes historical performance data across thousands of cycles to identify optimal parameters for specific load types and configurations, continuously refining cycle parameters based on empirical results.
Strengths: Exceptional energy efficiency while maintaining sterilization efficacy; rapid cycle times even with challenging loads; user-friendly interface with intuitive load selection options. Weaknesses: Limited chamber size compared to industrial autoclaves; higher maintenance requirements than simpler systems; premium pricing positioning in the market.
Critical Parameters Affecting Sterilization Consistency
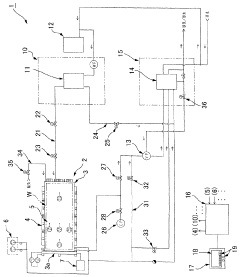
Process challenge device for a high-pressure steam sterilizer and sheet for a challenge device
PatentInactiveUS20070264683A1
Innovation
- A process challenge device comprising steam permeable bodies and a holder, where the steam permeable bodies form a cavity with an indicator that changes appearance upon exposure to a predetermined temperature, allowing for reusable testing without damaging the components and enabling user-selectable indicators.
Flow-type high-pressure steam sterilization method and flow-type sterilizer by soft hydrothermal process
PatentInactiveJPWO2017010525A1
Innovation
- A flow-type high-pressure steam sterilization method using a soft hydrothermal process that involves an air evacuation step, temperature and pressure increase, high-pressure steam sterilization with highly saturated steam circulation, and a controlled drying process to minimize condensation and shorten drying times.
Regulatory Standards for Autoclave Validation
Regulatory standards for autoclave validation are critical frameworks that ensure sterilization processes meet established safety and efficacy requirements. The FDA's Quality System Regulation (21 CFR Part 820) mandates that medical device manufacturers validate their sterilization processes, including autoclave operations. Similarly, the European Medical Device Regulation (EU MDR 2017/745) requires thorough validation of all sterilization methods used in medical device production.
ISO 17665-1:2006 specifically addresses moist heat sterilization validation, providing comprehensive guidelines for developing, validating, and routinely controlling sterilization processes. This standard emphasizes the importance of load configuration studies to ensure consistent sterilization across different load patterns and densities.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed TIR12:2020, which offers detailed guidance on steam sterilization validation in healthcare facilities. This technical information report specifically addresses load composition challenges and their impact on sterilization consistency.
Validation protocols typically require three consecutive successful cycles (Installation Qualification, Operational Qualification, and Performance Qualification) to demonstrate process reliability. These protocols must include worst-case scenarios that challenge the sterilization process, particularly focusing on load configuration variables.
Regulatory bodies increasingly emphasize parametric release approaches, where sterilization is validated based on measured physical parameters rather than biological indicators alone. This approach requires more rigorous initial validation of load studies but offers more consistent quality assurance during routine operation.
Documentation requirements for autoclave validation are extensive, including detailed load diagrams, temperature mapping studies, and biological indicator placement justifications. These records must demonstrate that all potential load configurations achieve the minimum required sterility assurance level (SAL), typically 10^-6 for critical medical devices.
Recent regulatory trends show increased scrutiny of load density validation, with authorities requiring manufacturers to establish clear maximum load densities and configurations. This focus stems from recognition that improper loading is a primary cause of sterilization failures in clinical settings.
Revalidation requirements are also becoming more stringent, with regulatory bodies mandating periodic revalidation of autoclave processes, especially when load compositions or configurations change. This ensures continued compliance with sterilization standards despite operational variations over time.
ISO 17665-1:2006 specifically addresses moist heat sterilization validation, providing comprehensive guidelines for developing, validating, and routinely controlling sterilization processes. This standard emphasizes the importance of load configuration studies to ensure consistent sterilization across different load patterns and densities.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed TIR12:2020, which offers detailed guidance on steam sterilization validation in healthcare facilities. This technical information report specifically addresses load composition challenges and their impact on sterilization consistency.
Validation protocols typically require three consecutive successful cycles (Installation Qualification, Operational Qualification, and Performance Qualification) to demonstrate process reliability. These protocols must include worst-case scenarios that challenge the sterilization process, particularly focusing on load configuration variables.
Regulatory bodies increasingly emphasize parametric release approaches, where sterilization is validated based on measured physical parameters rather than biological indicators alone. This approach requires more rigorous initial validation of load studies but offers more consistent quality assurance during routine operation.
Documentation requirements for autoclave validation are extensive, including detailed load diagrams, temperature mapping studies, and biological indicator placement justifications. These records must demonstrate that all potential load configurations achieve the minimum required sterility assurance level (SAL), typically 10^-6 for critical medical devices.
Recent regulatory trends show increased scrutiny of load density validation, with authorities requiring manufacturers to establish clear maximum load densities and configurations. This focus stems from recognition that improper loading is a primary cause of sterilization failures in clinical settings.
Revalidation requirements are also becoming more stringent, with regulatory bodies mandating periodic revalidation of autoclave processes, especially when load compositions or configurations change. This ensures continued compliance with sterilization standards despite operational variations over time.
Environmental Impact of Sterilization Processes
Sterilization processes, while essential for healthcare and industrial applications, carry significant environmental implications that warrant careful consideration. Autoclave sterilization, the focus of our load study on consistency effects, represents one of the most widely used methods globally. This process typically consumes substantial amounts of energy and water resources, with a standard autoclave cycle requiring 30-50 gallons of water and 6-15 kWh of electricity depending on load size and cycle parameters.
The environmental footprint of autoclave sterilization extends beyond resource consumption to include greenhouse gas emissions. Recent studies indicate that hospital sterilization departments contribute approximately 5-10% of a healthcare facility's total carbon footprint. When sterilization consistency is compromised due to improper loading techniques, these environmental impacts are magnified through repeated cycles, effectively doubling or tripling the resource utilization and emissions.
Chemical waste generation presents another environmental concern. While autoclaves primarily use steam, they often incorporate chemical indicators and cleaning agents that enter wastewater systems. Inconsistent sterilization may increase reliance on chemical sterilants as backup methods, introducing more persistent compounds into the environment. Research indicates that some sterilization chemicals, particularly those containing glutaraldehyde or formaldehyde, can remain biologically active in wastewater effluent.
Comparative analysis reveals that optimized autoclave loading practices can reduce environmental impact by 15-30% compared to suboptimal loading configurations. This improvement stems from enhanced steam penetration efficiency, reduced cycle times, and fewer failed cycles requiring reprocessing. Organizations implementing standardized loading protocols have documented significant reductions in water consumption and energy usage while maintaining sterilization efficacy.
Regulatory frameworks increasingly acknowledge these environmental considerations. The EU Medical Device Regulation and similar frameworks in other regions now incorporate sustainability requirements alongside safety standards. Healthcare facilities pursuing environmental certifications such as LEED or Practice Greenhealth must demonstrate sterilization process optimization as part of their sustainability initiatives.
Emerging technologies offer promising pathways to further reduce environmental impacts. Low-temperature hydrogen peroxide plasma systems and ozone-based sterilization methods demonstrate reduced resource requirements compared to traditional steam autoclaves, though their application remains limited to specific instrument types. Additionally, advanced monitoring systems that optimize cycle parameters based on actual load characteristics rather than worst-case scenarios show potential for significant resource conservation while maintaining sterilization consistency.
The environmental footprint of autoclave sterilization extends beyond resource consumption to include greenhouse gas emissions. Recent studies indicate that hospital sterilization departments contribute approximately 5-10% of a healthcare facility's total carbon footprint. When sterilization consistency is compromised due to improper loading techniques, these environmental impacts are magnified through repeated cycles, effectively doubling or tripling the resource utilization and emissions.
Chemical waste generation presents another environmental concern. While autoclaves primarily use steam, they often incorporate chemical indicators and cleaning agents that enter wastewater systems. Inconsistent sterilization may increase reliance on chemical sterilants as backup methods, introducing more persistent compounds into the environment. Research indicates that some sterilization chemicals, particularly those containing glutaraldehyde or formaldehyde, can remain biologically active in wastewater effluent.
Comparative analysis reveals that optimized autoclave loading practices can reduce environmental impact by 15-30% compared to suboptimal loading configurations. This improvement stems from enhanced steam penetration efficiency, reduced cycle times, and fewer failed cycles requiring reprocessing. Organizations implementing standardized loading protocols have documented significant reductions in water consumption and energy usage while maintaining sterilization efficacy.
Regulatory frameworks increasingly acknowledge these environmental considerations. The EU Medical Device Regulation and similar frameworks in other regions now incorporate sustainability requirements alongside safety standards. Healthcare facilities pursuing environmental certifications such as LEED or Practice Greenhealth must demonstrate sterilization process optimization as part of their sustainability initiatives.
Emerging technologies offer promising pathways to further reduce environmental impacts. Low-temperature hydrogen peroxide plasma systems and ozone-based sterilization methods demonstrate reduced resource requirements compared to traditional steam autoclaves, though their application remains limited to specific instrument types. Additionally, advanced monitoring systems that optimize cycle parameters based on actual load characteristics rather than worst-case scenarios show potential for significant resource conservation while maintaining sterilization consistency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!