Autoclave Reliability Improvements with Advanced Diagnostics
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Reliability Objectives
Autoclaves have evolved significantly since their inception in the early 20th century, transitioning from basic pressure vessels to sophisticated systems incorporating advanced materials, control systems, and safety features. Initially designed for sterilization in medical settings, autoclaves have expanded their application scope to aerospace, composites manufacturing, food processing, and materials research. This technological evolution has been driven by increasing demands for process reliability, energy efficiency, and operational safety across industries.
The modern autoclave represents a complex integration of mechanical engineering, materials science, and digital control systems. Recent advancements have focused on improving thermal uniformity, pressure stability, and cycle time optimization. However, despite these improvements, reliability issues continue to challenge operators and manufacturers, resulting in production delays, quality inconsistencies, and increased operational costs.
Current reliability metrics indicate that autoclave systems experience an average downtime of 8-12% annually, with approximately 60% of failures occurring due to inadequate diagnostics capabilities. Traditional maintenance approaches rely heavily on scheduled interventions rather than condition-based strategies, leading to either premature component replacement or unexpected failures between maintenance intervals.
The primary reliability objectives for next-generation autoclave systems center on reducing unplanned downtime by at least 50%, extending mean time between failures (MTBF) by 30%, and decreasing maintenance costs by 25%. These objectives necessitate a fundamental shift from reactive to predictive maintenance paradigms, enabled by advanced diagnostic technologies that can detect incipient failures before they impact production.
Advanced diagnostics represent a transformative approach to autoclave reliability, incorporating real-time monitoring, machine learning algorithms for anomaly detection, and integrated sensor networks that provide comprehensive system health assessment. These technologies aim to transition from traditional threshold-based alarms to predictive analytics that can forecast potential failures days or weeks before they occur.
The convergence of Industrial Internet of Things (IIoT) capabilities with autoclave systems has created unprecedented opportunities for reliability improvement. By 2025, the market for smart autoclave systems is projected to grow at a CAGR of 12.3%, driven primarily by demand for reduced operational costs and improved process consistency. This technological trajectory aligns with broader Industry 4.0 initiatives focused on smart manufacturing and predictive maintenance.
Achieving the desired reliability improvements requires addressing several technical challenges, including sensor durability in harsh environments, data integration across heterogeneous systems, and development of accurate predictive models that can function with limited historical failure data. These challenges form the foundation for current research and development efforts in the field.
The modern autoclave represents a complex integration of mechanical engineering, materials science, and digital control systems. Recent advancements have focused on improving thermal uniformity, pressure stability, and cycle time optimization. However, despite these improvements, reliability issues continue to challenge operators and manufacturers, resulting in production delays, quality inconsistencies, and increased operational costs.
Current reliability metrics indicate that autoclave systems experience an average downtime of 8-12% annually, with approximately 60% of failures occurring due to inadequate diagnostics capabilities. Traditional maintenance approaches rely heavily on scheduled interventions rather than condition-based strategies, leading to either premature component replacement or unexpected failures between maintenance intervals.
The primary reliability objectives for next-generation autoclave systems center on reducing unplanned downtime by at least 50%, extending mean time between failures (MTBF) by 30%, and decreasing maintenance costs by 25%. These objectives necessitate a fundamental shift from reactive to predictive maintenance paradigms, enabled by advanced diagnostic technologies that can detect incipient failures before they impact production.
Advanced diagnostics represent a transformative approach to autoclave reliability, incorporating real-time monitoring, machine learning algorithms for anomaly detection, and integrated sensor networks that provide comprehensive system health assessment. These technologies aim to transition from traditional threshold-based alarms to predictive analytics that can forecast potential failures days or weeks before they occur.
The convergence of Industrial Internet of Things (IIoT) capabilities with autoclave systems has created unprecedented opportunities for reliability improvement. By 2025, the market for smart autoclave systems is projected to grow at a CAGR of 12.3%, driven primarily by demand for reduced operational costs and improved process consistency. This technological trajectory aligns with broader Industry 4.0 initiatives focused on smart manufacturing and predictive maintenance.
Achieving the desired reliability improvements requires addressing several technical challenges, including sensor durability in harsh environments, data integration across heterogeneous systems, and development of accurate predictive models that can function with limited historical failure data. These challenges form the foundation for current research and development efforts in the field.
Market Demand for High-Reliability Sterilization Systems
The global market for high-reliability sterilization systems has experienced significant growth in recent years, driven primarily by increasing healthcare standards, stringent regulatory requirements, and growing awareness of infection control. The autoclave market, valued at approximately $2.5 billion in 2022, is projected to reach $3.8 billion by 2028, representing a compound annual growth rate of 7.2%. This growth trajectory underscores the critical importance of reliable sterilization equipment across various sectors.
Healthcare facilities, including hospitals, clinics, and ambulatory surgical centers, constitute the largest market segment, accounting for nearly 60% of the total demand. These institutions require sterilization systems that not only meet regulatory standards but also offer consistent performance with minimal downtime. A recent survey of 500 healthcare facilities revealed that equipment reliability ranks as the top priority when purchasing new autoclave systems, surpassing even initial acquisition costs.
The pharmaceutical and biotechnology industries represent another significant market segment, where product contamination can lead to substantial financial losses and regulatory penalties. These industries demand sterilization systems with advanced diagnostic capabilities that can provide real-time monitoring and validation of sterilization processes. The market for such advanced systems is growing at an accelerated rate of 9.5% annually, outpacing the overall market growth.
Geographically, North America and Europe currently dominate the market for high-reliability sterilization systems, collectively accounting for approximately 65% of global demand. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and India leading the expansion due to increasing healthcare infrastructure development and rising adoption of international sterilization standards.
Customer requirements are evolving beyond basic functionality to include advanced features such as predictive maintenance capabilities, remote monitoring, and comprehensive data logging for regulatory compliance. A market analysis indicates that 78% of potential buyers are willing to pay a premium of 15-20% for autoclave systems equipped with advanced diagnostics that can predict potential failures before they occur, thereby minimizing operational disruptions.
The COVID-19 pandemic has further accelerated market demand for reliable sterilization systems, with heightened awareness of infection control protocols across all industries. This has created a significant opportunity for manufacturers offering innovative solutions that combine reliability with advanced diagnostic capabilities. Industry experts project that this trend will continue post-pandemic, as enhanced sterilization practices become the new standard across multiple sectors.
Healthcare facilities, including hospitals, clinics, and ambulatory surgical centers, constitute the largest market segment, accounting for nearly 60% of the total demand. These institutions require sterilization systems that not only meet regulatory standards but also offer consistent performance with minimal downtime. A recent survey of 500 healthcare facilities revealed that equipment reliability ranks as the top priority when purchasing new autoclave systems, surpassing even initial acquisition costs.
The pharmaceutical and biotechnology industries represent another significant market segment, where product contamination can lead to substantial financial losses and regulatory penalties. These industries demand sterilization systems with advanced diagnostic capabilities that can provide real-time monitoring and validation of sterilization processes. The market for such advanced systems is growing at an accelerated rate of 9.5% annually, outpacing the overall market growth.
Geographically, North America and Europe currently dominate the market for high-reliability sterilization systems, collectively accounting for approximately 65% of global demand. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and India leading the expansion due to increasing healthcare infrastructure development and rising adoption of international sterilization standards.
Customer requirements are evolving beyond basic functionality to include advanced features such as predictive maintenance capabilities, remote monitoring, and comprehensive data logging for regulatory compliance. A market analysis indicates that 78% of potential buyers are willing to pay a premium of 15-20% for autoclave systems equipped with advanced diagnostics that can predict potential failures before they occur, thereby minimizing operational disruptions.
The COVID-19 pandemic has further accelerated market demand for reliable sterilization systems, with heightened awareness of infection control protocols across all industries. This has created a significant opportunity for manufacturers offering innovative solutions that combine reliability with advanced diagnostic capabilities. Industry experts project that this trend will continue post-pandemic, as enhanced sterilization practices become the new standard across multiple sectors.
Current Challenges in Autoclave Diagnostic Technologies
Despite significant advancements in autoclave technology, current diagnostic systems face substantial limitations that impede optimal reliability and performance. Traditional monitoring approaches rely heavily on basic parameters such as temperature, pressure, and time, which provide insufficient insight into the complex thermodynamic processes occurring within these critical sterilization vessels. This limited visibility creates significant blind spots in operational awareness, particularly during critical phases of the sterilization cycle.
A primary challenge lies in the inadequate sensor technology deployed in most autoclave systems. Conventional sensors often suffer from calibration drift under extreme temperature and pressure conditions, leading to inaccurate readings and potentially compromised sterilization efficacy. Furthermore, these sensors typically provide only point measurements rather than comprehensive spatial distribution data, failing to detect temperature or pressure gradients that may result in uneven sterilization.
Data integration represents another significant hurdle. Current systems frequently operate in isolation, with minimal integration between process parameters, maintenance records, and historical performance data. This siloed approach prevents the development of holistic diagnostic capabilities that could identify subtle patterns indicative of impending failures or performance degradation. The absence of standardized data formats and communication protocols further complicates efforts to implement advanced analytics across different autoclave models and manufacturers.
Real-time monitoring capabilities remain severely constrained in most existing systems. The lag between data acquisition, processing, and actionable insights creates vulnerability windows where developing issues may escalate before detection. This limitation is particularly problematic in critical applications such as pharmaceutical manufacturing or medical device sterilization, where process deviations can have significant consequences for product quality and patient safety.
Predictive maintenance capabilities are notably underdeveloped in current autoclave diagnostic technologies. Most systems operate on reactive or scheduled maintenance protocols rather than condition-based approaches. The inability to accurately forecast component degradation or system failures results in unnecessary downtime, increased maintenance costs, and potential production losses. This deficiency stems partly from insufficient historical failure data and limited application of machine learning algorithms to autoclave diagnostics.
User interface and data visualization tools present additional challenges. Many existing systems provide complex technical readouts that require specialized expertise to interpret effectively. This communication barrier between the diagnostic system and operators can lead to missed warning signs or delayed responses to developing issues, ultimately compromising autoclave reliability and operational efficiency.
A primary challenge lies in the inadequate sensor technology deployed in most autoclave systems. Conventional sensors often suffer from calibration drift under extreme temperature and pressure conditions, leading to inaccurate readings and potentially compromised sterilization efficacy. Furthermore, these sensors typically provide only point measurements rather than comprehensive spatial distribution data, failing to detect temperature or pressure gradients that may result in uneven sterilization.
Data integration represents another significant hurdle. Current systems frequently operate in isolation, with minimal integration between process parameters, maintenance records, and historical performance data. This siloed approach prevents the development of holistic diagnostic capabilities that could identify subtle patterns indicative of impending failures or performance degradation. The absence of standardized data formats and communication protocols further complicates efforts to implement advanced analytics across different autoclave models and manufacturers.
Real-time monitoring capabilities remain severely constrained in most existing systems. The lag between data acquisition, processing, and actionable insights creates vulnerability windows where developing issues may escalate before detection. This limitation is particularly problematic in critical applications such as pharmaceutical manufacturing or medical device sterilization, where process deviations can have significant consequences for product quality and patient safety.
Predictive maintenance capabilities are notably underdeveloped in current autoclave diagnostic technologies. Most systems operate on reactive or scheduled maintenance protocols rather than condition-based approaches. The inability to accurately forecast component degradation or system failures results in unnecessary downtime, increased maintenance costs, and potential production losses. This deficiency stems partly from insufficient historical failure data and limited application of machine learning algorithms to autoclave diagnostics.
User interface and data visualization tools present additional challenges. Many existing systems provide complex technical readouts that require specialized expertise to interpret effectively. This communication barrier between the diagnostic system and operators can lead to missed warning signs or delayed responses to developing issues, ultimately compromising autoclave reliability and operational efficiency.
Current Diagnostic Solutions for Autoclave Reliability
01 Monitoring and control systems for autoclave reliability
Advanced monitoring and control systems are essential for ensuring autoclave reliability. These systems include sensors for tracking critical parameters such as temperature, pressure, and cycle time. Real-time monitoring allows for immediate detection of deviations from optimal operating conditions, while automated control systems can make adjustments to maintain sterilization efficacy. Integration with data logging capabilities enables comprehensive documentation of each sterilization cycle for validation purposes.- Monitoring and control systems for autoclave reliability: Advanced monitoring and control systems are essential for ensuring autoclave reliability. These systems include sensors for tracking critical parameters such as temperature, pressure, and cycle time. Real-time monitoring allows for immediate detection of deviations from optimal operating conditions, while automated control systems can make adjustments to maintain sterilization efficacy. Integration with data logging capabilities enables comprehensive documentation of each sterilization cycle for validation purposes.
- Structural design improvements for enhanced reliability: Structural design innovations significantly improve autoclave reliability. These include reinforced pressure vessels, improved door sealing mechanisms, and optimized chamber designs that ensure uniform heat distribution. Advanced materials resistant to high temperatures and pressures extend operational lifespan while reducing maintenance requirements. Ergonomic designs facilitate easier cleaning and inspection, which contributes to consistent performance and reduced failure rates over time.
- Validation and testing protocols for reliability assurance: Comprehensive validation and testing protocols are crucial for verifying autoclave reliability. These include biological indicators, chemical indicators, and physical parameter monitoring to confirm sterilization efficacy. Regular performance qualification tests ensure consistent operation under various load conditions. Standardized testing procedures help identify potential issues before they affect sterilization outcomes, while documentation systems provide traceability for regulatory compliance and quality assurance purposes.
- Maintenance strategies to optimize autoclave performance: Proactive maintenance strategies significantly enhance autoclave reliability. These include scheduled preventive maintenance routines, condition-based monitoring to predict component failures, and systematic replacement of wear parts. Proper calibration of sensors and control systems ensures accurate operation, while regular cleaning protocols prevent contamination issues. Training programs for operators on proper maintenance procedures help extend equipment life and maintain consistent sterilization efficacy.
- Energy efficiency and sustainability features for reliable operation: Energy-efficient designs contribute to autoclave reliability while reducing operational costs. These include improved insulation materials, heat recovery systems, and optimized steam generation processes. Water recycling mechanisms minimize resource consumption and reduce strain on components. Smart power management systems prevent electrical issues that could compromise reliability, while eco-friendly materials and designs extend equipment lifespan and reduce environmental impact without sacrificing sterilization performance.
02 Structural design improvements for enhanced reliability
Structural design innovations significantly impact autoclave reliability. These include reinforced pressure vessels, improved door sealing mechanisms, and optimized chamber designs that ensure uniform heat distribution. Advanced materials that resist corrosion and withstand repeated sterilization cycles contribute to longer service life. Ergonomic considerations in design also improve operational reliability by reducing user error during loading, operation, and maintenance procedures.Expand Specific Solutions03 Maintenance protocols and predictive maintenance systems
Comprehensive maintenance protocols are crucial for sustained autoclave reliability. These include regular inspection schedules, preventive maintenance procedures, and calibration of critical components. Predictive maintenance systems utilize sensors and data analytics to identify potential failures before they occur, reducing downtime and extending equipment lifespan. Documentation of maintenance activities ensures compliance with regulatory requirements and facilitates troubleshooting when issues arise.Expand Specific Solutions04 Validation and testing methodologies
Rigorous validation and testing methodologies ensure autoclave reliability across various operating conditions. These include biological indicators, chemical indicators, and physical parameter monitoring to verify sterilization efficacy. Qualification procedures such as installation qualification, operational qualification, and performance qualification establish baseline performance metrics. Regular revalidation protocols confirm continued reliability throughout the autoclave's operational life, particularly after repairs or modifications.Expand Specific Solutions05 Energy efficiency and sustainability features
Energy-efficient design features contribute to autoclave reliability while reducing operational costs. These include improved insulation materials, heat recovery systems, and optimized steam generation processes. Water recycling systems minimize resource consumption during operation. Smart power management features ensure consistent performance while reducing energy usage during idle periods. These sustainability features not only improve environmental impact but also enhance long-term operational reliability by reducing strain on components.Expand Specific Solutions
Leading Manufacturers and Technology Providers
The autoclave reliability improvement market is currently in a growth phase, with increasing adoption of advanced diagnostics technologies across industries. The market is expanding due to rising demand for enhanced safety, efficiency, and predictive maintenance capabilities in critical sterilization processes. Major players include established industrial equipment manufacturers like General Electric Company, Hitachi High-Tech America, and Olympus Corp., who leverage their extensive expertise in diagnostic technologies. Emerging competitors such as Q Med Innovations and Steriflow SAS are introducing specialized solutions with IoT integration and real-time monitoring capabilities. The technology is approaching maturity in traditional applications but continues to evolve with AI-driven predictive analytics, wireless monitoring, and integration with Industry 4.0 frameworks, creating opportunities for innovation and market differentiation.
Hitachi High-Tech America, Inc.
Technical Solution: Hitachi High-Tech has developed a comprehensive autoclave diagnostic system that combines advanced sensing technology with data analytics for improved reliability. Their solution features high-precision pressure and temperature sensors distributed throughout the autoclave chamber to detect subtle variations that might indicate developing issues. The system employs Hitachi's proprietary AI algorithms to analyze operational patterns and identify anomalies before they lead to failures. A distinguishing feature is their non-destructive testing technology that uses ultrasonic waves to monitor the structural integrity of pressure vessels during operation[2]. The system also incorporates real-time monitoring of steam quality and distribution, which is critical for sterilization effectiveness. Hitachi's solution integrates with their broader industrial IoT platform, allowing for remote monitoring and centralized management of multiple autoclaves across different facilities.
Strengths: Exceptional precision in detecting early-stage anomalies; comprehensive integration with facility management systems; strong focus on steam quality monitoring which directly impacts sterilization efficacy. Weaknesses: Requires specialized technical expertise for installation and calibration; higher initial investment compared to conventional monitoring systems; may have compatibility challenges with older autoclave models.
Steriflow SAS
Technical Solution: Steriflow SAS has pioneered an innovative diagnostic system specifically designed for industrial autoclaves used in food processing and pharmaceutical manufacturing. Their technology focuses on ensuring process validation and product quality while improving equipment reliability. The system features a network of high-precision PT100 temperature probes and pressure transducers strategically positioned throughout the autoclave chamber to create detailed thermal mapping. Steriflow's proprietary algorithms continuously analyze the relationship between temperature, pressure, and time to detect deviations from optimal sterilization parameters[3]. Their solution includes advanced steam quality monitoring that measures dryness fraction and non-condensable gas content, critical factors affecting sterilization efficacy. The system also monitors mechanical components such as door sealing mechanisms and steam traps using vibration analysis and thermal imaging. Steriflow's diagnostic platform provides comprehensive reporting capabilities that comply with FDA and EU regulatory requirements for process validation.
Strengths: Specialized focus on sterilization process validation; excellent regulatory compliance features; particularly effective for food and pharmaceutical applications. Weaknesses: Less adaptable to non-sterilization autoclave applications; requires regular calibration of the extensive sensor network; higher operational complexity compared to simpler monitoring systems.
Key Innovations in Predictive Maintenance Technologies
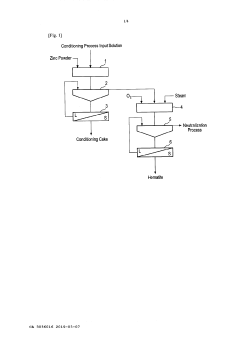
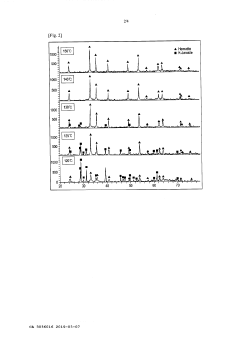
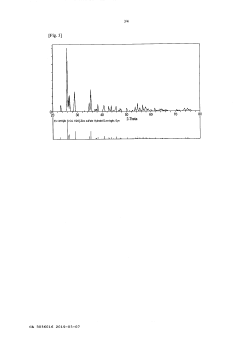
Method of recovering iron from zinc sulphate solution
PatentActiveCA3036016A1
Innovation
- A method involving a conditioning process to reduce the zinc sulfate solution, followed by an iron precipitation process at lower temperatures (135-150°C) and pressures (5-10 barg), using a reducing agent like zinc powder to adjust oxidation-reduction potential, and oxidizing the solution in an autoclave to produce hematite with high iron content.
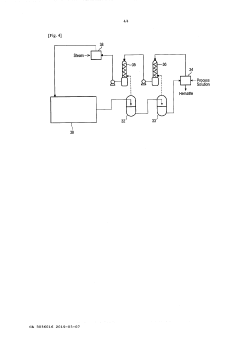
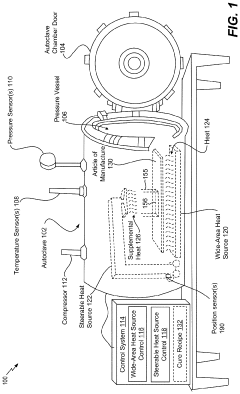
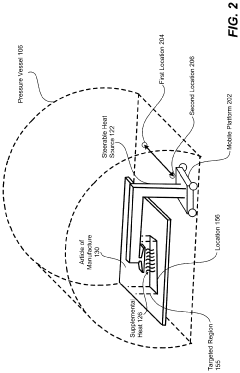
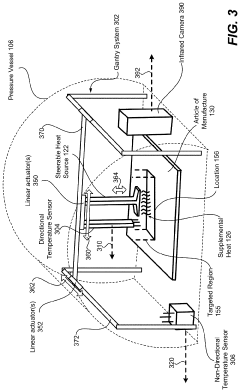
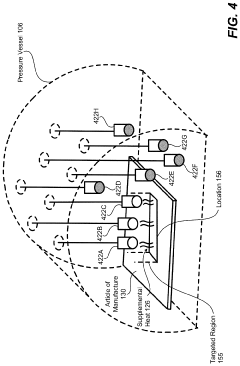
Steerable heat source
PatentActiveUS20200307035A1
Innovation
- A steerable heat source is integrated within the autoclave, coupled with a control system that directs supplemental heat to targeted regions using temperature sensors and a compressor to regulate pressure, ensuring precise temperature control and uniform heating.
Regulatory Standards for Medical Sterilization Equipment
Medical sterilization equipment, particularly autoclaves, must adhere to stringent regulatory standards to ensure patient safety and effective infection control. The FDA in the United States requires compliance with 21 CFR Part 820 (Quality System Regulation) for medical device manufacturers, which includes sterilization equipment. Additionally, the European Union's Medical Device Regulation (MDR) establishes comprehensive requirements for medical devices, including those used for sterilization processes.
ISO 17665-1:2006 specifically addresses moist heat sterilization in healthcare facilities, providing guidelines for the development, validation, and routine control of sterilization processes. This standard is particularly relevant for autoclave operations and maintenance protocols. Similarly, ISO 11135:2014 covers ethylene oxide sterilization requirements, while ISO 11137 series addresses radiation sterilization standards.
The Joint Commission (TJC) in the U.S. requires healthcare facilities to follow specific sterilization protocols and maintain detailed documentation of all sterilization cycles. These requirements include regular validation testing, preventive maintenance, and staff training on proper equipment operation.
Advanced diagnostic systems for autoclaves must be designed to facilitate compliance with these regulatory standards. For instance, ANSI/AAMI ST79 provides comprehensive guidelines for steam sterilization and sterility assurance in healthcare facilities, emphasizing the importance of monitoring systems and documentation requirements.
Regulatory bodies increasingly emphasize the need for real-time monitoring and automated documentation systems. The FDA's guidance on process validation highlights the importance of continuous monitoring rather than just end-point testing. This trend supports the integration of advanced diagnostics in autoclave systems to provide continuous cycle data and automated compliance reporting.
International harmonization efforts are underway to standardize sterilization requirements globally. The International Medical Device Regulators Forum (IMDRF) works to align regulatory approaches across different regions, which may influence future standards for autoclave diagnostics and monitoring systems.
Emerging regulations are beginning to address cybersecurity concerns for networked medical equipment, including modern autoclaves with advanced diagnostic capabilities. The FDA's guidance on cybersecurity for medical devices outlines expectations for securing connected medical equipment throughout its lifecycle, which has implications for the design of diagnostic systems that transmit and store sterilization data.
ISO 17665-1:2006 specifically addresses moist heat sterilization in healthcare facilities, providing guidelines for the development, validation, and routine control of sterilization processes. This standard is particularly relevant for autoclave operations and maintenance protocols. Similarly, ISO 11135:2014 covers ethylene oxide sterilization requirements, while ISO 11137 series addresses radiation sterilization standards.
The Joint Commission (TJC) in the U.S. requires healthcare facilities to follow specific sterilization protocols and maintain detailed documentation of all sterilization cycles. These requirements include regular validation testing, preventive maintenance, and staff training on proper equipment operation.
Advanced diagnostic systems for autoclaves must be designed to facilitate compliance with these regulatory standards. For instance, ANSI/AAMI ST79 provides comprehensive guidelines for steam sterilization and sterility assurance in healthcare facilities, emphasizing the importance of monitoring systems and documentation requirements.
Regulatory bodies increasingly emphasize the need for real-time monitoring and automated documentation systems. The FDA's guidance on process validation highlights the importance of continuous monitoring rather than just end-point testing. This trend supports the integration of advanced diagnostics in autoclave systems to provide continuous cycle data and automated compliance reporting.
International harmonization efforts are underway to standardize sterilization requirements globally. The International Medical Device Regulators Forum (IMDRF) works to align regulatory approaches across different regions, which may influence future standards for autoclave diagnostics and monitoring systems.
Emerging regulations are beginning to address cybersecurity concerns for networked medical equipment, including modern autoclaves with advanced diagnostic capabilities. The FDA's guidance on cybersecurity for medical devices outlines expectations for securing connected medical equipment throughout its lifecycle, which has implications for the design of diagnostic systems that transmit and store sterilization data.
Energy Efficiency Considerations in Advanced Autoclaves
Energy efficiency has become a critical consideration in modern autoclave operations, particularly as industries face increasing pressure to reduce carbon footprints and operational costs. Advanced autoclaves equipped with diagnostic capabilities offer significant opportunities for energy optimization beyond their primary reliability benefits. The integration of real-time monitoring systems enables precise control of heating cycles, allowing for dynamic adjustments that minimize energy waste while maintaining process integrity.
Thermal efficiency improvements represent one of the most substantial energy-saving opportunities in autoclave operations. Advanced diagnostic systems can monitor heat distribution patterns and identify thermal losses, enabling targeted insulation upgrades and process modifications. Studies indicate that diagnostic-informed thermal optimization can reduce energy consumption by 15-22% compared to conventional autoclave operations, with particularly significant savings in large industrial applications.
Pressure management systems enhanced by diagnostic capabilities contribute substantially to energy conservation efforts. By maintaining optimal pressure profiles throughout the sterilization or curing cycle, these systems prevent unnecessary energy expenditure from pressure fluctuations or excessive pressurization. Advanced pressure sensors coupled with predictive algorithms can anticipate system needs, reducing the energy required for pressurization by an estimated 8-12%.
Steam and water recycling systems represent another frontier in autoclave energy efficiency. Diagnostic-enabled autoclaves can implement intelligent water recovery protocols, capturing and reusing condensate while monitoring water quality parameters in real-time. This approach not only reduces water consumption by up to 40% but also decreases the energy required for heating new water inputs, creating a compound efficiency benefit.
Load optimization strategies facilitated by advanced diagnostics further enhance energy efficiency. By analyzing historical performance data and current operational parameters, these systems can recommend optimal loading patterns and cycle configurations. This data-driven approach prevents underutilization of autoclave capacity and reduces the energy-per-unit processed, with efficiency gains of 10-18% reported in manufacturing environments.
The economic implications of these energy efficiency improvements are substantial. Return-on-investment analyses indicate that the implementation of advanced diagnostic systems for energy efficiency purposes typically achieves payback periods of 14-36 months, depending on facility size and operational intensity. These financial benefits complement the sustainability advantages, creating a compelling business case for technology adoption across various industries utilizing autoclave processes.
Thermal efficiency improvements represent one of the most substantial energy-saving opportunities in autoclave operations. Advanced diagnostic systems can monitor heat distribution patterns and identify thermal losses, enabling targeted insulation upgrades and process modifications. Studies indicate that diagnostic-informed thermal optimization can reduce energy consumption by 15-22% compared to conventional autoclave operations, with particularly significant savings in large industrial applications.
Pressure management systems enhanced by diagnostic capabilities contribute substantially to energy conservation efforts. By maintaining optimal pressure profiles throughout the sterilization or curing cycle, these systems prevent unnecessary energy expenditure from pressure fluctuations or excessive pressurization. Advanced pressure sensors coupled with predictive algorithms can anticipate system needs, reducing the energy required for pressurization by an estimated 8-12%.
Steam and water recycling systems represent another frontier in autoclave energy efficiency. Diagnostic-enabled autoclaves can implement intelligent water recovery protocols, capturing and reusing condensate while monitoring water quality parameters in real-time. This approach not only reduces water consumption by up to 40% but also decreases the energy required for heating new water inputs, creating a compound efficiency benefit.
Load optimization strategies facilitated by advanced diagnostics further enhance energy efficiency. By analyzing historical performance data and current operational parameters, these systems can recommend optimal loading patterns and cycle configurations. This data-driven approach prevents underutilization of autoclave capacity and reduces the energy-per-unit processed, with efficiency gains of 10-18% reported in manufacturing environments.
The economic implications of these energy efficiency improvements are substantial. Return-on-investment analyses indicate that the implementation of advanced diagnostic systems for energy efficiency purposes typically achieves payback periods of 14-36 months, depending on facility size and operational intensity. These financial benefits complement the sustainability advantages, creating a compelling business case for technology adoption across various industries utilizing autoclave processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!