Autoclave Cycle Adjustments: Improving Sterilization Efficacy
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Background and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This method has become a cornerstone of infection control in healthcare settings, laboratories, and various industries requiring sterile equipment and materials. The fundamental principle—utilizing pressurized steam to eliminate microorganisms—remains unchanged, though technological advancements have dramatically improved efficiency, reliability, and process control.
The evolution of autoclave technology has progressed through several distinct phases: from basic pressure cooker-like devices to sophisticated computer-controlled systems with precise parameter monitoring capabilities. Modern autoclaves incorporate advanced sensors, programmable cycles, and validation systems that ensure sterilization efficacy while optimizing resource utilization. This technological progression reflects the growing understanding of sterilization science and increasing regulatory requirements for process validation.
Current autoclave sterilization faces several challenges, including the need to effectively sterilize increasingly complex medical devices with narrow lumens, hinges, or porous materials that may inhibit steam penetration. Additionally, there is growing pressure to reduce cycle times and resource consumption while maintaining or improving sterilization efficacy. The emergence of heat-sensitive materials in modern medical devices further complicates the sterilization landscape.
The primary objective of autoclave cycle adjustment research is to enhance sterilization efficacy while addressing these contemporary challenges. Specifically, this involves optimizing cycle parameters—including temperature, pressure, exposure time, and drying phases—to ensure complete microbial inactivation across diverse load configurations while minimizing cycle duration and utility consumption. This optimization must accommodate various sterilization challenges presented by modern medical devices and laboratory equipment.
Secondary objectives include developing more sophisticated monitoring and validation protocols to ensure consistent sterilization outcomes, reducing environmental impact through more efficient resource utilization, and extending equipment lifespan through optimized operational parameters. There is also significant interest in developing adaptive cycle technologies that can automatically adjust parameters based on load characteristics and real-time process feedback.
The technological trajectory suggests movement toward more intelligent, adaptive sterilization systems that can optimize cycles based on specific load requirements while maintaining compliance with increasingly stringent regulatory standards. This evolution aligns with broader healthcare trends toward process optimization, resource efficiency, and enhanced patient safety through improved infection control protocols.
The evolution of autoclave technology has progressed through several distinct phases: from basic pressure cooker-like devices to sophisticated computer-controlled systems with precise parameter monitoring capabilities. Modern autoclaves incorporate advanced sensors, programmable cycles, and validation systems that ensure sterilization efficacy while optimizing resource utilization. This technological progression reflects the growing understanding of sterilization science and increasing regulatory requirements for process validation.
Current autoclave sterilization faces several challenges, including the need to effectively sterilize increasingly complex medical devices with narrow lumens, hinges, or porous materials that may inhibit steam penetration. Additionally, there is growing pressure to reduce cycle times and resource consumption while maintaining or improving sterilization efficacy. The emergence of heat-sensitive materials in modern medical devices further complicates the sterilization landscape.
The primary objective of autoclave cycle adjustment research is to enhance sterilization efficacy while addressing these contemporary challenges. Specifically, this involves optimizing cycle parameters—including temperature, pressure, exposure time, and drying phases—to ensure complete microbial inactivation across diverse load configurations while minimizing cycle duration and utility consumption. This optimization must accommodate various sterilization challenges presented by modern medical devices and laboratory equipment.
Secondary objectives include developing more sophisticated monitoring and validation protocols to ensure consistent sterilization outcomes, reducing environmental impact through more efficient resource utilization, and extending equipment lifespan through optimized operational parameters. There is also significant interest in developing adaptive cycle technologies that can automatically adjust parameters based on load characteristics and real-time process feedback.
The technological trajectory suggests movement toward more intelligent, adaptive sterilization systems that can optimize cycles based on specific load requirements while maintaining compliance with increasingly stringent regulatory standards. This evolution aligns with broader healthcare trends toward process optimization, resource efficiency, and enhanced patient safety through improved infection control protocols.
Market Demand Analysis for Advanced Sterilization Solutions
The global sterilization market is experiencing significant growth, driven by increasing healthcare-associated infections, rising surgical procedures, and growing awareness of infection control protocols. The market for advanced sterilization solutions was valued at approximately $6.7 billion in 2021 and is projected to reach $9.8 billion by 2027, representing a compound annual growth rate of 6.5%. This growth trajectory underscores the substantial demand for more effective and efficient sterilization technologies.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, constitute the largest segment of end-users seeking advanced sterilization solutions. These institutions are increasingly prioritizing patient safety and regulatory compliance, creating a robust demand for sterilization technologies that can deliver consistent and validated results. The COVID-19 pandemic has further accelerated this trend, heightening awareness about sterilization protocols and creating unprecedented demand for reliable sterilization equipment.
Pharmaceutical and biotechnology companies represent another significant market segment, requiring stringent sterilization processes for manufacturing environments, research laboratories, and product development facilities. The expanding pharmaceutical industry, particularly in emerging economies, is creating substantial opportunities for advanced sterilization solution providers.
Market research indicates that autoclave sterilization remains the most widely adopted method globally, accounting for approximately 40% of the sterilization equipment market. However, end-users are increasingly demanding innovations that address the limitations of traditional autoclave cycles, including energy efficiency, cycle time reduction, and improved efficacy against resistant microorganisms.
Regional analysis reveals that North America currently dominates the market with a 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by healthcare infrastructure development, increasing surgical procedures, and growing awareness about infection control in countries like China and India.
Customer feedback and market surveys highlight several unmet needs in current sterilization solutions. These include demands for reduced cycle times without compromising efficacy, enhanced energy efficiency, improved validation methods, and better integration with tracking systems. Additionally, there is growing interest in sterilization solutions that can effectively handle complex medical devices with narrow lumens or sensitive electronic components.
The market is also witnessing a shift toward more environmentally sustainable sterilization methods, with healthcare facilities increasingly considering the environmental impact of their sterilization processes. This trend is creating opportunities for innovations in autoclave cycle adjustments that can reduce water consumption and energy usage while maintaining or improving sterilization efficacy.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, constitute the largest segment of end-users seeking advanced sterilization solutions. These institutions are increasingly prioritizing patient safety and regulatory compliance, creating a robust demand for sterilization technologies that can deliver consistent and validated results. The COVID-19 pandemic has further accelerated this trend, heightening awareness about sterilization protocols and creating unprecedented demand for reliable sterilization equipment.
Pharmaceutical and biotechnology companies represent another significant market segment, requiring stringent sterilization processes for manufacturing environments, research laboratories, and product development facilities. The expanding pharmaceutical industry, particularly in emerging economies, is creating substantial opportunities for advanced sterilization solution providers.
Market research indicates that autoclave sterilization remains the most widely adopted method globally, accounting for approximately 40% of the sterilization equipment market. However, end-users are increasingly demanding innovations that address the limitations of traditional autoclave cycles, including energy efficiency, cycle time reduction, and improved efficacy against resistant microorganisms.
Regional analysis reveals that North America currently dominates the market with a 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by healthcare infrastructure development, increasing surgical procedures, and growing awareness about infection control in countries like China and India.
Customer feedback and market surveys highlight several unmet needs in current sterilization solutions. These include demands for reduced cycle times without compromising efficacy, enhanced energy efficiency, improved validation methods, and better integration with tracking systems. Additionally, there is growing interest in sterilization solutions that can effectively handle complex medical devices with narrow lumens or sensitive electronic components.
The market is also witnessing a shift toward more environmentally sustainable sterilization methods, with healthcare facilities increasingly considering the environmental impact of their sterilization processes. This trend is creating opportunities for innovations in autoclave cycle adjustments that can reduce water consumption and energy usage while maintaining or improving sterilization efficacy.
Current Autoclave Technology Challenges and Limitations
Despite significant advancements in autoclave technology over recent decades, several persistent challenges and limitations continue to impact sterilization efficacy in both healthcare and industrial settings. Current autoclave systems face considerable difficulties in achieving uniform heat distribution throughout the sterilization chamber, particularly when processing dense loads or items with complex geometries. This non-uniform heat distribution creates potential "cold spots" where sterilization parameters may not be adequately met, compromising the overall efficacy of the process.
Temperature and pressure monitoring systems in many existing autoclaves lack the precision and spatial resolution necessary for comprehensive cycle validation. Most systems rely on single-point measurements that fail to capture temperature gradients across the chamber, leading to incomplete sterilization validation and potential safety risks. Additionally, the industry faces challenges with real-time monitoring capabilities that would enable dynamic cycle adjustments based on actual load conditions.
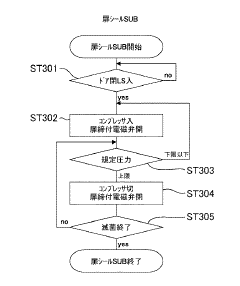
Air removal mechanisms represent another significant limitation in current autoclave technology. Residual air pockets within the chamber or within packaged items can insulate against steam penetration, creating areas where sterilization conditions are not achieved. Conventional pre-vacuum systems often prove insufficient for complex loads or densely packed materials, necessitating extended cycle times that reduce operational efficiency and potentially damage heat-sensitive items.
The relationship between cycle parameters (time, temperature, pressure) and sterilization efficacy remains incompletely characterized for many modern medical devices and materials. Standard cycles developed decades ago may not be optimal for new composite materials, complex electronic medical devices, or specialized surgical instruments. This knowledge gap leads to overly conservative cycle parameters that waste energy and time while potentially causing unnecessary material degradation.
Energy efficiency presents an ongoing challenge, with many autoclave systems consuming excessive water, steam, and electricity. The substantial resource requirements not only increase operational costs but also contribute to environmental impact concerns. Older autoclave models particularly suffer from poor insulation, inefficient steam generation, and wasteful water cooling systems that fail to recapture thermal energy.
Validation and documentation processes remain labor-intensive and prone to human error. Many facilities still rely on manual record-keeping and physical biological indicators rather than integrated electronic monitoring systems. This approach limits data analysis capabilities and makes it difficult to identify subtle cycle optimization opportunities or emerging equipment issues before they affect sterilization outcomes.
Compatibility with increasingly diverse and sensitive materials poses another significant limitation. As medical devices incorporate more electronics, adhesives, and composite materials with lower heat tolerance, standard autoclave cycles may cause damage while attempting to achieve sterilization parameters. This creates a challenging balance between ensuring sterility and preserving material integrity.
Temperature and pressure monitoring systems in many existing autoclaves lack the precision and spatial resolution necessary for comprehensive cycle validation. Most systems rely on single-point measurements that fail to capture temperature gradients across the chamber, leading to incomplete sterilization validation and potential safety risks. Additionally, the industry faces challenges with real-time monitoring capabilities that would enable dynamic cycle adjustments based on actual load conditions.
Air removal mechanisms represent another significant limitation in current autoclave technology. Residual air pockets within the chamber or within packaged items can insulate against steam penetration, creating areas where sterilization conditions are not achieved. Conventional pre-vacuum systems often prove insufficient for complex loads or densely packed materials, necessitating extended cycle times that reduce operational efficiency and potentially damage heat-sensitive items.
The relationship between cycle parameters (time, temperature, pressure) and sterilization efficacy remains incompletely characterized for many modern medical devices and materials. Standard cycles developed decades ago may not be optimal for new composite materials, complex electronic medical devices, or specialized surgical instruments. This knowledge gap leads to overly conservative cycle parameters that waste energy and time while potentially causing unnecessary material degradation.
Energy efficiency presents an ongoing challenge, with many autoclave systems consuming excessive water, steam, and electricity. The substantial resource requirements not only increase operational costs but also contribute to environmental impact concerns. Older autoclave models particularly suffer from poor insulation, inefficient steam generation, and wasteful water cooling systems that fail to recapture thermal energy.
Validation and documentation processes remain labor-intensive and prone to human error. Many facilities still rely on manual record-keeping and physical biological indicators rather than integrated electronic monitoring systems. This approach limits data analysis capabilities and makes it difficult to identify subtle cycle optimization opportunities or emerging equipment issues before they affect sterilization outcomes.
Compatibility with increasingly diverse and sensitive materials poses another significant limitation. As medical devices incorporate more electronics, adhesives, and composite materials with lower heat tolerance, standard autoclave cycles may cause damage while attempting to achieve sterilization parameters. This creates a challenging balance between ensuring sterility and preserving material integrity.
Current Cycle Adjustment Methodologies and Parameters
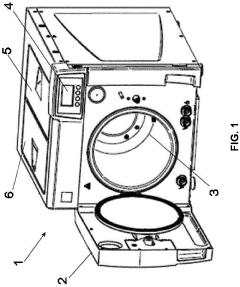
01 Sterilization parameters and validation methods
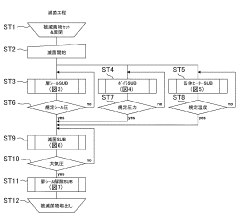
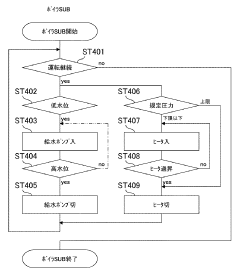
Autoclave sterilization efficacy depends on critical parameters such as temperature, pressure, and exposure time. Validation methods are essential to ensure these parameters are maintained throughout the sterilization cycle. Various monitoring systems and indicators can be used to verify that sterilization conditions have been achieved, including biological indicators, chemical indicators, and electronic monitoring systems that track and record cycle data for quality assurance purposes.- Sterilization parameters and process optimization: Autoclave sterilization efficacy depends on critical parameters such as temperature, pressure, and exposure time. Optimizing these parameters ensures complete sterilization while minimizing damage to materials. Advanced monitoring systems can track these parameters in real-time, allowing for process validation and adjustment. The relationship between these parameters affects sterilization efficacy for different types of microorganisms and materials.
- Biological indicators for sterilization validation: Biological indicators containing resistant microorganisms are used to validate autoclave sterilization processes. These indicators provide direct evidence of sterilization efficacy by demonstrating the destruction of highly resistant test organisms. Various types of biological indicators have been developed, including self-contained systems that offer rapid readout capabilities, allowing for quicker verification of sterilization cycles.
- Steam penetration and distribution techniques: Effective steam penetration and distribution within the autoclave chamber is crucial for sterilization efficacy. Various techniques have been developed to enhance steam penetration into complex medical devices and porous loads. These include pulsed vacuum systems, optimized chamber designs, and specialized loading configurations that ensure steam reaches all surfaces requiring sterilization.
- Material compatibility and load configuration: Different materials respond differently to autoclave conditions, affecting both sterilization efficacy and material integrity. Specialized cycles have been developed for heat-sensitive materials, dense loads, and complex medical devices. Proper load configuration ensures effective steam penetration while preventing the formation of air pockets that could compromise sterilization. Packaging materials must allow steam penetration while maintaining sterility after processing.
- Innovative autoclave technologies and designs: Recent innovations in autoclave technology have improved sterilization efficacy while reducing cycle times and energy consumption. These include advanced control systems, improved chamber designs, and novel heating methods. Some autoclaves incorporate rapid cooling mechanisms to reduce processing time, while others feature specialized cycles for specific applications. Smart autoclaves with connectivity features allow for remote monitoring and documentation of sterilization processes.
02 Biological indicators for efficacy verification
Biological indicators containing resistant microorganisms are used to verify autoclave sterilization efficacy. These indicators typically contain bacterial spores that are highly resistant to heat, such as Geobacillus stearothermophilus. After sterilization, these indicators are incubated to determine if the spores were successfully inactivated, providing a reliable method to confirm that sterilization conditions were sufficient to kill even the most resistant microorganisms.Expand Specific Solutions03 Steam penetration and load configuration
The efficacy of autoclave sterilization is significantly affected by steam penetration and load configuration. Proper arrangement of items within the autoclave chamber ensures that steam can reach all surfaces. Factors such as packaging materials, density of the load, and spacing between items influence steam penetration. Improper loading can create air pockets or cold spots where sterilization may be incomplete, compromising the overall efficacy of the process.Expand Specific Solutions04 Equipment design and maintenance
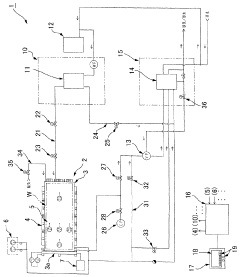
The design and maintenance of autoclave equipment significantly impact sterilization efficacy. Modern autoclaves incorporate features such as pre-vacuum cycles to remove air, steam quality monitoring, and uniform heat distribution systems. Regular maintenance, calibration, and testing of autoclave components ensure consistent performance. Proper door seals, steam generators, and control systems are critical for maintaining the required sterilization conditions throughout the entire cycle.Expand Specific Solutions05 Special applications and challenging materials
Certain materials and devices present unique challenges for autoclave sterilization efficacy. Heat-sensitive items, complex medical devices with lumens, porous materials, and dense loads may require modified sterilization protocols. Extended cycle times, specialized packaging, or positioning techniques may be necessary to ensure complete sterilization. For particularly challenging items, alternative sterilization methods might be considered in conjunction with autoclave processing to achieve optimal results.Expand Specific Solutions
Key Manufacturers and Competitors in Sterilization Equipment
The autoclave sterilization market is currently in a mature growth phase, characterized by steady technological advancements focused on improving efficacy and efficiency. The global market size is estimated at approximately $2.5 billion, with projected annual growth of 6-8% driven by increasing healthcare infrastructure investments and stringent sterilization regulations. From a technological maturity perspective, established players like Tuttnauer, Shinva Medical, and Stryker Corp. lead with advanced cycle optimization technologies, while emerging competitors such as W&H Sterilization and LTE Scientific are introducing innovations in automation and monitoring systems. Regional players including Cefla SC and Truking Technology are expanding their presence through specialized applications for pharmaceutical and medical device sterilization. The competitive landscape shows a balance between traditional market leaders and specialized niche providers focusing on specific industry segments.
Shinva Medical Instrument Co., Ltd.
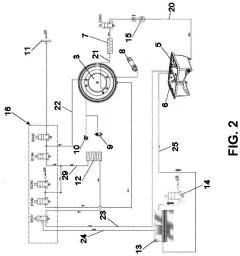
Technical Solution: Shinva Medical has pioneered the Intelligent Pressure Differential Control (IPDC) system for autoclave cycle optimization. This technology utilizes a network of distributed sensors to create a three-dimensional mapping of temperature and pressure conditions throughout the sterilization chamber. The IPDC system employs predictive modeling algorithms that anticipate sterilization needs based on load composition and density, automatically adjusting steam injection rates, pressure levels, and exposure times. Their proprietary SteamPulse technology creates controlled pressure oscillations during the sterilization phase, enhancing steam penetration into challenging areas such as lumens and porous materials. Shinva's system also incorporates real-time biological indicator simulation models that calculate theoretical kill rates throughout the cycle, allowing for dynamic adjustments to ensure sterilization efficacy while minimizing resource consumption. The company reports that this approach achieves consistent sterility assurance levels while reducing water consumption by up to 25% and energy usage by approximately 20% compared to conventional systems.
Strengths: Exceptional resource efficiency without compromising sterilization efficacy; sophisticated predictive modeling capabilities; excellent penetration for challenging loads; comprehensive data logging for validation. Weaknesses: Complex system requires specialized technical support; higher upfront costs; requires more extensive operator training compared to simpler systems.
ASP Global Manufacturing GmbH
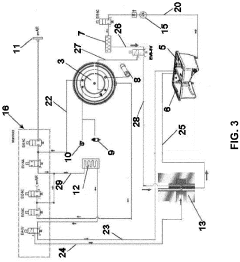
Technical Solution: ASP Global Manufacturing has developed the STERRAD NX Advanced Cycle Management System, which represents a significant innovation in low-temperature hydrogen peroxide gas plasma sterilization technology. Unlike traditional steam autoclaves, this system addresses the challenges of heat-sensitive instruments through precisely controlled hydrogen peroxide diffusion and plasma generation phases. The technology employs a multi-phase approach with dynamic concentration monitoring that continuously adjusts hydrogen peroxide injection rates based on chamber conditions and load characteristics. Their proprietary ALLClear technology incorporates impedance measurements to detect moisture levels in instruments, automatically extending drying phases when necessary to ensure complete sterilization. The system features adaptive algorithms that modify cycle parameters in real-time based on pressure response curves, optimizing gas diffusion into complex instrument geometries. Independent testing has shown this approach achieves consistent sterility assurance levels while reducing cycle times by up to 35% compared to first-generation hydrogen peroxide systems, with particular efficacy for instruments with long, narrow lumens.
Strengths: Excellent for heat-sensitive instruments; significantly reduced cycle times; effective sterilization of complex instrument geometries; minimal consumable usage. Weaknesses: Higher operational costs compared to steam systems; limited capacity per cycle; requires specialized ventilation systems; not suitable for all material types.
Critical Patents and Research in Sterilization Efficacy
Flow-type high-pressure steam sterilization method and flow-type sterilizer by soft hydrothermal process
PatentInactiveJPWO2017010525A1
Innovation
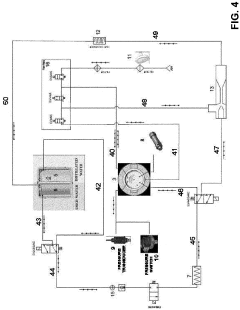
- A flow-type high-pressure steam sterilization method using a soft hydrothermal process that involves an air evacuation step, temperature and pressure increase, high-pressure steam sterilization with highly saturated steam circulation, and a controlled drying process to minimize condensation and shorten drying times.
autoclave
PatentActiveEP4190365A1
Innovation
- Replacing the vacuum pump with a Venturi ejector that utilizes the Venturi effect to generate suction for removing residual air and water steam from the sterilization chamber, either actuated by compressed air or steam generated within the autoclave, thereby eliminating the need for a vacuum pump and reducing costs.
Regulatory Compliance and Safety Standards
Regulatory compliance and safety standards form the cornerstone of autoclave sterilization processes across healthcare, pharmaceutical, and laboratory settings. The FDA in the United States, the European Medicines Agency (EMA) in Europe, and similar regulatory bodies worldwide have established comprehensive frameworks governing sterilization procedures. These regulations mandate specific validation protocols, documentation requirements, and performance standards that must be rigorously followed to ensure patient safety and product integrity.
ISO 17665 stands as the primary international standard specifically addressing moist heat sterilization processes, providing detailed guidelines for development, validation, and routine control. This standard emphasizes the critical importance of establishing and documenting the parameters that ensure consistent sterilization efficacy, including temperature, pressure, and exposure time across different load configurations.
Complementary standards such as ISO 11138 and ISO 11140 govern biological indicators and chemical indicators respectively, which are essential tools for verifying sterilization cycle effectiveness. These indicators provide visible confirmation that sterilization conditions have been achieved throughout the load, serving as critical quality control measures.
Recent regulatory trends have shifted toward more stringent requirements for cycle validation, with increased emphasis on parametric release methodologies that rely on precise measurement and control of physical parameters rather than post-sterilization testing alone. This approach demands more sophisticated monitoring systems and tighter process controls but offers enhanced assurance of sterilization efficacy.
Healthcare facilities must maintain detailed sterilization records for regulatory inspections and accreditation purposes. These records typically include cycle parameters, operator identification, load contents, biological indicator results, and maintenance documentation. The Joint Commission and similar accreditation bodies regularly evaluate compliance with these documentation requirements during facility inspections.
Risk management principles have become increasingly integrated into sterilization standards, requiring facilities to identify potential failure modes and implement appropriate mitigation strategies. This approach recognizes that sterilization efficacy depends not only on equipment performance but also on proper loading techniques, maintenance procedures, and operator training.
Emerging technologies for cycle monitoring and validation are being developed in response to evolving regulatory expectations. These include real-time parametric monitoring systems, wireless sensors for load mapping, and automated documentation platforms that enhance compliance while reducing administrative burden. As regulatory frameworks continue to evolve, these technologies will play an increasingly important role in demonstrating sterilization efficacy and maintaining regulatory compliance.
ISO 17665 stands as the primary international standard specifically addressing moist heat sterilization processes, providing detailed guidelines for development, validation, and routine control. This standard emphasizes the critical importance of establishing and documenting the parameters that ensure consistent sterilization efficacy, including temperature, pressure, and exposure time across different load configurations.
Complementary standards such as ISO 11138 and ISO 11140 govern biological indicators and chemical indicators respectively, which are essential tools for verifying sterilization cycle effectiveness. These indicators provide visible confirmation that sterilization conditions have been achieved throughout the load, serving as critical quality control measures.
Recent regulatory trends have shifted toward more stringent requirements for cycle validation, with increased emphasis on parametric release methodologies that rely on precise measurement and control of physical parameters rather than post-sterilization testing alone. This approach demands more sophisticated monitoring systems and tighter process controls but offers enhanced assurance of sterilization efficacy.
Healthcare facilities must maintain detailed sterilization records for regulatory inspections and accreditation purposes. These records typically include cycle parameters, operator identification, load contents, biological indicator results, and maintenance documentation. The Joint Commission and similar accreditation bodies regularly evaluate compliance with these documentation requirements during facility inspections.
Risk management principles have become increasingly integrated into sterilization standards, requiring facilities to identify potential failure modes and implement appropriate mitigation strategies. This approach recognizes that sterilization efficacy depends not only on equipment performance but also on proper loading techniques, maintenance procedures, and operator training.
Emerging technologies for cycle monitoring and validation are being developed in response to evolving regulatory expectations. These include real-time parametric monitoring systems, wireless sensors for load mapping, and automated documentation platforms that enhance compliance while reducing administrative burden. As regulatory frameworks continue to evolve, these technologies will play an increasingly important role in demonstrating sterilization efficacy and maintaining regulatory compliance.
Environmental Impact and Energy Efficiency Considerations
Autoclave sterilization processes, while essential for ensuring medical safety, have significant environmental and energy implications that warrant careful consideration. Traditional autoclave cycles consume substantial amounts of energy, with typical medical autoclaves requiring between 2-3 kWh per cycle and industrial-scale units consuming upwards of 15-20 kWh. This energy demand translates directly into considerable carbon emissions, particularly in regions where electricity generation relies heavily on fossil fuels.
Water consumption represents another critical environmental concern, with conventional autoclave systems using approximately 40-60 gallons per cycle for steam generation and cooling processes. This water usage becomes particularly problematic in regions experiencing water scarcity, where healthcare facilities must balance sterilization requirements against resource conservation imperatives.
Recent technological innovations have demonstrated promising pathways toward more sustainable autoclave operations. Pulse vacuum technology, for instance, has shown potential to reduce cycle times by 20-30% while maintaining sterilization efficacy, resulting in proportional energy savings. Similarly, advanced heat recovery systems can recapture up to 40% of thermal energy that would otherwise be wasted, significantly improving overall efficiency metrics.
The implementation of precise load-sensing technologies enables dynamic cycle adjustments based on actual sterilization requirements rather than standardized protocols. Studies indicate that such adaptive approaches can reduce energy consumption by 15-25% compared to fixed-cycle operations, while maintaining or even improving sterilization outcomes.
Material selection for autoclave chamber construction also impacts energy efficiency. Newer composite materials with superior insulation properties have demonstrated the ability to reduce heat loss by up to 35% compared to traditional stainless steel chambers, thereby decreasing energy requirements for maintaining sterilization temperatures.
Regulatory frameworks increasingly recognize the importance of environmental considerations in medical sterilization. The EU Medical Device Regulation and similar frameworks in other jurisdictions now incorporate sustainability metrics alongside traditional safety parameters, encouraging manufacturers to develop more resource-efficient sterilization technologies.
Cost-benefit analyses reveal that investments in energy-efficient autoclave technologies typically achieve return on investment within 2-4 years through operational savings, while simultaneously reducing environmental impact. Healthcare facilities implementing comprehensive energy management strategies for sterilization processes have reported carbon footprint reductions of 30-40% in this operational area.
Water consumption represents another critical environmental concern, with conventional autoclave systems using approximately 40-60 gallons per cycle for steam generation and cooling processes. This water usage becomes particularly problematic in regions experiencing water scarcity, where healthcare facilities must balance sterilization requirements against resource conservation imperatives.
Recent technological innovations have demonstrated promising pathways toward more sustainable autoclave operations. Pulse vacuum technology, for instance, has shown potential to reduce cycle times by 20-30% while maintaining sterilization efficacy, resulting in proportional energy savings. Similarly, advanced heat recovery systems can recapture up to 40% of thermal energy that would otherwise be wasted, significantly improving overall efficiency metrics.
The implementation of precise load-sensing technologies enables dynamic cycle adjustments based on actual sterilization requirements rather than standardized protocols. Studies indicate that such adaptive approaches can reduce energy consumption by 15-25% compared to fixed-cycle operations, while maintaining or even improving sterilization outcomes.
Material selection for autoclave chamber construction also impacts energy efficiency. Newer composite materials with superior insulation properties have demonstrated the ability to reduce heat loss by up to 35% compared to traditional stainless steel chambers, thereby decreasing energy requirements for maintaining sterilization temperatures.
Regulatory frameworks increasingly recognize the importance of environmental considerations in medical sterilization. The EU Medical Device Regulation and similar frameworks in other jurisdictions now incorporate sustainability metrics alongside traditional safety parameters, encouraging manufacturers to develop more resource-efficient sterilization technologies.
Cost-benefit analyses reveal that investments in energy-efficient autoclave technologies typically achieve return on investment within 2-4 years through operational savings, while simultaneously reducing environmental impact. Healthcare facilities implementing comprehensive energy management strategies for sterilization processes have reported carbon footprint reductions of 30-40% in this operational area.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!