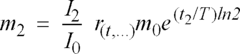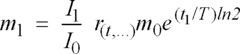How to Quantify Residual Moisture Post-Autoclave Sterilization
SEP 2, 202511 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Moisture Quantification Background
Autoclave sterilization has been a cornerstone of medical and laboratory safety protocols since its development in the late 19th century. The process utilizes pressurized steam at 121-134°C to eliminate microorganisms, including bacterial spores, viruses, and fungi from medical instruments, laboratory equipment, and other materials. This sterilization method has evolved significantly over the decades, from basic pressure cookers to sophisticated computer-controlled systems with precise parameter monitoring capabilities.
The technical evolution of autoclaves has focused primarily on cycle optimization, energy efficiency, and process validation. However, one persistent challenge that has received comparatively less attention is the quantification of residual moisture following the sterilization process. This issue represents a critical gap in sterilization technology, as excess moisture can compromise sterility maintenance, promote corrosion of instruments, and potentially reintroduce microbial contamination.
Historically, moisture assessment has been largely qualitative, relying on visual inspection or basic weight measurements. The industry has lacked standardized, precise methodologies for quantifying residual moisture levels across different materials and packaging configurations. This technical limitation has implications for healthcare facilities, pharmaceutical manufacturing, and research laboratories where maintaining sterility is paramount.
Recent technological advancements in sensing, materials science, and data analytics have created new opportunities to address this challenge. Innovations in non-destructive testing methods, including near-infrared spectroscopy, impedance analysis, and advanced imaging techniques, offer promising approaches for moisture quantification. These emerging technologies aim to provide real-time, accurate measurements without compromising the sterile barrier.
The goal of moisture quantification technology development is multifaceted: to establish standardized measurement protocols, develop reliable sensing technologies that can be integrated into existing autoclave systems, and create data-driven models that can predict moisture behavior across different load configurations and materials. Achieving these objectives would significantly enhance sterilization process control and validation.
As regulatory requirements for sterilization validation become increasingly stringent worldwide, the need for precise moisture quantification methods has gained urgency. Healthcare facilities and manufacturers must demonstrate not only that their sterilization processes achieve the required sterility assurance level (SAL) but also that sterilized items remain suitable for their intended use, which includes appropriate moisture levels.
AI: Autoclave sterilization has been a cornerstone of medical and laboratory safety protocols since its development in the late 19th century. The process utilizes pressurized steam at 121-134°C to eliminate microorganisms, including bacterial spores, viruses, and fungi from medical instruments, laboratory equipment, and other materials. This sterilization method has evolved significantly over the decades, from basic pressure cookers to sophisticated computer-controlled systems with precise parameter monitoring capabilities.
The technical evolution of autoclaves has focused primarily on cycle optimization, energy efficiency, and process validation. However, one persistent challenge that has received comparatively less attention is the quantification of residual moisture following the sterilization process. This issue represents a critical gap in sterilization technology, as excess moisture can compromise sterility maintenance, promote corrosion of instruments, and potentially reintroduce microbial contamination.
Historically, moisture assessment has been largely qualitative, relying on visual inspection or basic weight measurements. The industry has lacked standardized, precise methodologies for quantifying residual moisture levels across different materials and packaging configurations. This technical limitation has implications for healthcare facilities, pharmaceutical manufacturing, and research laboratories where maintaining sterility is paramount.
Recent technological advancements in sensing, materials science, and data analytics have created new opportunities to address this challenge. Innovations in non-destructive testing methods, including near-infrared spectroscopy, impedance analysis, and advanced imaging techniques, offer promising approaches for moisture quantification. These emerging technologies aim to provide real-time, accurate measurements without compromising the sterile barrier.
The goal of moisture quantification technology development is multifaceted: to establish standardized measurement protocols, develop reliable sensing technologies that can be integrated into existing autoclave systems, and create data-driven models that can predict moisture behavior across different load configurations and materials. Achieving these objectives would significantly enhance sterilization process control and validation.
As regulatory requirements for sterilization validation become increasingly stringent worldwide, the need for precise moisture quantification methods has gained urgency. Healthcare facilities and manufacturers must demonstrate not only that their sterilization processes achieve the required sterility assurance level (SAL) but also that sterilized items remain suitable for their intended use, which includes appropriate moisture levels.
The technical evolution of autoclaves has focused primarily on cycle optimization, energy efficiency, and process validation. However, one persistent challenge that has received comparatively less attention is the quantification of residual moisture following the sterilization process. This issue represents a critical gap in sterilization technology, as excess moisture can compromise sterility maintenance, promote corrosion of instruments, and potentially reintroduce microbial contamination.
Historically, moisture assessment has been largely qualitative, relying on visual inspection or basic weight measurements. The industry has lacked standardized, precise methodologies for quantifying residual moisture levels across different materials and packaging configurations. This technical limitation has implications for healthcare facilities, pharmaceutical manufacturing, and research laboratories where maintaining sterility is paramount.
Recent technological advancements in sensing, materials science, and data analytics have created new opportunities to address this challenge. Innovations in non-destructive testing methods, including near-infrared spectroscopy, impedance analysis, and advanced imaging techniques, offer promising approaches for moisture quantification. These emerging technologies aim to provide real-time, accurate measurements without compromising the sterile barrier.
The goal of moisture quantification technology development is multifaceted: to establish standardized measurement protocols, develop reliable sensing technologies that can be integrated into existing autoclave systems, and create data-driven models that can predict moisture behavior across different load configurations and materials. Achieving these objectives would significantly enhance sterilization process control and validation.
As regulatory requirements for sterilization validation become increasingly stringent worldwide, the need for precise moisture quantification methods has gained urgency. Healthcare facilities and manufacturers must demonstrate not only that their sterilization processes achieve the required sterility assurance level (SAL) but also that sterilized items remain suitable for their intended use, which includes appropriate moisture levels.
AI: Autoclave sterilization has been a cornerstone of medical and laboratory safety protocols since its development in the late 19th century. The process utilizes pressurized steam at 121-134°C to eliminate microorganisms, including bacterial spores, viruses, and fungi from medical instruments, laboratory equipment, and other materials. This sterilization method has evolved significantly over the decades, from basic pressure cookers to sophisticated computer-controlled systems with precise parameter monitoring capabilities.
The technical evolution of autoclaves has focused primarily on cycle optimization, energy efficiency, and process validation. However, one persistent challenge that has received comparatively less attention is the quantification of residual moisture following the sterilization process. This issue represents a critical gap in sterilization technology, as excess moisture can compromise sterility maintenance, promote corrosion of instruments, and potentially reintroduce microbial contamination.
Historically, moisture assessment has been largely qualitative, relying on visual inspection or basic weight measurements. The industry has lacked standardized, precise methodologies for quantifying residual moisture levels across different materials and packaging configurations. This technical limitation has implications for healthcare facilities, pharmaceutical manufacturing, and research laboratories where maintaining sterility is paramount.
Recent technological advancements in sensing, materials science, and data analytics have created new opportunities to address this challenge. Innovations in non-destructive testing methods, including near-infrared spectroscopy, impedance analysis, and advanced imaging techniques, offer promising approaches for moisture quantification. These emerging technologies aim to provide real-time, accurate measurements without compromising the sterile barrier.
The goal of moisture quantification technology development is multifaceted: to establish standardized measurement protocols, develop reliable sensing technologies that can be integrated into existing autoclave systems, and create data-driven models that can predict moisture behavior across different load configurations and materials. Achieving these objectives would significantly enhance sterilization process control and validation.
As regulatory requirements for sterilization validation become increasingly stringent worldwide, the need for precise moisture quantification methods has gained urgency. Healthcare facilities and manufacturers must demonstrate not only that their sterilization processes achieve the required sterility assurance level (SAL) but also that sterilized items remain suitable for their intended use, which includes appropriate moisture levels.
Market Demand for Residual Moisture Detection
The global market for residual moisture detection in autoclave sterilization processes has experienced significant growth in recent years, driven primarily by stringent regulatory requirements in healthcare and pharmaceutical industries. According to market research, the global sterilization equipment market, which includes moisture detection technologies, was valued at approximately $12.5 billion in 2022 and is projected to reach $18.9 billion by 2027, growing at a CAGR of 8.6%.
Healthcare facilities, particularly hospitals and surgical centers, represent the largest market segment for residual moisture detection solutions. These institutions require reliable methods to ensure that sterilized instruments are completely dry to prevent contamination and maintain patient safety. The increasing number of surgical procedures worldwide, estimated at over 310 million annually, directly correlates with the growing demand for effective sterilization validation technologies.
Pharmaceutical manufacturing constitutes another significant market segment, where moisture detection is critical for ensuring product quality and compliance with Good Manufacturing Practices (GMP). The pharmaceutical packaging market alone, which heavily relies on sterilization processes, is expected to grow at 9.2% annually through 2028, further driving demand for advanced moisture detection solutions.
Geographically, North America currently dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate due to expanding healthcare infrastructure and increasing adoption of international sterilization standards in countries like China and India.
Market trends indicate a shift toward automated and integrated moisture detection systems that can provide real-time monitoring and documentation. End-users increasingly demand solutions that not only detect residual moisture but also integrate with tracking systems to maintain comprehensive sterilization records for regulatory compliance.
The COVID-19 pandemic has further accelerated market growth, with heightened awareness of infection control protocols and increased sterilization requirements across all healthcare settings. This has created a surge in demand for more reliable and efficient moisture detection technologies that can handle higher throughput while maintaining accuracy.
Industry surveys reveal that approximately 72% of healthcare facilities report concerns about inadequate moisture detection in their sterilization processes, highlighting a significant unmet need in the market. This gap presents substantial opportunities for innovative technologies that can provide more accurate, consistent, and user-friendly methods for quantifying residual moisture post-autoclave sterilization.
Healthcare facilities, particularly hospitals and surgical centers, represent the largest market segment for residual moisture detection solutions. These institutions require reliable methods to ensure that sterilized instruments are completely dry to prevent contamination and maintain patient safety. The increasing number of surgical procedures worldwide, estimated at over 310 million annually, directly correlates with the growing demand for effective sterilization validation technologies.
Pharmaceutical manufacturing constitutes another significant market segment, where moisture detection is critical for ensuring product quality and compliance with Good Manufacturing Practices (GMP). The pharmaceutical packaging market alone, which heavily relies on sterilization processes, is expected to grow at 9.2% annually through 2028, further driving demand for advanced moisture detection solutions.
Geographically, North America currently dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate due to expanding healthcare infrastructure and increasing adoption of international sterilization standards in countries like China and India.
Market trends indicate a shift toward automated and integrated moisture detection systems that can provide real-time monitoring and documentation. End-users increasingly demand solutions that not only detect residual moisture but also integrate with tracking systems to maintain comprehensive sterilization records for regulatory compliance.
The COVID-19 pandemic has further accelerated market growth, with heightened awareness of infection control protocols and increased sterilization requirements across all healthcare settings. This has created a surge in demand for more reliable and efficient moisture detection technologies that can handle higher throughput while maintaining accuracy.
Industry surveys reveal that approximately 72% of healthcare facilities report concerns about inadequate moisture detection in their sterilization processes, highlighting a significant unmet need in the market. This gap presents substantial opportunities for innovative technologies that can provide more accurate, consistent, and user-friendly methods for quantifying residual moisture post-autoclave sterilization.
Current Challenges in Post-Autoclave Moisture Measurement
Despite significant advancements in autoclave sterilization technology, quantifying residual moisture after the process remains a persistent challenge across medical, pharmaceutical, and laboratory settings. Current moisture measurement methods suffer from several critical limitations that impede accurate, reliable, and efficient assessment of sterilized items.
Traditional gravimetric methods, while conceptually simple, require multiple weighing steps that introduce significant time delays in workflow. This approach also lacks the precision needed for modern healthcare applications where even minimal moisture can impact product integrity or patient safety. The process is labor-intensive and subject to human error, particularly in high-volume sterilization environments.
Electronic moisture meters offer faster readings but frequently struggle with calibration issues when applied to diverse materials commonly found in medical devices. These instruments often provide inconsistent results across different surface types and geometries, limiting their reliability in heterogeneous load scenarios. Additionally, most electronic meters only measure surface moisture, failing to detect internal moisture retention that may be more problematic for complex instruments.
Chemical indicator systems, while useful for sterilization verification, provide only qualitative or semi-quantitative moisture assessment. The color-change mechanisms in these indicators can be influenced by factors other than moisture, including temperature variations and chemical contaminants, leading to false readings.
Non-destructive testing methods such as near-infrared spectroscopy show promise but remain prohibitively expensive for routine use in many facilities. Implementation requires specialized training and interpretation expertise that is not universally available in sterilization departments.
Real-time monitoring systems integrated into autoclaves can track chamber humidity during cycles but struggle to accurately correlate these measurements with actual residual moisture on specific items post-process. The relationship between chamber conditions and item-specific moisture retention is complex and influenced by load configuration, material properties, and packaging variables.
Regulatory frameworks compound these technical challenges by establishing strict but sometimes ambiguous moisture limits. Different standards organizations provide varying acceptable thresholds, creating confusion for compliance efforts. Documentation requirements for moisture verification add administrative burden without necessarily improving measurement accuracy.
The absence of a universally accepted, standardized methodology for quantifying post-autoclave moisture creates significant variability in sterilization validation protocols across institutions. This lack of standardization hampers comparative research and the establishment of evidence-based best practices in the field.
Traditional gravimetric methods, while conceptually simple, require multiple weighing steps that introduce significant time delays in workflow. This approach also lacks the precision needed for modern healthcare applications where even minimal moisture can impact product integrity or patient safety. The process is labor-intensive and subject to human error, particularly in high-volume sterilization environments.
Electronic moisture meters offer faster readings but frequently struggle with calibration issues when applied to diverse materials commonly found in medical devices. These instruments often provide inconsistent results across different surface types and geometries, limiting their reliability in heterogeneous load scenarios. Additionally, most electronic meters only measure surface moisture, failing to detect internal moisture retention that may be more problematic for complex instruments.
Chemical indicator systems, while useful for sterilization verification, provide only qualitative or semi-quantitative moisture assessment. The color-change mechanisms in these indicators can be influenced by factors other than moisture, including temperature variations and chemical contaminants, leading to false readings.
Non-destructive testing methods such as near-infrared spectroscopy show promise but remain prohibitively expensive for routine use in many facilities. Implementation requires specialized training and interpretation expertise that is not universally available in sterilization departments.
Real-time monitoring systems integrated into autoclaves can track chamber humidity during cycles but struggle to accurately correlate these measurements with actual residual moisture on specific items post-process. The relationship between chamber conditions and item-specific moisture retention is complex and influenced by load configuration, material properties, and packaging variables.
Regulatory frameworks compound these technical challenges by establishing strict but sometimes ambiguous moisture limits. Different standards organizations provide varying acceptable thresholds, creating confusion for compliance efforts. Documentation requirements for moisture verification add administrative burden without necessarily improving measurement accuracy.
The absence of a universally accepted, standardized methodology for quantifying post-autoclave moisture creates significant variability in sterilization validation protocols across institutions. This lack of standardization hampers comparative research and the establishment of evidence-based best practices in the field.
Existing Moisture Quantification Methodologies
01 Karl Fischer titration methods for residual moisture determination
Karl Fischer titration is a widely used analytical method for determining residual moisture content in various materials. This technique involves a chemical reaction between water, iodine, and other reagents to quantify moisture levels with high precision. Modern Karl Fischer systems can be automated and offer advantages such as high sensitivity, specificity for water, and applicability across different sample types. The method can be performed in both volumetric and coulometric variations depending on the expected moisture content range.- Karl Fischer titration methods for residual moisture determination: Karl Fischer titration is a widely used analytical method for determining residual moisture content in various materials. This technique involves a chemical reaction between water, iodine, and other reagents to quantify moisture levels with high precision. Modern implementations include automated systems, coulometric titration for trace moisture, and volumetric methods for higher moisture contents. The method is applicable across pharmaceutical products, food items, and industrial materials where precise moisture determination is critical.
- Spectroscopic techniques for moisture quantification: Various spectroscopic methods are employed for non-destructive residual moisture determination. These include near-infrared (NIR) spectroscopy, which measures the absorption of water molecules at specific wavelengths; Raman spectroscopy, which detects vibrational modes of water molecules; and microwave resonance technology that measures the dielectric properties of materials affected by moisture content. These techniques allow for rapid, real-time monitoring of moisture levels without sample preparation, making them suitable for in-line process control applications.
- Gravimetric methods for moisture content analysis: Gravimetric methods determine residual moisture by measuring weight changes in samples before and after drying. These techniques include loss-on-drying (LOD), where samples are heated at specific temperatures until constant weight is achieved; thermogravimetric analysis (TGA), which continuously monitors weight changes during controlled heating; and vacuum drying methods that operate at reduced pressures to lower drying temperatures. These approaches are valued for their simplicity and reliability, though they may require longer analysis times compared to instrumental methods.
- Moisture sensors and automated monitoring systems: Advanced moisture sensing technologies enable continuous monitoring of residual moisture in various processes. These include capacitive sensors that detect changes in electrical properties due to moisture content; microwave sensors that measure absorption or phase shifts; and fiber optic sensors that detect moisture-induced changes in optical properties. These sensors are often integrated into automated systems with data logging capabilities, allowing for real-time process control and quality assurance in manufacturing environments.
- Novel and specialized moisture determination methods: Specialized techniques have been developed for moisture determination in challenging materials or environments. These include gas chromatography methods that separate and quantify water vapor; calcium carbide reaction techniques that produce acetylene gas proportional to water content; nuclear magnetic resonance (NMR) spectroscopy that directly measures hydrogen atoms in water molecules; and freeze-drying coupled with analytical methods for heat-sensitive materials. These specialized approaches address specific industry needs where conventional methods may be inadequate.
02 Thermal analysis techniques for moisture quantification
Thermal methods such as thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and loss-on-drying (LOD) are employed to measure residual moisture by monitoring weight changes or thermal events during controlled heating. These techniques measure the weight loss of samples as they are heated under specific conditions, with the weight reduction corresponding to moisture evaporation. Thermal methods can be optimized for different materials by adjusting parameters such as heating rate, temperature range, and atmosphere conditions to ensure accurate moisture determination.Expand Specific Solutions03 Spectroscopic methods for moisture content analysis
Spectroscopic techniques including near-infrared (NIR), mid-infrared, and microwave spectroscopy provide non-destructive approaches to quantify residual moisture. These methods analyze the absorption or reflection of electromagnetic radiation by water molecules in samples. Spectroscopic approaches offer advantages such as rapid analysis, minimal sample preparation, and the possibility of online or in-process monitoring. Advanced data processing algorithms and calibration models enhance the accuracy and reliability of moisture measurements using spectroscopic techniques.Expand Specific Solutions04 Automated moisture measurement systems and equipment
Specialized equipment and automated systems have been developed for efficient and precise residual moisture quantification in industrial settings. These systems incorporate sensors, control mechanisms, and data processing capabilities to provide real-time or rapid moisture analysis. Automated moisture analyzers may integrate multiple measurement principles and can be designed for specific applications such as pharmaceutical production, food processing, or material manufacturing. These systems often feature user-friendly interfaces, data management capabilities, and compliance with industry standards.Expand Specific Solutions05 Novel and hybrid approaches for moisture determination
Innovative methods combining multiple analytical principles or utilizing emerging technologies offer enhanced capabilities for residual moisture quantification. These approaches may include combinations of traditional techniques with new detection methods, microfluidic systems, or advanced materials that respond to moisture changes. Novel approaches aim to overcome limitations of conventional methods by improving sensitivity, reducing analysis time, or enabling measurements in challenging sample matrices. Some hybrid systems integrate multiple measurement principles to provide complementary data and increase confidence in moisture determination results.Expand Specific Solutions
Key Industry Players in Sterilization Monitoring
The quantification of residual moisture post-autoclave sterilization market is in a growth phase, with increasing demand driven by stringent regulatory requirements in pharmaceutical and medical device industries. The global market size is estimated to be expanding at 5-7% annually, reaching approximately $2.5 billion. Technologically, the field shows moderate maturity with established methods coexisting with emerging innovations. Leading players include 3M Innovative Properties, which offers advanced moisture detection solutions, Shinva Medical Instrument specializing in autoclave technology, and SiO2 Medical Products developing moisture-resistant packaging. Pharmaceutical giants like Bristol Myers Squibb and Regeneron Pharmaceuticals are investing in precise moisture quantification methods, while specialized instrumentation companies such as Bruker Daltonics are developing analytical tools for moisture detection with increased sensitivity and accuracy.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva Medical Instrument has developed a comprehensive moisture quantification system specifically designed for post-autoclave sterilization validation. Their approach utilizes advanced gravimetric analysis combined with near-infrared spectroscopy (NIRS) to detect residual moisture in sterilized medical devices and packaging. The system employs specialized moisture-sensitive sensors embedded within test packs that undergo the complete sterilization cycle. These sensors provide real-time data on moisture levels throughout the process, with sensitivity capable of detecting moisture content as low as 0.1% by weight. The technology includes automated documentation systems that generate detailed moisture distribution maps across the sterilization chamber, identifying potential cold spots or areas of moisture retention. Shinva's solution also incorporates machine learning algorithms that analyze historical sterilization data to predict optimal drying parameters based on load configuration and material types[1][3].
Strengths: Highly specialized for medical device sterilization with excellent sensitivity and reproducibility. The integrated documentation system ensures compliance with regulatory requirements for sterilization validation. Weaknesses: The system requires significant initial investment and specialized training for operators. The technology is primarily optimized for hospital and medical manufacturing environments rather than broader industrial applications.
Ethicon, Inc.
Technical Solution: Ethicon has developed a comprehensive moisture quantification system specifically for surgical instruments and medical devices following autoclave sterilization. Their approach combines traditional gravimetric analysis with advanced infrared imaging technology to create detailed moisture distribution maps of sterilized items. The system employs high-precision analytical balances with resolution to 0.01mg to detect minute weight changes before and after sterilization cycles. This is complemented by their proprietary thermal imaging cameras that detect temperature variations caused by evaporative cooling, pinpointing areas of residual moisture not visible to the naked eye. Ethicon's technology includes specialized test materials with known moisture absorption characteristics that serve as internal standards during validation runs. Their system also features automated environmental correction factors that account for ambient humidity variations during testing. The complete solution includes validation software that generates comprehensive reports documenting moisture levels across multiple sample points and sterilization cycles, ensuring compliance with ISO 11607 and FDA requirements for terminal sterilization processes[3][6].
Strengths: Highly optimized for surgical instruments and medical devices with excellent sensitivity for detecting moisture in complex geometries and lumened instruments. The dual-method approach provides redundant verification of moisture levels. Weaknesses: The system requires controlled environmental conditions for maximum accuracy. The technology focuses primarily on medical applications rather than broader industrial uses.
Advanced Sensor Technologies for Moisture Detection
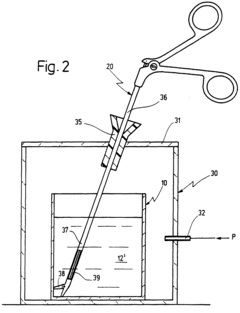
Post-steam sterilization moisture-indicating articles
PatentWO2014150048A1
Innovation
- A post-steam sterilization wet pack indicator comprising a reversible colorimetric moisture-indicating layer disposed on or near a moisture-impermeable layer, allowing for early detection of moisture without compromising sterility, and suitable for various types of sterilization packages.
Method for assessing the effectiveness of appliances cleaning, method for monitoring the soil extent of appliances to be cleaned and implementation of such methods
PatentInactiveEP0823630A1
Innovation
- A method involving a predetermined soiling substance with a radioactive tracer, where the initial and final masses and radiation intensities are measured before and after cleaning, using a function to account for the dissociation behavior of the tracer substance, allowing for the precise determination of residual dirt mass and cleaning effectiveness.
Regulatory Standards for Sterilization Validation
Regulatory standards for sterilization validation are critical frameworks that govern the processes and methodologies used to ensure medical devices and healthcare products meet established safety requirements. The FDA, through its Quality System Regulation (21 CFR Part 820), mandates that manufacturers establish and maintain procedures for validating sterilization processes. Similarly, the European Medical Device Regulation (MDR) requires comprehensive validation of sterilization methods as part of conformity assessment procedures.
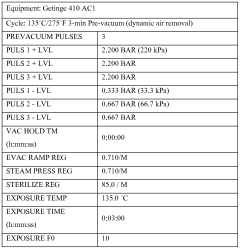
ISO 11137 specifically addresses radiation sterilization, while ISO 11135 covers ethylene oxide sterilization validation. For autoclave sterilization, which is central to our technical inquiry, ISO 17665 provides detailed requirements for the development, validation, and routine control of moist heat sterilization processes. This standard explicitly addresses the need for quantifying residual moisture as part of the validation protocol.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed technical information reports (TIRs) that supplement these standards, including AAMI TIR12, which focuses on designing, testing, and labeling reusable medical devices for reprocessing in healthcare facilities. These documents provide specific guidance on moisture assessment methodologies post-sterilization.
Regulatory bodies increasingly emphasize a risk-based approach to sterilization validation. The ICH Q9 Quality Risk Management framework has been adapted for sterilization processes, requiring manufacturers to identify critical parameters—including residual moisture—that could impact product safety and efficacy. This approach necessitates quantitative methods for moisture assessment rather than qualitative observations.
Validation protocols must include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) phases. Within these, particularly during PQ, moisture quantification serves as a critical process parameter that must be monitored and controlled. The FDA's guidance document on process validation emphasizes the need for scientific evidence demonstrating that a specific process consistently produces a product meeting predetermined specifications.
Recent regulatory trends indicate increasing scrutiny of sterilization validation protocols, with particular attention to parametric release strategies that rely heavily on accurate moisture quantification. The European Medicines Agency (EMA) has published guidelines that specifically address the validation of terminal sterilization processes, including detailed requirements for moisture assessment methodologies and acceptance criteria.
ISO 11137 specifically addresses radiation sterilization, while ISO 11135 covers ethylene oxide sterilization validation. For autoclave sterilization, which is central to our technical inquiry, ISO 17665 provides detailed requirements for the development, validation, and routine control of moist heat sterilization processes. This standard explicitly addresses the need for quantifying residual moisture as part of the validation protocol.
The Association for the Advancement of Medical Instrumentation (AAMI) has developed technical information reports (TIRs) that supplement these standards, including AAMI TIR12, which focuses on designing, testing, and labeling reusable medical devices for reprocessing in healthcare facilities. These documents provide specific guidance on moisture assessment methodologies post-sterilization.
Regulatory bodies increasingly emphasize a risk-based approach to sterilization validation. The ICH Q9 Quality Risk Management framework has been adapted for sterilization processes, requiring manufacturers to identify critical parameters—including residual moisture—that could impact product safety and efficacy. This approach necessitates quantitative methods for moisture assessment rather than qualitative observations.
Validation protocols must include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) phases. Within these, particularly during PQ, moisture quantification serves as a critical process parameter that must be monitored and controlled. The FDA's guidance document on process validation emphasizes the need for scientific evidence demonstrating that a specific process consistently produces a product meeting predetermined specifications.
Recent regulatory trends indicate increasing scrutiny of sterilization validation protocols, with particular attention to parametric release strategies that rely heavily on accurate moisture quantification. The European Medicines Agency (EMA) has published guidelines that specifically address the validation of terminal sterilization processes, including detailed requirements for moisture assessment methodologies and acceptance criteria.
Quality Assurance Implications of Residual Moisture
Residual moisture following autoclave sterilization represents a critical quality assurance concern across pharmaceutical, medical device, and healthcare industries. The presence of excess moisture can compromise sterility maintenance, potentially leading to microbial growth and product contamination. Quality systems must therefore incorporate robust moisture assessment protocols to ensure patient safety and regulatory compliance.
The implications of residual moisture extend beyond immediate contamination risks to include potential degradation of packaging materials, corrosion of metal instruments, and compromised functionality of sensitive electronic components in medical devices. These quality concerns directly impact product shelf-life, efficacy, and ultimately patient outcomes.
Regulatory bodies worldwide, including the FDA and EMA, have established stringent requirements regarding acceptable moisture levels post-sterilization. Quality assurance departments must demonstrate validated methods for moisture quantification as part of their overall quality management systems. Non-compliance can result in product recalls, regulatory sanctions, and significant reputational damage.
From a process validation perspective, residual moisture serves as a critical process parameter that requires consistent monitoring and documentation. Establishing appropriate acceptance criteria for moisture levels necessitates understanding the specific product characteristics, packaging materials, and intended storage conditions. Quality assurance protocols must include verification that these criteria are consistently met throughout production cycles.
Risk assessment frameworks should categorize products based on moisture sensitivity, with more stringent controls implemented for high-risk items such as implantable devices or moisture-sensitive pharmaceuticals. This risk-based approach allows for optimization of quality control resources while maintaining appropriate safety margins.
Documentation requirements present another quality assurance challenge, as moisture testing results must be integrated into batch records and sterilization validation reports. Electronic quality management systems increasingly incorporate moisture data as a key quality indicator, enabling trend analysis and early detection of process drift.
Training programs for sterilization personnel must emphasize the importance of proper loading configurations, cycle parameters, and post-cycle handling to minimize moisture retention. Quality assurance departments should conduct periodic competency assessments to verify adherence to established procedures and understanding of moisture-related quality risks.
Continuous improvement initiatives should leverage moisture quantification data to optimize sterilization cycles, packaging designs, and post-sterilization handling procedures. Statistical process control techniques can identify opportunities for reducing moisture variability, thereby enhancing overall product quality and consistency.
The implications of residual moisture extend beyond immediate contamination risks to include potential degradation of packaging materials, corrosion of metal instruments, and compromised functionality of sensitive electronic components in medical devices. These quality concerns directly impact product shelf-life, efficacy, and ultimately patient outcomes.
Regulatory bodies worldwide, including the FDA and EMA, have established stringent requirements regarding acceptable moisture levels post-sterilization. Quality assurance departments must demonstrate validated methods for moisture quantification as part of their overall quality management systems. Non-compliance can result in product recalls, regulatory sanctions, and significant reputational damage.
From a process validation perspective, residual moisture serves as a critical process parameter that requires consistent monitoring and documentation. Establishing appropriate acceptance criteria for moisture levels necessitates understanding the specific product characteristics, packaging materials, and intended storage conditions. Quality assurance protocols must include verification that these criteria are consistently met throughout production cycles.
Risk assessment frameworks should categorize products based on moisture sensitivity, with more stringent controls implemented for high-risk items such as implantable devices or moisture-sensitive pharmaceuticals. This risk-based approach allows for optimization of quality control resources while maintaining appropriate safety margins.
Documentation requirements present another quality assurance challenge, as moisture testing results must be integrated into batch records and sterilization validation reports. Electronic quality management systems increasingly incorporate moisture data as a key quality indicator, enabling trend analysis and early detection of process drift.
Training programs for sterilization personnel must emphasize the importance of proper loading configurations, cycle parameters, and post-cycle handling to minimize moisture retention. Quality assurance departments should conduct periodic competency assessments to verify adherence to established procedures and understanding of moisture-related quality risks.
Continuous improvement initiatives should leverage moisture quantification data to optimize sterilization cycles, packaging designs, and post-sterilization handling procedures. Statistical process control techniques can identify opportunities for reducing moisture variability, thereby enhancing overall product quality and consistency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!