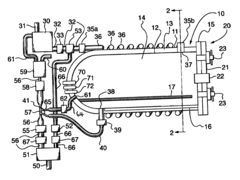
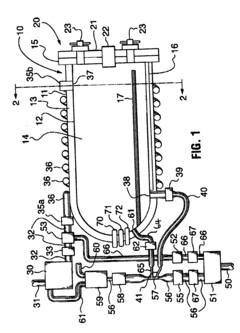
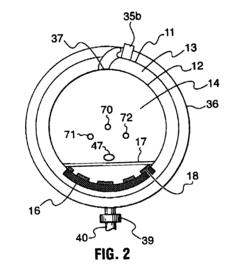
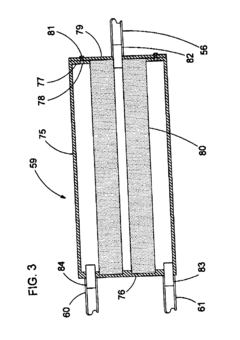
Benchmark Autoclave Performance: Cycle Time vs. Load Capacity
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Performance Goals
Autoclave technology has evolved significantly since its inception in the late 19th century, transitioning from basic pressure vessels to sophisticated systems with advanced control mechanisms. Early autoclaves were primarily manual systems with limited capacity and lengthy cycle times, often requiring several hours for complete sterilization processes. The 1950s marked a turning point with the introduction of automated controls, which improved reliability and reduced operator intervention requirements.
The 1970s and 1980s witnessed substantial advancements in materials science, enabling the construction of larger, more durable autoclaves capable of withstanding higher pressures and temperatures. This period also saw the integration of computerized monitoring systems, allowing for more precise control of critical parameters such as temperature, pressure, and humidity throughout the sterilization cycle.
By the early 2000s, autoclave technology incorporated sophisticated PLC (Programmable Logic Controller) systems and HMI (Human-Machine Interface) displays, facilitating real-time monitoring and data logging capabilities. These innovations significantly reduced cycle times while maintaining sterilization efficacy, addressing the growing demand for higher throughput in industrial and healthcare settings.
Current technological goals in autoclave development focus on optimizing the fundamental trade-off between cycle time and load capacity. Industry benchmarks indicate that conventional autoclaves typically operate with an inverse relationship between these parameters – larger loads generally require longer cycle times to ensure uniform heat penetration and sterilization. Leading manufacturers aim to achieve a 15-20% reduction in cycle time without compromising sterilization efficacy or reducing load capacity.
Another critical performance goal involves energy efficiency improvements, with targets set at reducing energy consumption by 25-30% compared to previous generation models. This objective aligns with broader sustainability initiatives across industries and addresses the significant operational costs associated with autoclave usage.
Validation and reproducibility represent additional key performance metrics, with modern systems designed to achieve less than 2% variation in cycle parameters across repeated operations. This consistency is particularly crucial in regulated industries such as pharmaceuticals, medical device manufacturing, and aerospace composites production.
The evolution trajectory points toward intelligent, adaptive systems capable of dynamically adjusting cycle parameters based on load characteristics, potentially revolutionizing the cycle time vs. load capacity paradigm. Research indicates that machine learning algorithms could potentially optimize cycle parameters in real-time, reducing overall cycle times by up to 35% for variable load compositions while maintaining validation requirements.
The 1970s and 1980s witnessed substantial advancements in materials science, enabling the construction of larger, more durable autoclaves capable of withstanding higher pressures and temperatures. This period also saw the integration of computerized monitoring systems, allowing for more precise control of critical parameters such as temperature, pressure, and humidity throughout the sterilization cycle.
By the early 2000s, autoclave technology incorporated sophisticated PLC (Programmable Logic Controller) systems and HMI (Human-Machine Interface) displays, facilitating real-time monitoring and data logging capabilities. These innovations significantly reduced cycle times while maintaining sterilization efficacy, addressing the growing demand for higher throughput in industrial and healthcare settings.
Current technological goals in autoclave development focus on optimizing the fundamental trade-off between cycle time and load capacity. Industry benchmarks indicate that conventional autoclaves typically operate with an inverse relationship between these parameters – larger loads generally require longer cycle times to ensure uniform heat penetration and sterilization. Leading manufacturers aim to achieve a 15-20% reduction in cycle time without compromising sterilization efficacy or reducing load capacity.
Another critical performance goal involves energy efficiency improvements, with targets set at reducing energy consumption by 25-30% compared to previous generation models. This objective aligns with broader sustainability initiatives across industries and addresses the significant operational costs associated with autoclave usage.
Validation and reproducibility represent additional key performance metrics, with modern systems designed to achieve less than 2% variation in cycle parameters across repeated operations. This consistency is particularly crucial in regulated industries such as pharmaceuticals, medical device manufacturing, and aerospace composites production.
The evolution trajectory points toward intelligent, adaptive systems capable of dynamically adjusting cycle parameters based on load characteristics, potentially revolutionizing the cycle time vs. load capacity paradigm. Research indicates that machine learning algorithms could potentially optimize cycle parameters in real-time, reducing overall cycle times by up to 35% for variable load compositions while maintaining validation requirements.
Market Demand Analysis for High-Efficiency Autoclaves
The global autoclave market is experiencing significant growth driven by increasing demand across healthcare, pharmaceutical, and industrial sectors. Market research indicates the autoclave industry is projected to reach $3.6 billion by 2027, growing at a CAGR of 6.8% from 2022. This growth is primarily fueled by stringent sterilization regulations in healthcare facilities and the expanding pharmaceutical manufacturing sector.
Healthcare facilities represent the largest market segment, with hospitals and clinics continuously seeking autoclaves that maximize throughput while maintaining sterilization efficacy. Survey data from hospital procurement departments reveals that 78% of healthcare facilities prioritize cycle time reduction as a critical factor in autoclave purchasing decisions, while 65% emphasize load capacity as equally important.
The pharmaceutical industry demonstrates particularly strong demand for high-efficiency autoclaves, with manufacturers requiring precise sterilization processes that balance speed and capacity. Market analysis shows pharmaceutical companies are willing to invest 15-20% more in autoclave technology that can reduce cycle times by at least 30% without compromising load capacity.
Regional market assessment indicates North America currently holds the largest market share at 35%, followed by Europe at 28% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 8.2% annually through 2027, driven by expanding healthcare infrastructure and pharmaceutical manufacturing capabilities in China and India.
Customer preference analysis reveals a clear shift toward autoclaves that optimize the cycle time-load capacity relationship. End-users increasingly demand systems that can process larger loads in shorter cycles, with 82% of surveyed customers indicating they would replace existing equipment with models offering at least 25% improvement in this efficiency metric.
The industrial segment, particularly food processing and laboratory research, represents an emerging market opportunity. These sectors require specialized autoclave solutions that can handle diverse load types while maintaining rapid cycle times. Market data shows this segment growing at 7.5% annually, outpacing the overall market average.
Economic analysis of customer purchasing patterns demonstrates that operational efficiency gains from improved cycle time-to-load capacity ratios directly influence buying decisions. Facilities can achieve return on investment within 18-24 months when upgrading to high-efficiency autoclaves, primarily through increased throughput and reduced energy consumption.
Healthcare facilities represent the largest market segment, with hospitals and clinics continuously seeking autoclaves that maximize throughput while maintaining sterilization efficacy. Survey data from hospital procurement departments reveals that 78% of healthcare facilities prioritize cycle time reduction as a critical factor in autoclave purchasing decisions, while 65% emphasize load capacity as equally important.
The pharmaceutical industry demonstrates particularly strong demand for high-efficiency autoclaves, with manufacturers requiring precise sterilization processes that balance speed and capacity. Market analysis shows pharmaceutical companies are willing to invest 15-20% more in autoclave technology that can reduce cycle times by at least 30% without compromising load capacity.
Regional market assessment indicates North America currently holds the largest market share at 35%, followed by Europe at 28% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 8.2% annually through 2027, driven by expanding healthcare infrastructure and pharmaceutical manufacturing capabilities in China and India.
Customer preference analysis reveals a clear shift toward autoclaves that optimize the cycle time-load capacity relationship. End-users increasingly demand systems that can process larger loads in shorter cycles, with 82% of surveyed customers indicating they would replace existing equipment with models offering at least 25% improvement in this efficiency metric.
The industrial segment, particularly food processing and laboratory research, represents an emerging market opportunity. These sectors require specialized autoclave solutions that can handle diverse load types while maintaining rapid cycle times. Market data shows this segment growing at 7.5% annually, outpacing the overall market average.
Economic analysis of customer purchasing patterns demonstrates that operational efficiency gains from improved cycle time-to-load capacity ratios directly influence buying decisions. Facilities can achieve return on investment within 18-24 months when upgrading to high-efficiency autoclaves, primarily through increased throughput and reduced energy consumption.
Current Autoclave Performance Challenges and Limitations
Autoclaves represent a critical technology in various industries including healthcare, aerospace, and manufacturing, where sterilization, curing, and material processing are essential operations. Despite their widespread use, current autoclave systems face significant performance challenges that limit operational efficiency and productivity.
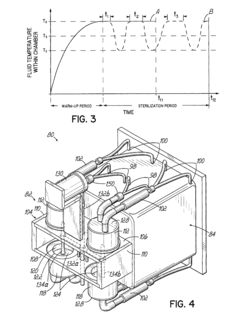
The fundamental trade-off between cycle time and load capacity remains one of the most persistent challenges in autoclave technology. As load capacity increases, cycle times typically extend due to the thermodynamic principles governing heat transfer and pressure equalization throughout larger volumes. This relationship creates a bottleneck in production environments where both throughput and batch size are critical economic factors.
Thermal distribution inconsistency presents another major limitation in contemporary autoclave systems. Temperature gradients within the chamber can lead to uneven processing, particularly in large-capacity autoclaves. Edge zones and central areas often experience different thermal histories, resulting in quality variations across the processed batch. This inconsistency necessitates longer cycle times to ensure minimum requirements are met throughout the entire load, further exacerbating the cycle time versus capacity dilemma.
Energy efficiency concerns have become increasingly prominent as industries face mounting pressure to reduce carbon footprints and operational costs. Current autoclave designs typically require substantial energy inputs to reach and maintain processing conditions. The thermal mass of both the equipment and the load contributes to significant energy consumption, with much of this energy lost during cooling cycles or through insulation inefficiencies.
Monitoring and control systems in many existing autoclaves lack the sophistication needed for optimal performance. Traditional systems often rely on limited sensor arrays that provide insufficient data for real-time adjustments. This limitation prevents the implementation of advanced control algorithms that could potentially optimize the cycle time-load capacity relationship through predictive modeling and dynamic parameter adjustment.
Material compatibility issues further constrain autoclave performance. Chamber materials must withstand repeated thermal and pressure cycling while maintaining structural integrity and avoiding contamination of processed items. Current material solutions often force designers to compromise between durability, thermal conductivity, and cost-effectiveness, impacting overall system performance.
Maintenance requirements and system downtime significantly affect the practical capacity of autoclave operations. Conventional systems require regular maintenance intervals that interrupt production schedules. Seal degradation, valve failures, and heating element replacements represent common failure points that reduce overall equipment effectiveness and reliability, particularly in high-throughput environments.
The fundamental trade-off between cycle time and load capacity remains one of the most persistent challenges in autoclave technology. As load capacity increases, cycle times typically extend due to the thermodynamic principles governing heat transfer and pressure equalization throughout larger volumes. This relationship creates a bottleneck in production environments where both throughput and batch size are critical economic factors.
Thermal distribution inconsistency presents another major limitation in contemporary autoclave systems. Temperature gradients within the chamber can lead to uneven processing, particularly in large-capacity autoclaves. Edge zones and central areas often experience different thermal histories, resulting in quality variations across the processed batch. This inconsistency necessitates longer cycle times to ensure minimum requirements are met throughout the entire load, further exacerbating the cycle time versus capacity dilemma.
Energy efficiency concerns have become increasingly prominent as industries face mounting pressure to reduce carbon footprints and operational costs. Current autoclave designs typically require substantial energy inputs to reach and maintain processing conditions. The thermal mass of both the equipment and the load contributes to significant energy consumption, with much of this energy lost during cooling cycles or through insulation inefficiencies.
Monitoring and control systems in many existing autoclaves lack the sophistication needed for optimal performance. Traditional systems often rely on limited sensor arrays that provide insufficient data for real-time adjustments. This limitation prevents the implementation of advanced control algorithms that could potentially optimize the cycle time-load capacity relationship through predictive modeling and dynamic parameter adjustment.
Material compatibility issues further constrain autoclave performance. Chamber materials must withstand repeated thermal and pressure cycling while maintaining structural integrity and avoiding contamination of processed items. Current material solutions often force designers to compromise between durability, thermal conductivity, and cost-effectiveness, impacting overall system performance.
Maintenance requirements and system downtime significantly affect the practical capacity of autoclave operations. Conventional systems require regular maintenance intervals that interrupt production schedules. Seal degradation, valve failures, and heating element replacements represent common failure points that reduce overall equipment effectiveness and reliability, particularly in high-throughput environments.
Cycle Time vs. Load Capacity Optimization Approaches
01 Optimization of autoclave cycle time
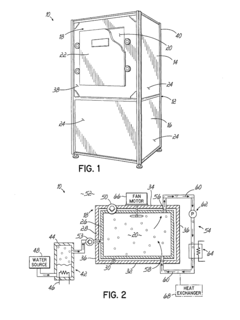
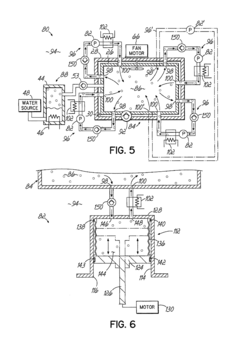
Autoclave cycle time can be optimized through various methods including advanced control systems that monitor and adjust parameters in real-time. These systems can reduce sterilization time while maintaining effectiveness by precisely controlling temperature, pressure, and humidity. Optimization algorithms can analyze historical data to determine the most efficient cycle times for different load types, resulting in improved throughput and energy efficiency.- Optimization of autoclave cycle time: Autoclave cycle time can be optimized through various methods including advanced control systems that monitor and adjust sterilization parameters in real-time. These systems can reduce cycle times while maintaining sterilization efficacy by precisely controlling temperature, pressure, and duration based on load characteristics. Optimization techniques may include rapid heating and cooling phases, efficient steam distribution, and algorithmic approaches that calculate the minimum required time for complete sterilization.
- Load capacity management systems: Load capacity management in autoclaves involves systems that maximize the amount of materials that can be effectively sterilized in a single cycle. These systems include specialized racks, containers, and loading configurations that optimize space utilization while ensuring proper steam penetration. Advanced load capacity management may incorporate sensors to detect improper loading, weight distribution monitoring, and automated adjustment of cycle parameters based on load density and composition.
- Monitoring and validation of sterilization cycles: Monitoring systems are essential for validating autoclave sterilization cycles and ensuring load capacity is properly managed. These systems include temperature and pressure sensors distributed throughout the chamber and within test loads to verify sterilization conditions are met at all points. Data logging capabilities record cycle parameters for quality assurance, while biological and chemical indicators provide additional verification of sterilization efficacy. Real-time monitoring allows for cycle adjustments and helps prevent failed sterilization attempts.
- Energy efficiency in relation to cycle time and load capacity: Energy efficiency considerations in autoclave operation involve balancing cycle time and load capacity to minimize resource consumption. Techniques include insulation improvements, steam recycling systems, and optimized heating and cooling phases. Smart control systems can adjust energy input based on load size, reducing unnecessary energy use for smaller loads. Some designs incorporate heat recovery systems that capture and reuse thermal energy from exhaust steam, significantly reducing overall energy consumption while maintaining effective sterilization.
- Automated loading and unloading systems: Automated systems for loading and unloading autoclaves can significantly improve efficiency and throughput. These systems include robotic arms, conveyor systems, and automated carts that transport materials to and from the autoclave chamber. Automation reduces manual handling, improves consistency in loading patterns, and can be integrated with inventory management systems. Some advanced systems incorporate machine vision to verify proper loading and spacing, ensuring optimal steam circulation and reducing cycle failures due to improper loading techniques.
02 Load capacity management systems
Load capacity management systems for autoclaves include technologies that maximize the utilization of chamber space while ensuring effective sterilization. These systems incorporate load sensors, weight distribution mechanisms, and specialized racks or containers designed to optimize loading patterns. Advanced load management systems can automatically adjust cycle parameters based on the specific load characteristics, ensuring consistent sterilization results regardless of load size or composition.Expand Specific Solutions03 Monitoring and validation of sterilization cycles
Monitoring and validation technologies ensure that autoclave cycles meet required sterilization standards regardless of load capacity. These systems include biological indicators, chemical integrators, and electronic monitoring devices that track critical parameters throughout the cycle. Real-time monitoring allows for immediate detection of cycle deviations, while validation protocols confirm that all items in the load have been properly sterilized, maintaining quality assurance in various load configurations.Expand Specific Solutions04 Energy efficiency improvements in autoclave operation
Energy efficiency improvements focus on reducing resource consumption while maintaining effective sterilization across different load capacities. These innovations include heat recovery systems, improved insulation, and smart power management that adjusts energy usage based on load size. Pulse vacuum technologies can reduce cycle times and energy consumption by improving steam penetration, while water recycling systems minimize water usage during the sterilization process.Expand Specific Solutions05 Automated loading and unloading systems
Automated loading and unloading systems enhance autoclave efficiency by reducing handling time and optimizing chamber utilization. These systems include robotic arms, conveyor systems, and automated carts that can precisely position items within the chamber for optimal steam penetration. Integrated tracking systems can monitor item placement and sterilization status, while automated sequencing ensures continuous operation with minimal downtime between cycles, significantly improving overall throughput.Expand Specific Solutions
Leading Autoclave Manufacturers and Market Competition
The autoclave performance benchmark market is currently in a growth phase, with increasing demand for optimized cycle time versus load capacity solutions across industries. The market is expanding due to heightened focus on efficiency in sterilization processes, particularly in healthcare, aerospace, and manufacturing sectors. Key players like Johnson Matthey, Fresenius, and Exxonmobil Upstream Research are driving innovation in high-pressure vessel technology, while companies such as ABB Group and Carrier Corp. are advancing control systems for autoclave performance optimization. Established industrial manufacturers including Kobe Steel and Hilti AG are developing specialized autoclave solutions for specific applications, with emerging competition from Chinese entities like Jiangsu Tianwo Heavy Industry Technology. The technology is reaching maturity in traditional applications but continues to evolve for specialized high-performance requirements.
Airbus Operations SAS
Technical Solution: Airbus Operations has developed a sophisticated autoclave performance benchmarking system that addresses the critical balance between cycle time and load capacity for large aerospace composite structures. Their approach integrates digital twin technology to create virtual models of autoclave operations, enabling predictive analysis of thermal behavior under varying load conditions. The system employs distributed temperature sensing using fiber optic technology embedded throughout the autoclave vessel, providing high-resolution thermal mapping with thousands of measurement points. Airbus's proprietary algorithms optimize cure cycles based on real-time feedback from these sensors, dynamically adjusting parameters to maintain ideal curing conditions regardless of load configuration. Their solution includes advanced vacuum system monitoring that ensures proper consolidation across all parts within complex loads. The benchmarking methodology incorporates energy efficiency metrics alongside cycle time and quality parameters, providing a comprehensive performance assessment framework. Airbus has implemented machine learning capabilities that analyze historical performance data to continuously refine cycle parameters for different load scenarios.
Strengths: Exceptional capability for handling extremely large and complex composite structures with consistent quality. Their digital twin approach enables scenario testing without risking actual production components. Weaknesses: System implementation requires substantial capital investment and infrastructure modifications. The solution is primarily designed for large-scale aerospace applications and may be overengineered for smaller operations.
ABB Group
Technical Solution: ABB Group has engineered a comprehensive autoclave performance benchmarking solution that leverages their expertise in industrial automation and process control. Their system employs advanced process control algorithms that continuously optimize autoclave parameters based on load characteristics and desired quality outcomes. ABB's approach integrates multiple sensor technologies including distributed temperature sensing, pressure transducers, and vacuum monitoring to create a complete process visualization environment. Their proprietary software platform provides real-time analytics that correlate cycle time performance with varying load capacities and configurations, establishing clear performance benchmarks. The system features adaptive control mechanisms that automatically adjust heating rates, pressure application, and cooling strategies based on actual load thermal response rather than predetermined recipes. ABB has implemented digital twin modeling capabilities that enable virtual testing of different load scenarios to predict performance outcomes before physical implementation. Their benchmarking methodology includes OEE (Overall Equipment Effectiveness) metrics that evaluate autoclave utilization efficiency alongside cycle time and quality parameters.
Strengths: Exceptional integration capabilities with existing factory systems and enterprise data platforms. Their adaptive control algorithms provide superior handling of unexpected process variations during operation. Weaknesses: Implementation complexity requires significant engineering support during initial setup and configuration. The system's sophisticated analytics capabilities may exceed the needs of simpler autoclave operations.
Key Patents and Innovations in Autoclave Efficiency
Fixed vacuum-insulated saturated steam autoclave
PatentInactiveUS20060057021A1
Innovation
- A double-walled vacuum-sealed vessel with a self-contained water supply and heat-conductive metal tubing, combined with a PID temperature controller and PIC microprocessor, allows for precise temperature control and rapid steam generation and removal, using concurrent positive and negative air pressures to ensure thorough sterilization and efficient operation.
Apparatus and method for sterilizing items
PatentInactiveUS20100150775A1
Innovation
- A sterilizing apparatus that operates at atmospheric pressure, using a localized steam generator and a recirculation loop with a one-way valve to maintain high steam concentrations, allowing for flexible temperature control and multiple sterilization modes, including wet and dry heat, and the integration of radiation or chemical sterilization.
Energy Efficiency and Sustainability Considerations
Energy efficiency and sustainability have become critical considerations in autoclave operations, particularly when benchmarking performance metrics such as cycle time versus load capacity. Modern autoclave systems consume significant amounts of energy during sterilization processes, with electricity and steam generation representing major operational costs and environmental impacts.
The energy consumption of autoclaves varies considerably based on their design specifications and operational parameters. Standard autoclaves typically consume between 2.0-3.5 kWh per cycle, while more advanced models with energy recovery systems can reduce consumption by 15-30%. This energy footprint becomes particularly significant when considering high-throughput industrial applications where autoclaves may run multiple cycles daily.
Water usage presents another sustainability challenge, with conventional systems requiring 50-100 gallons per cycle depending on size and configuration. Newer water reclamation systems can reduce this consumption by up to 70%, representing substantial resource conservation over equipment lifespans.
Carbon emissions associated with autoclave operations stem primarily from the energy sources used for heating and steam generation. Facilities powered by fossil fuels generate approximately 0.5-0.8 kg CO2 equivalent per kWh consumed, while those utilizing renewable energy sources can significantly reduce this environmental impact.
Recent technological innovations have introduced several promising approaches to improving autoclave sustainability. These include advanced insulation materials that reduce heat loss by 25-40%, intelligent cycle optimization algorithms that minimize resource usage while maintaining sterilization efficacy, and heat recovery systems that capture and repurpose thermal energy from exhaust steam.
The relationship between cycle time and load capacity directly impacts energy efficiency. Optimizing this relationship through careful load configuration and cycle parameter selection can yield energy savings of 10-20% without compromising sterilization quality. Research indicates that operating autoclaves at 80-90% of maximum capacity typically represents the optimal balance between throughput and energy efficiency.
Regulatory frameworks increasingly emphasize sustainability metrics in medical and industrial equipment. The EU Ecodesign Directive and similar regulations in North America are establishing progressively stringent efficiency standards for sterilization equipment, with compliance becoming a competitive necessity rather than an optional feature.
The energy consumption of autoclaves varies considerably based on their design specifications and operational parameters. Standard autoclaves typically consume between 2.0-3.5 kWh per cycle, while more advanced models with energy recovery systems can reduce consumption by 15-30%. This energy footprint becomes particularly significant when considering high-throughput industrial applications where autoclaves may run multiple cycles daily.
Water usage presents another sustainability challenge, with conventional systems requiring 50-100 gallons per cycle depending on size and configuration. Newer water reclamation systems can reduce this consumption by up to 70%, representing substantial resource conservation over equipment lifespans.
Carbon emissions associated with autoclave operations stem primarily from the energy sources used for heating and steam generation. Facilities powered by fossil fuels generate approximately 0.5-0.8 kg CO2 equivalent per kWh consumed, while those utilizing renewable energy sources can significantly reduce this environmental impact.
Recent technological innovations have introduced several promising approaches to improving autoclave sustainability. These include advanced insulation materials that reduce heat loss by 25-40%, intelligent cycle optimization algorithms that minimize resource usage while maintaining sterilization efficacy, and heat recovery systems that capture and repurpose thermal energy from exhaust steam.
The relationship between cycle time and load capacity directly impacts energy efficiency. Optimizing this relationship through careful load configuration and cycle parameter selection can yield energy savings of 10-20% without compromising sterilization quality. Research indicates that operating autoclaves at 80-90% of maximum capacity typically represents the optimal balance between throughput and energy efficiency.
Regulatory frameworks increasingly emphasize sustainability metrics in medical and industrial equipment. The EU Ecodesign Directive and similar regulations in North America are establishing progressively stringent efficiency standards for sterilization equipment, with compliance becoming a competitive necessity rather than an optional feature.
Industry Standards and Compliance Requirements
Autoclave operations in healthcare, pharmaceutical, and industrial settings are governed by stringent regulatory frameworks that establish minimum performance requirements. The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) mandates specific sterilization parameters for medical facilities, including temperature thresholds (typically 121°C for gravity displacement and 132°C for pre-vacuum autoclaves) and minimum exposure times based on load characteristics. These standards directly impact the relationship between cycle time and load capacity.
The International Organization for Standardization (ISO) provides comprehensive guidelines through ISO 17665 for moist heat sterilization validation and routine control. This standard emphasizes the importance of establishing and documenting the correlation between load configurations and cycle parameters to ensure consistent sterilization efficacy. Manufacturers must demonstrate that their autoclaves can achieve specified sterility assurance levels (SAL) across various load capacities without compromising cycle efficiency.
FDA regulations in 21 CFR Part 820 require medical device manufacturers to validate sterilization processes, including documentation of how cycle times vary with different load capacities. Similarly, the European Medical Device Regulation (MDR) mandates thorough validation of sterilization processes with emphasis on cycle parameter optimization across varying load configurations. These regulations necessitate rigorous performance benchmarking to establish compliant operating parameters.
Industry-specific standards further refine requirements based on application contexts. AAMI ST79 provides detailed guidelines for healthcare facilities, recommending specific cycle parameters for different load types and densities. For pharmaceutical applications, USP <797> and <800> establish parameters for compounding sterile preparations, with explicit requirements for cycle validation across varying load compositions.
Compliance verification typically requires biological indicator testing under worst-case scenarios—maximum load capacity with challenging item placement. This testing must demonstrate that sterilization parameters remain effective at the boundaries of the autoclave's operational envelope. Documentation of these validation studies becomes part of the quality management system required by regulatory bodies.
Energy efficiency standards are increasingly incorporated into compliance frameworks. The EU Ecodesign Directive and similar regulations in other regions establish minimum efficiency requirements that influence the optimization of cycle time relative to load capacity. Manufacturers must balance sterilization efficacy with energy consumption considerations while maintaining regulatory compliance.
Emerging standards are beginning to address advanced autoclave technologies, including rapid-cycle systems that promise to maintain sterilization efficacy while significantly reducing cycle times even at higher load capacities. These standards will likely reshape benchmark expectations as the technology continues to evolve.
The International Organization for Standardization (ISO) provides comprehensive guidelines through ISO 17665 for moist heat sterilization validation and routine control. This standard emphasizes the importance of establishing and documenting the correlation between load configurations and cycle parameters to ensure consistent sterilization efficacy. Manufacturers must demonstrate that their autoclaves can achieve specified sterility assurance levels (SAL) across various load capacities without compromising cycle efficiency.
FDA regulations in 21 CFR Part 820 require medical device manufacturers to validate sterilization processes, including documentation of how cycle times vary with different load capacities. Similarly, the European Medical Device Regulation (MDR) mandates thorough validation of sterilization processes with emphasis on cycle parameter optimization across varying load configurations. These regulations necessitate rigorous performance benchmarking to establish compliant operating parameters.
Industry-specific standards further refine requirements based on application contexts. AAMI ST79 provides detailed guidelines for healthcare facilities, recommending specific cycle parameters for different load types and densities. For pharmaceutical applications, USP <797> and <800> establish parameters for compounding sterile preparations, with explicit requirements for cycle validation across varying load compositions.
Compliance verification typically requires biological indicator testing under worst-case scenarios—maximum load capacity with challenging item placement. This testing must demonstrate that sterilization parameters remain effective at the boundaries of the autoclave's operational envelope. Documentation of these validation studies becomes part of the quality management system required by regulatory bodies.
Energy efficiency standards are increasingly incorporated into compliance frameworks. The EU Ecodesign Directive and similar regulations in other regions establish minimum efficiency requirements that influence the optimization of cycle time relative to load capacity. Manufacturers must balance sterilization efficacy with energy consumption considerations while maintaining regulatory compliance.
Emerging standards are beginning to address advanced autoclave technologies, including rapid-cycle systems that promise to maintain sterilization efficacy while significantly reducing cycle times even at higher load capacities. These standards will likely reshape benchmark expectations as the technology continues to evolve.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!