Benchmarking Autoclave Performance: Steam Penetration Best Practices
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Performance Goals
Autoclave technology has evolved significantly since its inception in the late 19th century. Originally developed for medical sterilization, autoclaves have transformed from simple pressure cookers to sophisticated computerized systems with precise control mechanisms. The evolution trajectory shows three distinct phases: mechanical systems (1880s-1950s), electromechanical systems (1960s-1990s), and digital systems (2000s-present). Each phase marked substantial improvements in reliability, efficiency, and process control capabilities.
The modern autoclave industry has been shaped by stringent regulatory requirements across healthcare, pharmaceutical, and food processing sectors. These regulations have driven technological advancements focused on validation, documentation, and reproducibility of sterilization processes. Steam penetration, as a critical performance parameter, has received particular attention due to its direct correlation with sterilization efficacy.
Current performance goals for autoclave technology center around five key dimensions: energy efficiency, process reliability, cycle time optimization, validation capabilities, and environmental impact. Energy efficiency has become increasingly important as organizations seek to reduce operational costs and carbon footprints. Modern autoclaves aim to achieve optimal steam generation and distribution while minimizing energy consumption through improved insulation, steam recovery systems, and intelligent heating algorithms.
Process reliability remains paramount, with contemporary performance standards demanding consistent steam penetration across all load configurations. This has led to innovations in chamber design, steam distribution systems, and load positioning protocols. The industry benchmark now includes achieving uniform temperature distribution with variations not exceeding ±1°C throughout the sterilization cycle.
Cycle time optimization represents another critical performance goal, particularly in high-throughput environments. Advanced autoclaves employ rapid heating and cooling technologies, optimized vacuum systems, and load-specific cycle parameters to reduce overall processing time while maintaining sterilization efficacy. The benchmark for modern systems includes complete cycles for standard loads in under 45 minutes, a significant improvement from historical 1-2 hour cycles.
Validation capabilities have evolved to include real-time monitoring, automated documentation, and predictive analytics. Contemporary performance goals include comprehensive data capture, wireless connectivity for remote monitoring, and integration with facility management systems. The ability to demonstrate consistent steam penetration through biological indicators and physical measurements has become a standard requirement rather than an optional feature.
Environmental sustainability has emerged as a newer performance goal, with reduced water consumption, lower chemical usage, and smaller physical footprints becoming important benchmarks. Leading manufacturers now target water consumption reductions of 20-30% compared to previous generation systems.
The modern autoclave industry has been shaped by stringent regulatory requirements across healthcare, pharmaceutical, and food processing sectors. These regulations have driven technological advancements focused on validation, documentation, and reproducibility of sterilization processes. Steam penetration, as a critical performance parameter, has received particular attention due to its direct correlation with sterilization efficacy.
Current performance goals for autoclave technology center around five key dimensions: energy efficiency, process reliability, cycle time optimization, validation capabilities, and environmental impact. Energy efficiency has become increasingly important as organizations seek to reduce operational costs and carbon footprints. Modern autoclaves aim to achieve optimal steam generation and distribution while minimizing energy consumption through improved insulation, steam recovery systems, and intelligent heating algorithms.
Process reliability remains paramount, with contemporary performance standards demanding consistent steam penetration across all load configurations. This has led to innovations in chamber design, steam distribution systems, and load positioning protocols. The industry benchmark now includes achieving uniform temperature distribution with variations not exceeding ±1°C throughout the sterilization cycle.
Cycle time optimization represents another critical performance goal, particularly in high-throughput environments. Advanced autoclaves employ rapid heating and cooling technologies, optimized vacuum systems, and load-specific cycle parameters to reduce overall processing time while maintaining sterilization efficacy. The benchmark for modern systems includes complete cycles for standard loads in under 45 minutes, a significant improvement from historical 1-2 hour cycles.
Validation capabilities have evolved to include real-time monitoring, automated documentation, and predictive analytics. Contemporary performance goals include comprehensive data capture, wireless connectivity for remote monitoring, and integration with facility management systems. The ability to demonstrate consistent steam penetration through biological indicators and physical measurements has become a standard requirement rather than an optional feature.
Environmental sustainability has emerged as a newer performance goal, with reduced water consumption, lower chemical usage, and smaller physical footprints becoming important benchmarks. Leading manufacturers now target water consumption reductions of 20-30% compared to previous generation systems.
Market Demand for Effective Sterilization Solutions
The global sterilization market has experienced significant growth in recent years, driven primarily by increasing healthcare-associated infections, growing surgical procedures, and heightened awareness of infection control protocols. The market for autoclave sterilization solutions specifically was valued at approximately $2.1 billion in 2022 and is projected to reach $3.4 billion by 2028, representing a compound annual growth rate of 8.3%.
Healthcare facilities worldwide are facing mounting pressure to ensure absolute sterility of medical instruments and equipment. This demand is particularly acute in hospitals, where the consequences of inadequate sterilization can be life-threatening. A recent survey of 500 healthcare facilities across North America and Europe revealed that 78% consider improving sterilization processes a high or critical priority, with steam autoclaves remaining the preferred method for 82% of respondents.
The pharmaceutical and biotechnology sectors represent another significant market segment, with stringent regulatory requirements driving demand for validated sterilization processes. As these industries expand their production capabilities, particularly for injectable medications and biological products, the need for reliable steam penetration in autoclaves has become paramount.
Emerging economies present substantial growth opportunities, with healthcare infrastructure development in countries like India, China, and Brazil creating new demand centers. Market analysis indicates that these regions will account for approximately 35% of global sterilization equipment purchases by 2025, up from 22% in 2020.
The COVID-19 pandemic has further accelerated market demand, highlighting the critical importance of effective sterilization protocols. A 2021 industry report noted a 43% increase in autoclave purchases during the pandemic, with healthcare facilities upgrading existing equipment to meet enhanced infection control standards.
Consumer expectations are also evolving, with patients increasingly aware of infection risks and demanding evidence of robust sterilization practices. This has prompted healthcare providers to not only implement effective sterilization solutions but also to communicate these practices as a competitive advantage.
Regulatory bodies worldwide continue to strengthen sterilization standards, with recent updates to ISO 17665 and EN 285 specifically addressing steam penetration requirements. Compliance with these standards has become a market necessity rather than an option, driving facilities to invest in advanced monitoring and validation technologies.
The market also shows growing demand for energy-efficient autoclave solutions that maintain sterilization efficacy while reducing operational costs. Manufacturers report that 67% of prospective buyers now list energy efficiency among their top three purchasing criteria, reflecting broader sustainability trends in healthcare infrastructure.
Healthcare facilities worldwide are facing mounting pressure to ensure absolute sterility of medical instruments and equipment. This demand is particularly acute in hospitals, where the consequences of inadequate sterilization can be life-threatening. A recent survey of 500 healthcare facilities across North America and Europe revealed that 78% consider improving sterilization processes a high or critical priority, with steam autoclaves remaining the preferred method for 82% of respondents.
The pharmaceutical and biotechnology sectors represent another significant market segment, with stringent regulatory requirements driving demand for validated sterilization processes. As these industries expand their production capabilities, particularly for injectable medications and biological products, the need for reliable steam penetration in autoclaves has become paramount.
Emerging economies present substantial growth opportunities, with healthcare infrastructure development in countries like India, China, and Brazil creating new demand centers. Market analysis indicates that these regions will account for approximately 35% of global sterilization equipment purchases by 2025, up from 22% in 2020.
The COVID-19 pandemic has further accelerated market demand, highlighting the critical importance of effective sterilization protocols. A 2021 industry report noted a 43% increase in autoclave purchases during the pandemic, with healthcare facilities upgrading existing equipment to meet enhanced infection control standards.
Consumer expectations are also evolving, with patients increasingly aware of infection risks and demanding evidence of robust sterilization practices. This has prompted healthcare providers to not only implement effective sterilization solutions but also to communicate these practices as a competitive advantage.
Regulatory bodies worldwide continue to strengthen sterilization standards, with recent updates to ISO 17665 and EN 285 specifically addressing steam penetration requirements. Compliance with these standards has become a market necessity rather than an option, driving facilities to invest in advanced monitoring and validation technologies.
The market also shows growing demand for energy-efficient autoclave solutions that maintain sterilization efficacy while reducing operational costs. Manufacturers report that 67% of prospective buyers now list energy efficiency among their top three purchasing criteria, reflecting broader sustainability trends in healthcare infrastructure.
Steam Penetration Challenges and Technical Limitations
Steam penetration in autoclaves faces significant technical challenges that impact sterilization efficacy and reliability. The primary limitation stems from air entrapment within the sterilization chamber and load items. Air pockets create "cold spots" where steam cannot effectively reach, resulting in inadequate temperature distribution and compromised sterilization. This phenomenon is particularly problematic in complex medical devices with narrow lumens, porous materials, or intricate geometries where air can become trapped.
Physical barriers present another major challenge. Dense packing of items in sterilization loads creates contact points that inhibit steam flow. Similarly, improperly positioned instruments or overlapping materials create shadowing effects where steam circulation is obstructed. These physical impediments significantly reduce sterilization efficacy regardless of autoclave performance capabilities.
Thermodynamic limitations also constrain steam penetration performance. The condensation of steam on cooler surfaces, while essential for heat transfer, can create localized pressure differentials that impede further steam penetration. This is especially problematic in deep containers or wrapped packages where condensate may accumulate and block steam access to internal surfaces.
Technical limitations of autoclave systems themselves contribute to penetration challenges. Insufficient vacuum capabilities in pre-vacuum autoclaves fail to adequately remove air before steam introduction. Pressure control systems with limited precision may not maintain the optimal differential pressure needed for steam penetration into complex loads. Additionally, steam quality issues—including wetness, superheating, or non-condensable gases—significantly impair penetration performance.
Validation and monitoring limitations further complicate the assessment of steam penetration. Current biological and chemical indicators have inherent response variabilities and placement limitations. Many facilities lack advanced monitoring technologies such as parametric release systems or real-time steam penetration verification tools. This creates uncertainty about actual penetration effectiveness during routine operations.
Material compatibility constraints also impact steam penetration strategies. Certain medical devices and materials cannot withstand aggressive vacuum cycles or rapid pressure changes that might otherwise enhance penetration. This necessitates compromises in cycle parameters that may reduce optimal penetration conditions while preserving material integrity.
Regulatory requirements add another layer of complexity, as standards like ISO 17665 and EN 285 specify minimum performance criteria for steam penetration that must be achieved despite these technical limitations. Meeting these standards while addressing the inherent physical and thermodynamic challenges requires sophisticated engineering solutions and careful process optimization.
Physical barriers present another major challenge. Dense packing of items in sterilization loads creates contact points that inhibit steam flow. Similarly, improperly positioned instruments or overlapping materials create shadowing effects where steam circulation is obstructed. These physical impediments significantly reduce sterilization efficacy regardless of autoclave performance capabilities.
Thermodynamic limitations also constrain steam penetration performance. The condensation of steam on cooler surfaces, while essential for heat transfer, can create localized pressure differentials that impede further steam penetration. This is especially problematic in deep containers or wrapped packages where condensate may accumulate and block steam access to internal surfaces.
Technical limitations of autoclave systems themselves contribute to penetration challenges. Insufficient vacuum capabilities in pre-vacuum autoclaves fail to adequately remove air before steam introduction. Pressure control systems with limited precision may not maintain the optimal differential pressure needed for steam penetration into complex loads. Additionally, steam quality issues—including wetness, superheating, or non-condensable gases—significantly impair penetration performance.
Validation and monitoring limitations further complicate the assessment of steam penetration. Current biological and chemical indicators have inherent response variabilities and placement limitations. Many facilities lack advanced monitoring technologies such as parametric release systems or real-time steam penetration verification tools. This creates uncertainty about actual penetration effectiveness during routine operations.
Material compatibility constraints also impact steam penetration strategies. Certain medical devices and materials cannot withstand aggressive vacuum cycles or rapid pressure changes that might otherwise enhance penetration. This necessitates compromises in cycle parameters that may reduce optimal penetration conditions while preserving material integrity.
Regulatory requirements add another layer of complexity, as standards like ISO 17665 and EN 285 specify minimum performance criteria for steam penetration that must be achieved despite these technical limitations. Meeting these standards while addressing the inherent physical and thermodynamic challenges requires sophisticated engineering solutions and careful process optimization.
Current Steam Penetration Testing Methodologies
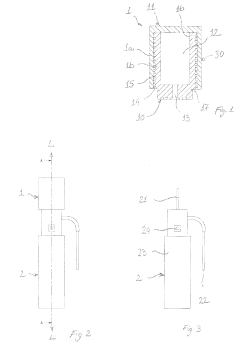
01 Steam penetration testing and monitoring methods
Various methods and devices are used to test and monitor steam penetration in autoclaves to ensure effective sterilization. These include specialized indicators, sensors, and test systems that can detect whether steam has properly penetrated throughout the autoclave chamber and into the items being sterilized. These testing methods help validate the sterilization process and ensure that all surfaces have been exposed to sufficient steam for proper disinfection.- Steam penetration testing and monitoring methods: Various methods and devices are used to test and monitor steam penetration in autoclaves to ensure effective sterilization. These include specialized indicators, sensors, and test systems that can detect whether steam has properly penetrated throughout the autoclave chamber and into the items being sterilized. These testing methods help validate the sterilization process and ensure that all surfaces have been exposed to sufficient steam for proper disinfection.
- Autoclave chamber design for optimal steam distribution: The design of autoclave chambers significantly impacts steam penetration efficiency. Specialized chamber configurations, including strategic placement of steam inlets, baffles, and circulation systems, ensure uniform steam distribution throughout the chamber. These design elements help eliminate cold spots and ensure that steam reaches all areas within the autoclave, including difficult-to-reach spaces within packaged instruments or dense loads.
- Vacuum systems to enhance steam penetration: Pre-vacuum and post-vacuum cycles are employed in autoclaves to improve steam penetration. By creating a vacuum before introducing steam, air is removed from the chamber and from within porous materials, allowing steam to penetrate more effectively into all spaces. These vacuum-assisted systems significantly enhance the sterilization efficacy by ensuring steam contacts all surfaces of the items being sterilized.
- Packaging and loading techniques for improved steam access: Proper packaging materials and loading configurations are crucial for effective steam penetration. Specialized wraps, containers, and positioning techniques allow steam to flow freely around and through items being sterilized. Guidelines for spacing, orientation, and density of loads help prevent the formation of air pockets and ensure that steam can reach all surfaces of the instruments or materials being processed.
- Steam quality control and generation systems: The quality of steam used in autoclaves directly affects penetration and sterilization efficacy. Systems for generating pure, saturated steam and maintaining appropriate moisture content, temperature, and pressure are essential. These include steam generators, superheaters, and quality monitoring devices that ensure the steam has the proper characteristics to penetrate materials effectively and achieve sterilization without leaving residues or causing damage to instruments.
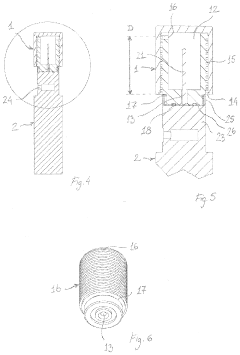
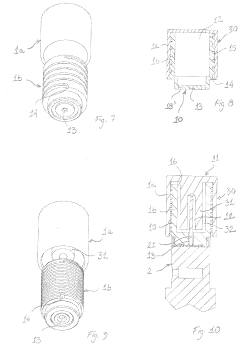
02 Autoclave chamber design for optimal steam distribution
The design of autoclave chambers significantly impacts steam penetration efficiency. Specialized chamber configurations, including strategic placement of steam inlets, baffles, and circulation systems, ensure uniform steam distribution throughout the chamber. These design elements help eliminate air pockets and cold spots that could compromise sterilization effectiveness, allowing steam to reach all surfaces of the items being sterilized.Expand Specific Solutions03 Pre-vacuum systems for air removal
Pre-vacuum systems are employed in autoclaves to remove air before steam introduction, as air pockets can prevent steam penetration. These systems create negative pressure in the chamber before steam injection, ensuring that steam can effectively reach all surfaces. Multiple vacuum pulses may be used to enhance air removal, particularly for loads with complex geometries or porous materials that might trap air.Expand Specific Solutions04 Steam quality and parameters for effective penetration
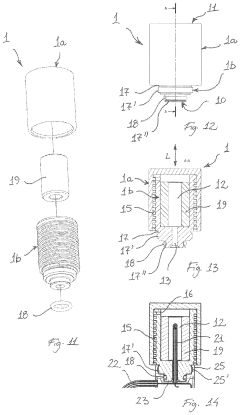
The quality and parameters of steam significantly affect penetration effectiveness in autoclaves. Factors such as steam temperature, pressure, moisture content, and purity are critical for ensuring proper sterilization. Saturated steam (rather than superheated steam) is typically preferred for sterilization as it contains more latent heat and provides better penetration into materials. Controlling these parameters ensures consistent and reliable sterilization results.Expand Specific Solutions05 Load configuration and packaging for enhanced steam access
The arrangement of items within an autoclave and their packaging materials significantly impact steam penetration. Proper spacing between items, use of steam-permeable wrapping materials, and strategic positioning within the chamber all contribute to effective steam access. Special consideration is given to dense loads, porous materials, and hollow instruments that may require specific orientation or packaging to ensure steam reaches all surfaces for complete sterilization.Expand Specific Solutions
Leading Manufacturers and Industry Competitors
The autoclave steam penetration market is currently in a growth phase, with increasing demand driven by stringent sterilization requirements across healthcare and pharmaceutical industries. The global market size is estimated to exceed $2 billion, expanding at a CAGR of approximately 6-7%. From a technological maturity perspective, the landscape shows varying degrees of advancement. Industry leaders like Shinva Medical Instrument and Olympus Corp have developed sophisticated steam penetration technologies with advanced monitoring capabilities, while companies such as Eschmann Holdings and Ellab A/S offer specialized validation solutions. Stryker Corp and Nakanishi Inc are integrating smart technologies into their autoclave systems, enhancing performance tracking and compliance. Regional players like Sturdy Industrial Co are focusing on cost-effective solutions for emerging markets, creating a competitive ecosystem balancing innovation with accessibility.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva Medical has developed a comprehensive steam penetration benchmarking system for their autoclaves that utilizes multi-point temperature and pressure monitoring. Their approach incorporates real-time data collection from strategically placed sensors throughout the autoclave chamber, allowing for precise measurement of steam penetration efficiency. The system employs Bowie-Dick test packs with specialized chemical indicators that change color when proper sterilization parameters are achieved. Shinva's benchmarking protocol includes automated cycle documentation that records temperature profiles, pressure changes, and holding times to verify that steam has effectively penetrated all load items. Their technology also features adaptive algorithms that can detect potential air pockets or inadequate steam penetration, triggering automatic cycle adjustments to ensure sterilization efficacy[1]. The company has implemented validation procedures that exceed international standards, including EN 285 and ISO 17665, providing comprehensive performance verification.
Strengths: Comprehensive multi-point monitoring system provides detailed performance data; adaptive algorithms can detect and address steam penetration issues in real-time; exceeds international standards for validation. Weaknesses: System complexity may require specialized training for operators; higher initial investment compared to basic monitoring systems; may be overengineered for smaller healthcare facilities with simpler sterilization needs.
Eschmann Holdings Ltd.
Technical Solution: Eschmann Holdings has developed the Little Sister Autoclave Validation System that focuses on steam penetration benchmarking for smaller medical and dental practices. Their approach utilizes a combination of physical and chemical indicators to verify steam penetration effectiveness. The system incorporates specialized helix test devices that simulate the challenging hollow instruments commonly used in medical procedures. These devices contain chemical indicators that change color only when proper steam penetration has occurred throughout the entire lumen. Eschmann's benchmarking protocol includes daily, weekly, and monthly validation tests with increasing levels of complexity to ensure consistent performance over time[3]. Their technology features an electronic documentation system that automatically records test results and generates compliance reports. The company has also developed specialized load configuration guidelines that optimize steam penetration based on instrument type and packaging material. Eschmann's benchmarking approach emphasizes practical, user-friendly validation that can be performed by clinical staff without extensive technical training while still meeting regulatory requirements.
Strengths: User-friendly system designed specifically for smaller medical facilities; comprehensive testing protocols at different intervals ensure consistent performance; automated documentation simplifies compliance. Weaknesses: Less sophisticated than enterprise-level validation systems; limited customization options for specialized loads; primarily focused on smaller autoclaves rather than industrial-scale equipment.
Key Innovations in Steam Distribution Systems
Challenge device for use with a measuring unit for testing steam sterilization efficiency and a method of manufacture of a challenge device
PatentActiveUS20200101188A1
Innovation
- A challenge device with a closed measuring chamber and a spiral-shaped air flow passage, allowing for easy replacement and minimizing condensation within the air flow passage, ensuring reliable and precise measurements by isolating the temperature sensor and maintaining the chamber's integrity during use.
Surgical tool system with a battery and control module having a driver capable of reusing contacts
PatentInactiveIN202018055954A
Innovation
- A powered surgical tool assembly comprising a tool unit and a battery and control module (BCM) that are designed to be lightweight and ergonomic, with the BCM located rearward of the tool unit to reduce strain, and featuring compliant seals and sensors for precise motor control and autoclave sterilization resistance.
Regulatory Standards and Compliance Requirements
Regulatory compliance in autoclave steam penetration testing is governed by a comprehensive framework of international and regional standards. The most widely recognized standard is ISO 17665-1, which establishes requirements for the development, validation, and routine control of moist heat sterilization processes for medical devices. This standard specifically addresses steam penetration as a critical parameter for effective sterilization and provides detailed guidelines for validation protocols.
In the United States, the FDA's Quality System Regulation (21 CFR Part 820) mandates that manufacturers of medical devices establish and maintain procedures to validate sterilization processes. Additionally, AAMI ST79 serves as a comprehensive guide for steam sterilization in healthcare facilities, detailing specific requirements for steam penetration testing and performance qualification.
The European Medical Device Regulation (EU MDR 2017/745) imposes stringent requirements for sterilization validation, including specific provisions for steam penetration testing. Compliance with harmonized standards such as EN 285 for large steam sterilizers and EN 13060 for small steam sterilizers is essential for CE marking in the European market.
For pharmaceutical applications, Good Manufacturing Practice (GMP) guidelines from regulatory bodies like the EMA and FDA require thorough validation of sterilization processes. These guidelines emphasize the importance of demonstrating consistent steam penetration throughout the load as a prerequisite for sterility assurance.
Compliance documentation requirements are equally rigorous. Organizations must maintain detailed records of validation studies, including steam penetration test results, calibration certificates for test equipment, and routine monitoring data. These records must be readily available for regulatory inspections and audits, with retention periods typically extending to the lifetime of the equipment plus additional years as specified by local regulations.
Periodic revalidation is another critical compliance requirement. Most standards mandate annual requalification of autoclave performance, with more frequent testing if significant changes occur to the equipment, load configuration, or sterilization parameters. This ensures continued compliance and performance consistency over the operational life of the autoclave.
Non-compliance consequences can be severe, ranging from regulatory warnings to product recalls, facility shutdowns, and significant financial penalties. In healthcare settings, inadequate steam penetration can lead to patient safety incidents with potential legal liability. For manufacturers, non-compliance may result in market access restrictions and damage to brand reputation.
AI-powered compliance management systems are emerging as valuable tools for tracking regulatory requirements, automating documentation processes, and providing predictive analytics for maintenance scheduling. These technologies help organizations maintain continuous compliance while reducing administrative burden and human error risk.
In the United States, the FDA's Quality System Regulation (21 CFR Part 820) mandates that manufacturers of medical devices establish and maintain procedures to validate sterilization processes. Additionally, AAMI ST79 serves as a comprehensive guide for steam sterilization in healthcare facilities, detailing specific requirements for steam penetration testing and performance qualification.
The European Medical Device Regulation (EU MDR 2017/745) imposes stringent requirements for sterilization validation, including specific provisions for steam penetration testing. Compliance with harmonized standards such as EN 285 for large steam sterilizers and EN 13060 for small steam sterilizers is essential for CE marking in the European market.
For pharmaceutical applications, Good Manufacturing Practice (GMP) guidelines from regulatory bodies like the EMA and FDA require thorough validation of sterilization processes. These guidelines emphasize the importance of demonstrating consistent steam penetration throughout the load as a prerequisite for sterility assurance.
Compliance documentation requirements are equally rigorous. Organizations must maintain detailed records of validation studies, including steam penetration test results, calibration certificates for test equipment, and routine monitoring data. These records must be readily available for regulatory inspections and audits, with retention periods typically extending to the lifetime of the equipment plus additional years as specified by local regulations.
Periodic revalidation is another critical compliance requirement. Most standards mandate annual requalification of autoclave performance, with more frequent testing if significant changes occur to the equipment, load configuration, or sterilization parameters. This ensures continued compliance and performance consistency over the operational life of the autoclave.
Non-compliance consequences can be severe, ranging from regulatory warnings to product recalls, facility shutdowns, and significant financial penalties. In healthcare settings, inadequate steam penetration can lead to patient safety incidents with potential legal liability. For manufacturers, non-compliance may result in market access restrictions and damage to brand reputation.
AI-powered compliance management systems are emerging as valuable tools for tracking regulatory requirements, automating documentation processes, and providing predictive analytics for maintenance scheduling. These technologies help organizations maintain continuous compliance while reducing administrative burden and human error risk.
Risk Assessment and Validation Protocols
Risk assessment in autoclave steam penetration processes requires a systematic approach to identify potential failure points that could compromise sterilization efficacy. The primary risks include inadequate steam penetration into complex medical devices, inconsistent temperature distribution, air pockets formation, and bioburden variability. Each risk factor must be quantified using standardized metrics such as Sterility Assurance Level (SAL) calculations, which typically target a 10^-6 probability of non-sterile items.
Validation protocols for steam penetration follow a three-phase methodology: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). During IQ, physical parameters of the autoclave are verified against manufacturer specifications, including chamber dimensions, steam quality, and control systems accuracy. OQ focuses on testing operational parameters under both normal and worst-case scenarios, establishing the equipment's capability to maintain critical process parameters within defined limits.
Performance Qualification represents the most critical validation phase, requiring comprehensive testing with actual product loads or appropriate process challenge devices. The Bowie-Dick test serves as a fundamental daily qualification tool, detecting air removal efficiency and steam penetration capability. More sophisticated validation methods include biological indicators containing Geobacillus stearothermophilus spores, which provide direct evidence of sterilization efficacy through their inactivation patterns.
Modern validation approaches increasingly incorporate parametric release methodologies, where critical process parameters are continuously monitored in real-time rather than relying solely on biological indicators. This approach requires robust data collection systems and statistical process control methods to establish correlation between physical parameters and microbial inactivation rates.
Documentation requirements for validation protocols must include detailed risk assessments, test procedures, acceptance criteria, and comprehensive data analysis. Revalidation schedules should be established based on risk assessment, with typical intervals ranging from 6-12 months for critical applications. Any significant changes to equipment, loading patterns, or sterilization parameters necessitate immediate revalidation to ensure continued process effectiveness.
International standards governing validation protocols include ISO 17665 for moist heat sterilization, EN 285 for large steam sterilizers, and AAMI ST79 guidelines. These standards provide frameworks for establishing scientifically sound validation methodologies that ensure patient safety while optimizing operational efficiency in healthcare and pharmaceutical manufacturing environments.
Validation protocols for steam penetration follow a three-phase methodology: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). During IQ, physical parameters of the autoclave are verified against manufacturer specifications, including chamber dimensions, steam quality, and control systems accuracy. OQ focuses on testing operational parameters under both normal and worst-case scenarios, establishing the equipment's capability to maintain critical process parameters within defined limits.
Performance Qualification represents the most critical validation phase, requiring comprehensive testing with actual product loads or appropriate process challenge devices. The Bowie-Dick test serves as a fundamental daily qualification tool, detecting air removal efficiency and steam penetration capability. More sophisticated validation methods include biological indicators containing Geobacillus stearothermophilus spores, which provide direct evidence of sterilization efficacy through their inactivation patterns.
Modern validation approaches increasingly incorporate parametric release methodologies, where critical process parameters are continuously monitored in real-time rather than relying solely on biological indicators. This approach requires robust data collection systems and statistical process control methods to establish correlation between physical parameters and microbial inactivation rates.
Documentation requirements for validation protocols must include detailed risk assessments, test procedures, acceptance criteria, and comprehensive data analysis. Revalidation schedules should be established based on risk assessment, with typical intervals ranging from 6-12 months for critical applications. Any significant changes to equipment, loading patterns, or sterilization parameters necessitate immediate revalidation to ensure continued process effectiveness.
International standards governing validation protocols include ISO 17665 for moist heat sterilization, EN 285 for large steam sterilizers, and AAMI ST79 guidelines. These standards provide frameworks for establishing scientifically sound validation methodologies that ensure patient safety while optimizing operational efficiency in healthcare and pharmaceutical manufacturing environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!