Autoclave Contamination Sources and Mitigation Techniques
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Background and Sterilization Goals
Autoclaves have been a cornerstone of sterilization technology since their invention in the 19th century by Charles Chamberland, a colleague of Louis Pasteur. These pressure vessels utilize saturated steam under pressure to eliminate microorganisms through protein denaturation and coagulation. The evolution of autoclave technology has progressed from simple pressure cookers to sophisticated computer-controlled systems capable of precise parameter management and validation.
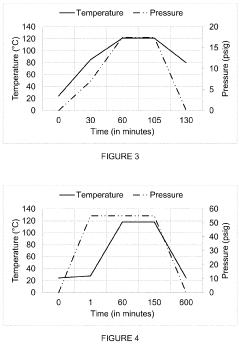
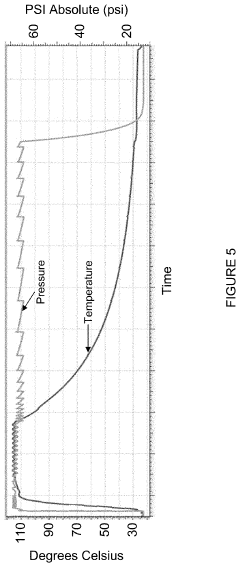
The fundamental principle of autoclave sterilization remains consistent: achieving and maintaining specific combinations of temperature, pressure, and time to ensure complete microbial inactivation. Modern autoclaves typically operate at 121°C (250°F) at 15 psi for 15-20 minutes or 134°C (273°F) at 30 psi for 3-4 minutes, depending on the load characteristics and sterilization requirements.
The primary goal of autoclave sterilization is to achieve a Sterility Assurance Level (SAL) of 10^-6, meaning a probability of less than one in a million that a viable microorganism remains after processing. This standard is particularly critical in healthcare, pharmaceutical manufacturing, and laboratory settings where sterility is paramount for patient safety and experimental integrity.
Recent technological advancements have focused on enhancing efficiency, reducing cycle times, improving energy consumption, and developing more sophisticated monitoring systems. The integration of IoT capabilities has enabled real-time monitoring, remote operation, and comprehensive data logging for regulatory compliance and quality assurance purposes.
Contamination control represents a significant challenge in autoclave operations. Sources of contamination can originate from inadequate cleaning of items before sterilization, improper packaging, biofilm formation within the autoclave chamber or plumbing, poor steam quality, or procedural errors during loading and unloading. These contamination risks directly undermine the primary sterilization goals and can lead to serious consequences in clinical or production environments.
The evolution of autoclave technology has been driven by increasingly stringent regulatory requirements from bodies such as the FDA, EU MDR, and ISO standards. These regulations have pushed manufacturers to develop more reliable validation methods, better documentation systems, and enhanced fail-safe mechanisms to ensure consistent sterilization outcomes.
Current research in autoclave technology focuses on developing more environmentally friendly systems with reduced water and energy consumption, faster cycle times without compromising efficacy, and advanced materials that can better withstand repeated sterilization cycles. Additionally, there is growing interest in alternative sterilization methods that might complement traditional autoclaving for heat-sensitive materials.
The fundamental principle of autoclave sterilization remains consistent: achieving and maintaining specific combinations of temperature, pressure, and time to ensure complete microbial inactivation. Modern autoclaves typically operate at 121°C (250°F) at 15 psi for 15-20 minutes or 134°C (273°F) at 30 psi for 3-4 minutes, depending on the load characteristics and sterilization requirements.
The primary goal of autoclave sterilization is to achieve a Sterility Assurance Level (SAL) of 10^-6, meaning a probability of less than one in a million that a viable microorganism remains after processing. This standard is particularly critical in healthcare, pharmaceutical manufacturing, and laboratory settings where sterility is paramount for patient safety and experimental integrity.
Recent technological advancements have focused on enhancing efficiency, reducing cycle times, improving energy consumption, and developing more sophisticated monitoring systems. The integration of IoT capabilities has enabled real-time monitoring, remote operation, and comprehensive data logging for regulatory compliance and quality assurance purposes.
Contamination control represents a significant challenge in autoclave operations. Sources of contamination can originate from inadequate cleaning of items before sterilization, improper packaging, biofilm formation within the autoclave chamber or plumbing, poor steam quality, or procedural errors during loading and unloading. These contamination risks directly undermine the primary sterilization goals and can lead to serious consequences in clinical or production environments.
The evolution of autoclave technology has been driven by increasingly stringent regulatory requirements from bodies such as the FDA, EU MDR, and ISO standards. These regulations have pushed manufacturers to develop more reliable validation methods, better documentation systems, and enhanced fail-safe mechanisms to ensure consistent sterilization outcomes.
Current research in autoclave technology focuses on developing more environmentally friendly systems with reduced water and energy consumption, faster cycle times without compromising efficacy, and advanced materials that can better withstand repeated sterilization cycles. Additionally, there is growing interest in alternative sterilization methods that might complement traditional autoclaving for heat-sensitive materials.
Market Demand for Contamination-Free Sterilization
The global market for contamination-free sterilization solutions has experienced significant growth in recent years, driven primarily by heightened awareness of infection control and stringent regulatory requirements across healthcare, pharmaceutical, and biotechnology sectors. The autoclave sterilization market, valued at approximately $2.5 billion in 2022, is projected to grow at a compound annual growth rate of 6.8% through 2028, reflecting the increasing demand for reliable sterilization technologies.
Healthcare facilities represent the largest market segment, accounting for over 45% of the total demand. Hospitals, clinics, and ambulatory surgical centers require consistent, validated sterilization processes to prevent healthcare-associated infections (HAIs), which affect millions of patients annually and result in substantial healthcare costs. The economic burden of HAIs in the United States alone exceeds $30 billion yearly, creating a compelling financial incentive for investment in advanced contamination prevention technologies.
The pharmaceutical and biotechnology industries constitute the fastest-growing segment, with increasing requirements for sterile manufacturing environments. The production of biologics, vaccines, and cell therapies demands exceptionally high standards of sterility, driving investment in advanced autoclave systems with enhanced contamination control features. Market research indicates that 78% of pharmaceutical manufacturers plan to upgrade their sterilization infrastructure within the next three years.
Regional analysis reveals that North America currently dominates the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the most rapid growth, with China and India emerging as key markets due to expanding healthcare infrastructure and increasing pharmaceutical manufacturing capacity.
Consumer preferences are shifting toward autoclave systems with integrated contamination monitoring and prevention features. End-users increasingly demand systems with automated cycle documentation, real-time monitoring capabilities, and predictive maintenance functions to ensure consistent sterilization outcomes. A recent industry survey indicated that 67% of procurement decision-makers consider contamination prevention features as "very important" or "critical" when selecting new autoclave equipment.
Regulatory trends are further shaping market demand, with agencies worldwide implementing more stringent requirements for sterilization validation and documentation. The FDA's emphasis on quality risk management and the EU Medical Device Regulation's focus on process validation have created additional market pressure for contamination-resistant autoclave technologies. These regulatory developments have accelerated the adoption of advanced sterilization solutions, particularly in regulated industries where non-compliance can result in significant penalties and reputational damage.
Healthcare facilities represent the largest market segment, accounting for over 45% of the total demand. Hospitals, clinics, and ambulatory surgical centers require consistent, validated sterilization processes to prevent healthcare-associated infections (HAIs), which affect millions of patients annually and result in substantial healthcare costs. The economic burden of HAIs in the United States alone exceeds $30 billion yearly, creating a compelling financial incentive for investment in advanced contamination prevention technologies.
The pharmaceutical and biotechnology industries constitute the fastest-growing segment, with increasing requirements for sterile manufacturing environments. The production of biologics, vaccines, and cell therapies demands exceptionally high standards of sterility, driving investment in advanced autoclave systems with enhanced contamination control features. Market research indicates that 78% of pharmaceutical manufacturers plan to upgrade their sterilization infrastructure within the next three years.
Regional analysis reveals that North America currently dominates the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the most rapid growth, with China and India emerging as key markets due to expanding healthcare infrastructure and increasing pharmaceutical manufacturing capacity.
Consumer preferences are shifting toward autoclave systems with integrated contamination monitoring and prevention features. End-users increasingly demand systems with automated cycle documentation, real-time monitoring capabilities, and predictive maintenance functions to ensure consistent sterilization outcomes. A recent industry survey indicated that 67% of procurement decision-makers consider contamination prevention features as "very important" or "critical" when selecting new autoclave equipment.
Regulatory trends are further shaping market demand, with agencies worldwide implementing more stringent requirements for sterilization validation and documentation. The FDA's emphasis on quality risk management and the EU Medical Device Regulation's focus on process validation have created additional market pressure for contamination-resistant autoclave technologies. These regulatory developments have accelerated the adoption of advanced sterilization solutions, particularly in regulated industries where non-compliance can result in significant penalties and reputational damage.
Current Challenges in Autoclave Contamination Control
Despite significant advancements in autoclave technology, contamination control remains a persistent challenge across various industries, particularly in healthcare, pharmaceutical manufacturing, and laboratory settings. Current autoclave contamination issues stem from multiple sources that compromise sterilization efficacy and potentially endanger patients or product integrity.
Water quality represents a primary concern, as mineral deposits from hard water can accumulate on instruments and autoclave chambers, creating protective barriers for microorganisms and reducing steam penetration. These deposits also accelerate corrosion of instruments and autoclave components, further complicating sterilization processes.
Biofilm formation presents another significant challenge, with microorganisms creating protective extracellular matrices that demonstrate remarkable resistance to standard sterilization parameters. These biofilms can develop in water lines, drains, and hard-to-reach areas of the autoclave chamber, requiring specialized cleaning protocols beyond routine maintenance.
Improper packaging and loading practices continue to undermine sterilization effectiveness. When instruments are improperly wrapped or autoclave chambers are overloaded, steam cannot adequately penetrate all surfaces, creating "cold spots" where microorganisms may survive. Similarly, inadequate cleaning of instruments prior to autoclaving leaves organic residues that can shield microorganisms from steam exposure.
Maintenance deficiencies represent a systemic challenge across facilities. Worn door gaskets, calibration drift in temperature and pressure sensors, and malfunctioning steam generators directly impact sterilization efficacy. Many facilities lack comprehensive preventive maintenance programs, relying instead on reactive approaches when failures occur.
Documentation and validation gaps further complicate contamination control efforts. Inconsistent monitoring practices, inadequate record-keeping of cycle parameters, and insufficient biological indicator testing make it difficult to identify contamination sources when they arise. This is particularly problematic in facilities without electronic tracking systems for sterilization cycles.
Cross-contamination between loads represents an emerging concern, especially in high-throughput environments where rapid turnaround is prioritized over thorough cleaning between cycles. Residual contaminants from previous loads can be transferred to subsequent batches, creating persistent contamination issues that prove difficult to trace and eliminate.
Air removal inefficiencies in pre-vacuum autoclaves can create air pockets that prevent steam contact with all surfaces. Modern autoclaves incorporate sophisticated air removal systems, but older models or improperly maintained units often struggle with this fundamental requirement for effective sterilization.
Water quality represents a primary concern, as mineral deposits from hard water can accumulate on instruments and autoclave chambers, creating protective barriers for microorganisms and reducing steam penetration. These deposits also accelerate corrosion of instruments and autoclave components, further complicating sterilization processes.
Biofilm formation presents another significant challenge, with microorganisms creating protective extracellular matrices that demonstrate remarkable resistance to standard sterilization parameters. These biofilms can develop in water lines, drains, and hard-to-reach areas of the autoclave chamber, requiring specialized cleaning protocols beyond routine maintenance.
Improper packaging and loading practices continue to undermine sterilization effectiveness. When instruments are improperly wrapped or autoclave chambers are overloaded, steam cannot adequately penetrate all surfaces, creating "cold spots" where microorganisms may survive. Similarly, inadequate cleaning of instruments prior to autoclaving leaves organic residues that can shield microorganisms from steam exposure.
Maintenance deficiencies represent a systemic challenge across facilities. Worn door gaskets, calibration drift in temperature and pressure sensors, and malfunctioning steam generators directly impact sterilization efficacy. Many facilities lack comprehensive preventive maintenance programs, relying instead on reactive approaches when failures occur.
Documentation and validation gaps further complicate contamination control efforts. Inconsistent monitoring practices, inadequate record-keeping of cycle parameters, and insufficient biological indicator testing make it difficult to identify contamination sources when they arise. This is particularly problematic in facilities without electronic tracking systems for sterilization cycles.
Cross-contamination between loads represents an emerging concern, especially in high-throughput environments where rapid turnaround is prioritized over thorough cleaning between cycles. Residual contaminants from previous loads can be transferred to subsequent batches, creating persistent contamination issues that prove difficult to trace and eliminate.
Air removal inefficiencies in pre-vacuum autoclaves can create air pockets that prevent steam contact with all surfaces. Modern autoclaves incorporate sophisticated air removal systems, but older models or improperly maintained units often struggle with this fundamental requirement for effective sterilization.
Existing Contamination Mitigation Solutions
01 Sterilization methods to prevent autoclave contamination
Various sterilization methods can be employed to prevent contamination in autoclaves. These include steam sterilization, chemical sterilization, and radiation sterilization. Proper sterilization protocols ensure that microorganisms are effectively eliminated, reducing the risk of contamination during the autoclave process. These methods can be optimized for different types of materials and equipment to ensure thorough decontamination.- Methods for detecting and preventing autoclave contamination: Various methods and systems have been developed to detect and prevent contamination in autoclaves. These include monitoring systems that can identify potential contaminants before they affect sterilization processes, preventive maintenance protocols, and specialized indicators that change color or appearance when contamination is present. These detection methods help ensure the integrity of the sterilization process and prevent cross-contamination of medical instruments and equipment.
- Sterilization validation and monitoring systems: Validation and monitoring systems are essential for ensuring autoclave effectiveness and preventing contamination. These systems include biological indicators, chemical indicators, and electronic monitoring devices that verify sterilization parameters such as temperature, pressure, and exposure time. Regular validation testing helps identify potential contamination sources and ensures that sterilization processes meet required standards for medical and laboratory applications.
- Cleaning agents and procedures for autoclave decontamination: Specialized cleaning agents and procedures have been developed to remove and prevent contamination in autoclaves. These include enzymatic cleaners, descaling agents, and antimicrobial solutions designed to eliminate biofilms, mineral deposits, and other contaminants that can affect sterilization efficacy. Regular cleaning protocols and maintenance schedules help maintain autoclave performance and prevent cross-contamination between sterilization cycles.
- Design improvements to prevent autoclave contamination: Innovative autoclave designs incorporate features specifically aimed at preventing contamination. These include improved sealing mechanisms, advanced filtration systems, optimized chamber designs, and materials resistant to biofilm formation. Some designs also feature self-cleaning capabilities, improved drainage systems, and better air removal mechanisms to ensure complete sterilization and minimize the risk of contamination during operation.
- Contamination control in specialized autoclave applications: Specific industries and applications require specialized contamination control measures for autoclaves. These include pharmaceutical manufacturing, laboratory research, medical device production, and food processing. Tailored approaches address unique contamination risks in these environments, such as particulate control in cleanrooms, prevention of cross-contamination between product batches, and specialized validation protocols for high-risk applications.
02 Monitoring and detection systems for autoclave contamination
Advanced monitoring and detection systems can be implemented to identify contamination in autoclaves. These systems may include sensors that detect biological indicators, chemical indicators, or physical parameters that suggest contamination. Real-time monitoring allows for immediate intervention when contamination is detected, preventing the processing of improperly sterilized items. These systems enhance the reliability and safety of autoclave operations.Expand Specific Solutions03 Design features to minimize autoclave contamination
Specific design features can be incorporated into autoclaves to minimize contamination risks. These include sealed chambers, filtered air systems, and specialized materials that resist microbial growth. Proper drainage systems prevent the accumulation of water that could harbor contaminants. The design may also include separate clean and dirty areas to prevent cross-contamination during loading and unloading processes.Expand Specific Solutions04 Cleaning and maintenance protocols for autoclaves
Regular cleaning and maintenance protocols are essential for preventing contamination in autoclaves. These protocols may include daily cleaning procedures, periodic deep cleaning, and scheduled maintenance checks. Proper cleaning agents that effectively remove organic matter and microbial biofilms should be used. Maintenance includes checking seals, valves, and filters to ensure they are functioning correctly and not contributing to contamination issues.Expand Specific Solutions05 Validation and quality control for autoclave processes
Validation and quality control measures are crucial for ensuring that autoclave processes effectively prevent contamination. These measures include regular testing of autoclave performance, validation of sterilization cycles, and quality control checks of processed items. Standardized protocols for validation ensure consistency and reliability in autoclave operations. Documentation and record-keeping of validation results provide evidence of compliance with sterilization standards.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The autoclave contamination management market is in a growth phase, driven by increasing healthcare standards and industrial sterilization needs. The market is expanding at approximately 6-8% annually, with significant potential in healthcare, pharmaceutical, and food processing sectors. Technologically, the field shows moderate maturity with ongoing innovation in contamination detection and prevention systems. Leading players include STERIS, Inc. and Olympus Corp., who focus on advanced monitoring solutions, while Shimadzu Corp. and Nakanishi, Inc. are developing precision contamination detection technologies. Chinese manufacturers like Shinva Medical and Zealway Instrument are rapidly gaining market share through cost-effective solutions, while research institutions such as Université Paris-Saclay and CNRS are advancing fundamental contamination science and mitigation techniques.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva has developed a multi-layered approach to autoclave contamination control focusing on both prevention and detection. Their technology includes specialized water pretreatment systems designed specifically for autoclave feed water, incorporating multi-stage filtration, UV treatment, and continuous conductivity monitoring. Shinva's autoclaves feature proprietary chamber designs with electro-polished surfaces (Ra<0.4μm) that minimize biofilm attachment points and facilitate more effective cleaning. They've implemented automated detergent-based cleaning cycles with programmable parameters for different contamination scenarios. Their systems include real-time monitoring of steam quality parameters with automated cycle abortion when parameters fall outside acceptable ranges, preventing contaminated loads from being processed as sterile.
Strengths: Strong focus on preventative design elements and comprehensive water quality management. Their systems are designed with consideration for the specific contamination challenges in developing markets. Weaknesses: Their detection technologies are less sophisticated than some Western competitors, and their documentation/validation support is less comprehensive.
Olympus Corp.
Technical Solution: Olympus has focused on contamination control specifically for endoscope reprocessing autoclaves, addressing the unique challenges of these complex medical devices. Their technology includes specialized detergent formulations designed to break down biofilms in narrow lumens and channels of endoscopes. Olympus has developed automated leak testing systems integrated into their autoclaves to detect microscopic breaches that could harbor contaminants. Their OER-Pro system incorporates high-resolution flow sensors to verify proper irrigation of all endoscope channels during sterilization. Olympus's contamination mitigation approach includes disposable valves and caps to eliminate cross-contamination points, and their autoclaves feature RFID tracking of endoscopes to ensure proper reprocessing protocols are followed for each specific device type.
Strengths: Unparalleled expertise in endoscope reprocessing with technologies specifically designed for these challenging devices. Their systems address the full reprocessing workflow rather than just the autoclave cycle itself. Weaknesses: Their solutions are highly specialized for flexible endoscopes and less applicable to general autoclave applications. Their proprietary consumables create significant ongoing operational costs.
Critical Patents and Research on Contamination Prevention
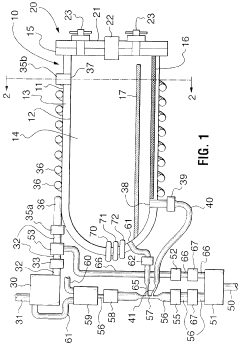
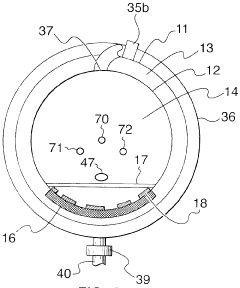
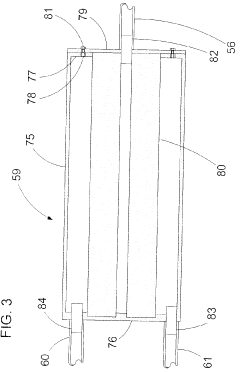
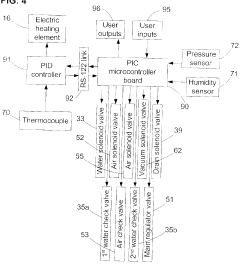
Fixed vacuum-insulated saturated steam autoclave
PatentInactiveCA2559406A1
Innovation
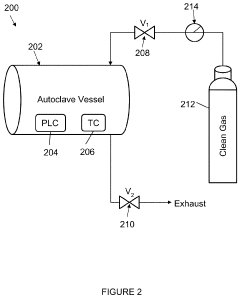
- A double-walled vacuum-sealed vessel with a self-contained water supply and airflow system, using positive and negative air pressures to rapidly generate and remove steam, combined with a PID temperature controller and PIC microprocessor for precise temperature control, ensures a consistent sterilization environment within a portable, insulated chamber.
Autoclave operation to accommodate fluid sterilization cycles
PatentInactiveUS20200179548A1
Innovation
- The autoclave system pre-pressurizes to an initial pressure before heating, maintaining pressure within ±10% of the initial level during temperature increase, and then cools, using a programmable logic controller and temperature controller to manage pressure and temperature profiles.
Validation Protocols and Quality Assurance Standards
Validation protocols and quality assurance standards form the cornerstone of effective autoclave contamination control strategies. The implementation of robust validation methodologies ensures that sterilization processes consistently achieve their intended outcomes while maintaining compliance with regulatory requirements. Current industry standards, including ISO 17665 for moist heat sterilization and USP <1211> for sterilization and sterility assurance, provide comprehensive frameworks for validation procedures specific to autoclave operations.
The validation lifecycle for autoclave processes typically encompasses installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) phases. During IQ, all autoclave components are verified against design specifications, with particular attention to steam quality parameters and chamber integrity that could impact contamination risk. OQ procedures focus on demonstrating that the autoclave operates within established parameters across its intended operating range, including temperature distribution studies that identify potential cold spots where contamination might persist.
Performance qualification represents the most critical validation phase for contamination control, requiring biological indicator challenges using appropriate test organisms such as Geobacillus stearothermophilus. These microorganisms, selected for their high resistance to steam sterilization, provide definitive evidence of sterilization efficacy. Modern validation approaches increasingly incorporate parametric release methodologies, where critical process parameters are continuously monitored to provide real-time assurance of sterility without relying solely on biological indicators.
Quality assurance programs supporting autoclave operations must include regular revalidation schedules, typically conducted annually or after significant maintenance events. Comprehensive documentation systems track all validation activities, deviations, and corrective actions, establishing an audit trail that demonstrates ongoing compliance. Risk-based approaches to validation, as outlined in ICH Q9 guidelines, enable organizations to focus resources on the most critical contamination control points within the autoclave process.
Emerging trends in validation protocols include the implementation of Process Analytical Technology (PAT) for real-time monitoring of critical parameters and rapid microbial detection methods that can significantly reduce the time required for sterility confirmation. Additionally, the development of validation master plans (VMPs) specific to autoclave operations provides a strategic framework for ensuring comprehensive coverage of all contamination risks throughout the equipment lifecycle.
For organizations seeking to optimize their validation approaches, the integration of contamination risk assessment methodologies with validation protocols offers significant advantages. This integrated approach ensures that validation activities specifically target identified contamination sources and verify the effectiveness of implemented mitigation strategies, creating a continuous improvement cycle that enhances overall sterility assurance.
The validation lifecycle for autoclave processes typically encompasses installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) phases. During IQ, all autoclave components are verified against design specifications, with particular attention to steam quality parameters and chamber integrity that could impact contamination risk. OQ procedures focus on demonstrating that the autoclave operates within established parameters across its intended operating range, including temperature distribution studies that identify potential cold spots where contamination might persist.
Performance qualification represents the most critical validation phase for contamination control, requiring biological indicator challenges using appropriate test organisms such as Geobacillus stearothermophilus. These microorganisms, selected for their high resistance to steam sterilization, provide definitive evidence of sterilization efficacy. Modern validation approaches increasingly incorporate parametric release methodologies, where critical process parameters are continuously monitored to provide real-time assurance of sterility without relying solely on biological indicators.
Quality assurance programs supporting autoclave operations must include regular revalidation schedules, typically conducted annually or after significant maintenance events. Comprehensive documentation systems track all validation activities, deviations, and corrective actions, establishing an audit trail that demonstrates ongoing compliance. Risk-based approaches to validation, as outlined in ICH Q9 guidelines, enable organizations to focus resources on the most critical contamination control points within the autoclave process.
Emerging trends in validation protocols include the implementation of Process Analytical Technology (PAT) for real-time monitoring of critical parameters and rapid microbial detection methods that can significantly reduce the time required for sterility confirmation. Additionally, the development of validation master plans (VMPs) specific to autoclave operations provides a strategic framework for ensuring comprehensive coverage of all contamination risks throughout the equipment lifecycle.
For organizations seeking to optimize their validation approaches, the integration of contamination risk assessment methodologies with validation protocols offers significant advantages. This integrated approach ensures that validation activities specifically target identified contamination sources and verify the effectiveness of implemented mitigation strategies, creating a continuous improvement cycle that enhances overall sterility assurance.
Environmental Impact and Sustainability Considerations
Autoclave sterilization processes, while effective for ensuring sterility in medical and industrial settings, carry significant environmental implications that warrant careful consideration. The energy consumption of autoclaves represents a major environmental concern, with standard cycles requiring substantial electricity or steam generation, contributing to carbon emissions and resource depletion. A typical medical autoclave can consume between 2-5 kWh per cycle, with larger industrial units consuming considerably more, highlighting the need for energy-efficient designs and operational protocols.
Water usage presents another critical environmental challenge, particularly in water-stressed regions. Conventional autoclaves may use 50-100 gallons per cycle for steam generation and cooling processes. This consumption can be reduced through water recycling systems and condensate recovery technologies, which can recapture up to 80% of process water for reuse, significantly reducing the environmental footprint of sterilization operations.
Chemical additives used in autoclave processes, including cleaning agents, descaling compounds, and corrosion inhibitors, often contain environmentally persistent substances that may enter wastewater streams. Modern formulations increasingly incorporate biodegradable alternatives with reduced environmental persistence, while advanced wastewater treatment systems can neutralize or remove these compounds before discharge, minimizing ecological impact.
Sustainable autoclave design has emerged as a priority for manufacturers, with innovations focusing on reduced resource consumption and improved efficiency. Heat recovery systems can capture and repurpose waste heat, potentially reducing energy requirements by 15-30%. Additionally, vacuum-assisted cycles can decrease cycle times and associated resource consumption, while programmable controls optimize resource usage based on load characteristics and sterilization requirements.
The lifecycle assessment of autoclave equipment reveals opportunities for sustainability improvements through materials selection and end-of-life considerations. Manufacturers increasingly employ recyclable materials in construction, design for disassembly, and offer take-back programs for equipment refurbishment or responsible disposal. These approaches extend equipment lifespan and reduce waste generation, aligning with circular economy principles.
Regulatory frameworks worldwide are evolving to address the environmental impact of sterilization processes. Standards such as ISO 14001 provide guidelines for environmental management systems in healthcare facilities, while regional regulations increasingly mandate energy efficiency benchmarks and waste management protocols for sterilization equipment. Compliance with these frameworks drives continuous improvement in environmental performance across the autoclave industry.
Water usage presents another critical environmental challenge, particularly in water-stressed regions. Conventional autoclaves may use 50-100 gallons per cycle for steam generation and cooling processes. This consumption can be reduced through water recycling systems and condensate recovery technologies, which can recapture up to 80% of process water for reuse, significantly reducing the environmental footprint of sterilization operations.
Chemical additives used in autoclave processes, including cleaning agents, descaling compounds, and corrosion inhibitors, often contain environmentally persistent substances that may enter wastewater streams. Modern formulations increasingly incorporate biodegradable alternatives with reduced environmental persistence, while advanced wastewater treatment systems can neutralize or remove these compounds before discharge, minimizing ecological impact.
Sustainable autoclave design has emerged as a priority for manufacturers, with innovations focusing on reduced resource consumption and improved efficiency. Heat recovery systems can capture and repurpose waste heat, potentially reducing energy requirements by 15-30%. Additionally, vacuum-assisted cycles can decrease cycle times and associated resource consumption, while programmable controls optimize resource usage based on load characteristics and sterilization requirements.
The lifecycle assessment of autoclave equipment reveals opportunities for sustainability improvements through materials selection and end-of-life considerations. Manufacturers increasingly employ recyclable materials in construction, design for disassembly, and offer take-back programs for equipment refurbishment or responsible disposal. These approaches extend equipment lifespan and reduce waste generation, aligning with circular economy principles.
Regulatory frameworks worldwide are evolving to address the environmental impact of sterilization processes. Standards such as ISO 14001 provide guidelines for environmental management systems in healthcare facilities, while regional regulations increasingly mandate energy efficiency benchmarks and waste management protocols for sterilization equipment. Compliance with these frameworks drives continuous improvement in environmental performance across the autoclave industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!