Autoclave Validation: Minimum Load Temperature Achievement
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Validation Background and Objectives
Autoclave sterilization represents one of the most critical processes in medical device manufacturing, pharmaceutical production, and healthcare settings. Since its inception in the late 19th century, this technology has evolved from basic pressure cookers to sophisticated computer-controlled systems capable of precise temperature and pressure regulation. The fundamental principle remains unchanged: using saturated steam under pressure to achieve microbial inactivation through protein denaturation and coagulation.
The validation of autoclave processes has become increasingly important as regulatory requirements have tightened across industries. Organizations such as the FDA, ISO, and various pharmacopeias have established stringent guidelines for sterilization validation, with temperature achievement being a critical parameter. Historically, validation methodologies have evolved from simple biological indicators to comprehensive approaches incorporating physical, chemical, and biological monitoring systems.
Minimum load temperature achievement represents a particularly challenging aspect of autoclave validation. This parameter ensures that even the coldest point within the load reaches the minimum temperature required for sterilization efficacy. The technical objective of this validation is to demonstrate with statistical confidence that all items within the sterilization load consistently achieve the specified minimum temperature (typically 121°C for standard cycles) for the required duration.
Current validation protocols focus on identifying and monitoring cold spots within loads through strategic thermocouple placement. These protocols have evolved significantly over the past decades, moving from empirical approaches to science-based methodologies that incorporate heat transfer principles, load configuration analysis, and statistical process control techniques.
The technological trajectory in this field is moving toward more sophisticated monitoring systems, including wireless sensors, real-time monitoring capabilities, and advanced data analytics for predictive modeling. These innovations aim to enhance the reliability of temperature achievement verification while reducing validation time and resources.
Industry trends indicate growing interest in parametric release methodologies, where sterilization efficacy is determined primarily through physical and chemical parameters rather than biological indicators. This approach requires exceptionally robust temperature monitoring and validation protocols to ensure minimum temperature achievement throughout the load.
The ultimate goal of autoclave validation technology development is to establish methodologies that provide absolute assurance of sterility while optimizing cycle efficiency, reducing energy consumption, and accommodating increasingly complex medical devices and pharmaceutical products. Achieving these objectives requires continued innovation in temperature monitoring technology, load configuration analysis, and validation protocols.
The validation of autoclave processes has become increasingly important as regulatory requirements have tightened across industries. Organizations such as the FDA, ISO, and various pharmacopeias have established stringent guidelines for sterilization validation, with temperature achievement being a critical parameter. Historically, validation methodologies have evolved from simple biological indicators to comprehensive approaches incorporating physical, chemical, and biological monitoring systems.
Minimum load temperature achievement represents a particularly challenging aspect of autoclave validation. This parameter ensures that even the coldest point within the load reaches the minimum temperature required for sterilization efficacy. The technical objective of this validation is to demonstrate with statistical confidence that all items within the sterilization load consistently achieve the specified minimum temperature (typically 121°C for standard cycles) for the required duration.
Current validation protocols focus on identifying and monitoring cold spots within loads through strategic thermocouple placement. These protocols have evolved significantly over the past decades, moving from empirical approaches to science-based methodologies that incorporate heat transfer principles, load configuration analysis, and statistical process control techniques.
The technological trajectory in this field is moving toward more sophisticated monitoring systems, including wireless sensors, real-time monitoring capabilities, and advanced data analytics for predictive modeling. These innovations aim to enhance the reliability of temperature achievement verification while reducing validation time and resources.
Industry trends indicate growing interest in parametric release methodologies, where sterilization efficacy is determined primarily through physical and chemical parameters rather than biological indicators. This approach requires exceptionally robust temperature monitoring and validation protocols to ensure minimum temperature achievement throughout the load.
The ultimate goal of autoclave validation technology development is to establish methodologies that provide absolute assurance of sterility while optimizing cycle efficiency, reducing energy consumption, and accommodating increasingly complex medical devices and pharmaceutical products. Achieving these objectives requires continued innovation in temperature monitoring technology, load configuration analysis, and validation protocols.
Market Requirements for Sterilization Process Validation
The sterilization process validation market is experiencing significant growth driven by stringent regulatory requirements across healthcare, pharmaceutical, and medical device industries. Current global market valuation exceeds $2.5 billion with projected annual growth rates of 8-10% through 2028, reflecting the critical importance of validated sterilization processes in ensuring patient safety and product efficacy.
Regulatory bodies worldwide, including the FDA, EMA, and ISO, have established increasingly rigorous standards for sterilization validation, particularly for autoclave processes. FDA's 21 CFR Part 820 and ISO 17665 specifically mandate comprehensive validation protocols that demonstrate consistent achievement of minimum load temperatures throughout the sterilization cycle. These regulations have created substantial market demand for advanced validation technologies and services.
Healthcare facilities represent the largest market segment, accounting for approximately 45% of the total market share. These institutions require regular validation of their sterilization equipment to maintain accreditation and ensure patient safety. The pharmaceutical manufacturing sector follows at 30%, driven by GMP requirements and the critical need to prevent contamination in drug production environments.
End-users increasingly demand validation solutions that offer real-time monitoring capabilities, automated documentation, and integration with existing quality management systems. The market shows a clear preference for validation technologies that can demonstrate temperature uniformity across various load configurations and identify cold spots within autoclaves. This has spurred innovation in temperature mapping systems and validation software that can generate comprehensive reports for regulatory submissions.
The COVID-19 pandemic has accelerated market growth by highlighting the importance of properly validated sterilization processes in preventing healthcare-associated infections. This has resulted in increased investment in validation technologies and services, particularly in regions with developing healthcare infrastructure.
Geographic analysis reveals North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the fastest growth is occurring in emerging markets where healthcare infrastructure is rapidly expanding and regulatory frameworks are maturing.
Key customer requirements include validation solutions that minimize operational disruption, reduce validation cycle time, and provide clear documentation of minimum temperature achievement throughout the load. There is also growing demand for validation services that can accommodate increasingly complex medical devices and pharmaceutical products with challenging geometries and thermal properties.
Regulatory bodies worldwide, including the FDA, EMA, and ISO, have established increasingly rigorous standards for sterilization validation, particularly for autoclave processes. FDA's 21 CFR Part 820 and ISO 17665 specifically mandate comprehensive validation protocols that demonstrate consistent achievement of minimum load temperatures throughout the sterilization cycle. These regulations have created substantial market demand for advanced validation technologies and services.
Healthcare facilities represent the largest market segment, accounting for approximately 45% of the total market share. These institutions require regular validation of their sterilization equipment to maintain accreditation and ensure patient safety. The pharmaceutical manufacturing sector follows at 30%, driven by GMP requirements and the critical need to prevent contamination in drug production environments.
End-users increasingly demand validation solutions that offer real-time monitoring capabilities, automated documentation, and integration with existing quality management systems. The market shows a clear preference for validation technologies that can demonstrate temperature uniformity across various load configurations and identify cold spots within autoclaves. This has spurred innovation in temperature mapping systems and validation software that can generate comprehensive reports for regulatory submissions.
The COVID-19 pandemic has accelerated market growth by highlighting the importance of properly validated sterilization processes in preventing healthcare-associated infections. This has resulted in increased investment in validation technologies and services, particularly in regions with developing healthcare infrastructure.
Geographic analysis reveals North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the fastest growth is occurring in emerging markets where healthcare infrastructure is rapidly expanding and regulatory frameworks are maturing.
Key customer requirements include validation solutions that minimize operational disruption, reduce validation cycle time, and provide clear documentation of minimum temperature achievement throughout the load. There is also growing demand for validation services that can accommodate increasingly complex medical devices and pharmaceutical products with challenging geometries and thermal properties.
Current Challenges in Minimum Load Temperature Achievement
Achieving minimum load temperature in autoclave validation processes presents several significant challenges that impact sterilization efficacy and regulatory compliance. The primary obstacle remains the identification and consistent monitoring of cold spots within complex load configurations. These cold spots, which represent the areas last to reach the target sterilization temperature, can vary substantially depending on load density, composition, and arrangement, making their prediction and measurement technically demanding.
Temperature distribution heterogeneity continues to challenge validation teams, particularly when dealing with mixed loads containing materials with different thermal properties. Dense materials like metals conduct heat differently than porous items or liquids, creating temperature gradients that must be carefully mapped and understood. This variability necessitates comprehensive temperature mapping studies that are both resource-intensive and time-consuming.
Sensor placement optimization represents another critical challenge. Determining the optimal number and positioning of temperature probes to accurately capture the thermal profile of the entire load requires significant expertise. Insufficient or improperly placed sensors may fail to detect cold spots, while excessive sensors can complicate validation procedures without providing proportional benefits to data quality.
Equipment limitations further compound these challenges. Many autoclaves, particularly older models, exhibit inherent temperature variations across their chamber volume. These variations, often resulting from steam distribution inefficiencies or chamber design limitations, create additional complexity in achieving uniform minimum temperatures throughout the load. Even modern autoclaves with advanced control systems cannot fully eliminate these physical constraints.
Validation protocols themselves present methodological challenges. The balance between statistical confidence and practical feasibility often forces compromises in sampling approaches. Questions regarding appropriate cycle repetitions, acceptable temperature ranges, and hold time calculations remain subjects of ongoing debate among validation specialists.
Regulatory expectations add another layer of complexity, with different regions and agencies sometimes applying varying interpretations of validation requirements. The transition from traditional F0-based approaches to more sophisticated parametric release methodologies has created uncertainty regarding minimum temperature achievement documentation and justification.
Finally, the increasing complexity of medical devices and pharmaceutical products introduces new validation challenges. Novel materials, intricate geometries, and specialized packaging can create unprecedented thermal barriers that traditional validation approaches may struggle to address adequately. As product innovation accelerates, validation methodologies must evolve correspondingly to ensure sterilization efficacy while maintaining practical feasibility.
Temperature distribution heterogeneity continues to challenge validation teams, particularly when dealing with mixed loads containing materials with different thermal properties. Dense materials like metals conduct heat differently than porous items or liquids, creating temperature gradients that must be carefully mapped and understood. This variability necessitates comprehensive temperature mapping studies that are both resource-intensive and time-consuming.
Sensor placement optimization represents another critical challenge. Determining the optimal number and positioning of temperature probes to accurately capture the thermal profile of the entire load requires significant expertise. Insufficient or improperly placed sensors may fail to detect cold spots, while excessive sensors can complicate validation procedures without providing proportional benefits to data quality.
Equipment limitations further compound these challenges. Many autoclaves, particularly older models, exhibit inherent temperature variations across their chamber volume. These variations, often resulting from steam distribution inefficiencies or chamber design limitations, create additional complexity in achieving uniform minimum temperatures throughout the load. Even modern autoclaves with advanced control systems cannot fully eliminate these physical constraints.
Validation protocols themselves present methodological challenges. The balance between statistical confidence and practical feasibility often forces compromises in sampling approaches. Questions regarding appropriate cycle repetitions, acceptable temperature ranges, and hold time calculations remain subjects of ongoing debate among validation specialists.
Regulatory expectations add another layer of complexity, with different regions and agencies sometimes applying varying interpretations of validation requirements. The transition from traditional F0-based approaches to more sophisticated parametric release methodologies has created uncertainty regarding minimum temperature achievement documentation and justification.
Finally, the increasing complexity of medical devices and pharmaceutical products introduces new validation challenges. Novel materials, intricate geometries, and specialized packaging can create unprecedented thermal barriers that traditional validation approaches may struggle to address adequately. As product innovation accelerates, validation methodologies must evolve correspondingly to ensure sterilization efficacy while maintaining practical feasibility.
Current Technical Approaches to Minimum Load Temperature Validation
01 Temperature monitoring and control systems for autoclave validation
Effective temperature monitoring and control systems are essential for autoclave validation to ensure that minimum load temperatures are consistently achieved. These systems typically include temperature sensors, data loggers, and control algorithms that can accurately measure and maintain the required temperature throughout the sterilization cycle. Advanced systems may incorporate real-time monitoring capabilities and automated adjustments to ensure that the minimum temperature threshold is maintained across the entire load.- Temperature monitoring and control systems for autoclave validation: Effective temperature monitoring and control systems are essential for autoclave validation to ensure that minimum load temperatures are consistently achieved. These systems typically include temperature sensors, data loggers, and control mechanisms that can accurately measure and maintain the required temperature throughout the sterilization cycle. Advanced monitoring systems can provide real-time feedback and automatically adjust parameters to ensure that the minimum load temperature is maintained for the required duration.
- Minimum load temperature requirements for different sterilization cycles: Different types of sterilization cycles require specific minimum load temperatures to ensure effective sterilization. For standard steam sterilization, the minimum load temperature typically ranges from 121°C to 134°C depending on the cycle type and duration. The minimum temperature must be maintained for a specified period to achieve the required sterility assurance level. Validation protocols must verify that these minimum temperature requirements are consistently met throughout the load.
- Load configuration and placement for temperature uniformity: The configuration and placement of items within an autoclave significantly impact temperature distribution and the achievement of minimum load temperatures. Validation protocols must consider worst-case scenarios, including dense loads and challenging locations within the chamber. Proper spacing between items allows steam penetration and ensures uniform heat distribution. Strategic placement of temperature sensors at the coldest points of the load is crucial for accurate validation of minimum load temperatures.
- Validation protocols and documentation for minimum load temperature: Comprehensive validation protocols are essential to verify that autoclaves consistently achieve minimum load temperatures. These protocols typically include installation qualification, operational qualification, and performance qualification phases. Documentation must include temperature mapping studies, cycle development data, and routine monitoring records. Regular revalidation is necessary to ensure ongoing compliance with minimum temperature requirements, especially after repairs or modifications to the autoclave system.
- Technological innovations for improving minimum load temperature achievement: Recent technological innovations have enhanced the ability to achieve and verify minimum load temperatures during autoclave sterilization. These include advanced steam distribution systems, improved chamber designs, and sophisticated control algorithms. Some systems incorporate artificial intelligence to optimize cycle parameters based on load characteristics. Wireless temperature sensors and cloud-based monitoring platforms allow for more precise temperature mapping and data analysis, ensuring that minimum load temperatures are consistently achieved throughout the sterilization process.
02 Minimum load temperature requirements for different sterilization cycles
Different types of materials and loads require specific minimum temperature thresholds during autoclave sterilization. The validation process must account for these variations by establishing appropriate minimum load temperatures based on the nature of the items being sterilized. For standard medical equipment, minimum temperatures typically range from 121°C to 134°C, with corresponding pressure requirements. The validation protocol must specify these minimum temperature requirements for different load configurations to ensure effective sterilization.Expand Specific Solutions03 Cold spot identification and temperature distribution analysis
Identifying cold spots within the autoclave chamber is crucial for validation as these areas are most likely to fall below the minimum required temperature. Temperature mapping studies using multiple sensors placed throughout the load help identify these cold spots and analyze temperature distribution patterns. This analysis ensures that even the coolest areas of the load reach and maintain the minimum required temperature for the specified time period, which is essential for validation compliance.Expand Specific Solutions04 Load configuration impact on minimum temperature achievement
The arrangement and composition of items within the autoclave significantly affect temperature distribution and the ability to reach minimum load temperatures. Validation protocols must account for various load configurations, including maximum and minimum loads, to ensure that sterilization parameters are consistently achieved. Proper spacing between items, appropriate packaging materials, and strategic placement within the chamber all contribute to ensuring that the minimum required temperature is reached throughout the entire load.Expand Specific Solutions05 Documentation and validation protocols for minimum load temperature
Comprehensive documentation is essential for autoclave validation, particularly regarding minimum load temperature requirements. Validation protocols should include detailed procedures for temperature monitoring, acceptance criteria, and corrective actions when minimum temperatures are not achieved. These protocols typically require documentation of temperature readings at multiple locations within the load, verification that minimum temperatures were maintained for the required duration, and regular revalidation to ensure ongoing compliance with sterilization standards.Expand Specific Solutions
Leading Manufacturers and Regulatory Bodies in Sterilization
The autoclave validation market is in a mature growth phase, characterized by established protocols and regulatory frameworks. The global market size for sterilization equipment, including autoclaves, is estimated at $12-15 billion with steady annual growth of 6-8%. Technologically, minimum load temperature achievement represents a critical validation parameter that has reached high maturity levels. Leading players like Fedegari Autoclavi demonstrate advanced capabilities in precision temperature control systems, while medical equipment manufacturers such as Covidien (Medtronic) and Olympus have integrated sophisticated validation protocols. Shinva Medical Instrument and 3D Systems offer innovative approaches to temperature monitoring and validation. Research institutions like CSIR and Shandong University contribute to technical standards development, while regulatory testing organizations like Vkan Certification ensure compliance across pharmaceutical and medical device industries.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva Medical Instrument has developed an innovative autoclave validation system that combines traditional temperature mapping with advanced computational fluid dynamics (CFD) modeling. Their approach utilizes a network of high-precision temperature sensors strategically placed throughout the chamber and load based on thermal simulation results that identify potential cold spots. The system features adaptive cycle control technology that continuously monitors temperature distribution patterns and automatically adjusts steam injection parameters, pressure levels, and cycle duration to ensure minimum temperature achievement across all load components. Shinva's validation methodology incorporates a proprietary algorithm that calculates heat penetration rates for different load materials and configurations, enabling predictive temperature achievement verification. Their technology includes automated load density analysis that identifies potential cold spots before cycle initiation, allowing for proactive adjustment of validation parameters. The system generates comprehensive validation reports that track temperature profiles at multiple points throughout the sterilization cycle, providing documented evidence of minimum temperature achievement for regulatory compliance.
Strengths: Strong integration of theoretical modeling with practical validation techniques, particularly suited for varied load compositions. Cost-effective implementation compared to Western competitors while maintaining compliance with international standards. Weaknesses: Less extensive global support network compared to European and American competitors, and some advanced features may have less robust documentation in multiple languages.
Fedegari Autoclavi SpA
Technical Solution: Fedegari has developed an advanced autoclave validation system that utilizes distributed temperature sensing technology with multiple strategically placed probes throughout the chamber and load. Their Thema4 process controller implements predictive algorithms to ensure minimum load temperature achievement across all critical points. The system features real-time monitoring capabilities that track temperature gradients between different load components, automatically adjusting cycle parameters to maintain optimal sterilization conditions. Fedegari's validation approach incorporates F0 value calculations that account for both time and temperature parameters, ensuring that the most challenging locations within the load reach and maintain the minimum required temperature. Their technology includes automated load pattern recognition that adjusts validation protocols based on specific load configurations, density, and thermal properties, significantly improving cycle efficiency while maintaining compliance with international standards for sterilization validation.
Strengths: Highly specialized in pharmaceutical and biomedical sterilization with proprietary algorithms for temperature prediction and control. Superior data integration capabilities for comprehensive validation documentation. Weaknesses: Premium pricing structure may limit accessibility for smaller facilities, and system complexity requires specialized training for operators.
Critical Patents and Research in Autoclave Validation Technology
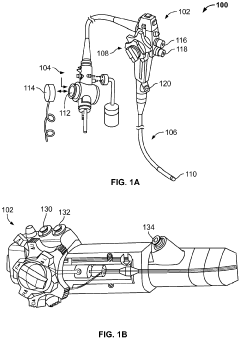
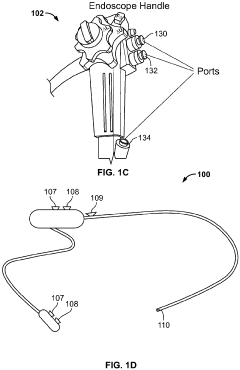
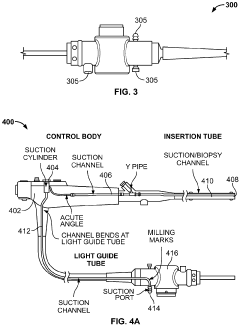
Method and system for steam sterilization of endoscopes
PatentInactiveEP3915459A1
Innovation
- A method and apparatus utilizing superheated steam delivered through pressure-resistant fittings and controlled by a microprocessor, with optional suction mechanisms and temperature/pressure sensors, to ensure thorough sterilization of endoscope channels while minimizing damage to the equipment.
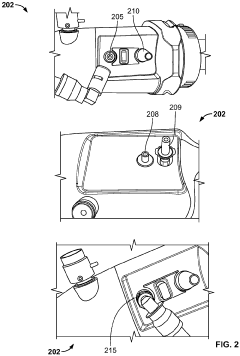
Endoscope suitable for autoclaving
PatentInactiveUS20040082835A1
Innovation
- The endoscope design incorporates a soft barrier to manage pressure and thermal loads during autoclaving, using a pressure-regulating valve and waterproof cap to prevent steam invasion and ensure a fluid-tight seal, while maintaining the integrity of resin tubes and switches.
Regulatory Compliance and Standards for Autoclave Validation
Autoclave validation processes are governed by a comprehensive framework of regulatory requirements and industry standards that ensure sterilization efficacy and patient safety. The FDA's Quality System Regulation (21 CFR Part 820) establishes fundamental requirements for medical device manufacturers, specifically addressing process validation including sterilization processes. For pharmaceutical applications, FDA's Current Good Manufacturing Practice (cGMP) regulations (21 CFR Parts 210 and 211) provide similar oversight, with specific attention to terminal sterilization processes.
International standards play a crucial role in defining validation methodologies. ISO 17665-1:2006 "Sterilization of health care products - Moist heat - Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices" provides detailed guidelines for steam sterilization validation, including specific parameters for minimum load temperature achievement. This standard emphasizes the importance of temperature mapping and load configuration studies to ensure all items reach the minimum required temperature.
The European standard EN 285 specifically addresses large steam sterilizers and provides detailed specifications for temperature distribution and penetration testing. It requires that the temperature difference between any two points in the chamber should not exceed 2°C during the sterilization phase, a critical parameter for ensuring minimum load temperature achievement across all items.
AAMI ST79 "Comprehensive guide to steam sterilization and sterility assurance in health care facilities" offers practical guidance for healthcare facilities, detailing validation protocols including temperature monitoring requirements. This standard recommends strategic placement of temperature sensors at potential cold spots within loads to verify minimum temperature achievement.
PDA Technical Report No. 1 provides specific guidance on validation of moist heat sterilization processes in the pharmaceutical industry, with detailed protocols for temperature mapping studies. It emphasizes the importance of worst-case scenarios testing, including minimum load configurations and challenging load compositions.
Compliance with these standards requires documented evidence that all items within an autoclave load consistently achieve the minimum specified temperature (typically 121°C for standard cycles) for the required duration. This documentation must include calibration records for all temperature monitoring devices, detailed load configuration diagrams, and comprehensive temperature mapping data.
Regulatory bodies increasingly expect risk-based approaches to validation, with particular attention to identifying and monitoring potential cold spots within loads. Recent regulatory trends show increased scrutiny of validation data integrity and statistical analysis of temperature distribution patterns across multiple validation runs.
International standards play a crucial role in defining validation methodologies. ISO 17665-1:2006 "Sterilization of health care products - Moist heat - Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices" provides detailed guidelines for steam sterilization validation, including specific parameters for minimum load temperature achievement. This standard emphasizes the importance of temperature mapping and load configuration studies to ensure all items reach the minimum required temperature.
The European standard EN 285 specifically addresses large steam sterilizers and provides detailed specifications for temperature distribution and penetration testing. It requires that the temperature difference between any two points in the chamber should not exceed 2°C during the sterilization phase, a critical parameter for ensuring minimum load temperature achievement across all items.
AAMI ST79 "Comprehensive guide to steam sterilization and sterility assurance in health care facilities" offers practical guidance for healthcare facilities, detailing validation protocols including temperature monitoring requirements. This standard recommends strategic placement of temperature sensors at potential cold spots within loads to verify minimum temperature achievement.
PDA Technical Report No. 1 provides specific guidance on validation of moist heat sterilization processes in the pharmaceutical industry, with detailed protocols for temperature mapping studies. It emphasizes the importance of worst-case scenarios testing, including minimum load configurations and challenging load compositions.
Compliance with these standards requires documented evidence that all items within an autoclave load consistently achieve the minimum specified temperature (typically 121°C for standard cycles) for the required duration. This documentation must include calibration records for all temperature monitoring devices, detailed load configuration diagrams, and comprehensive temperature mapping data.
Regulatory bodies increasingly expect risk-based approaches to validation, with particular attention to identifying and monitoring potential cold spots within loads. Recent regulatory trends show increased scrutiny of validation data integrity and statistical analysis of temperature distribution patterns across multiple validation runs.
Risk Assessment and Failure Mode Analysis in Sterilization Processes
Sterilization processes, particularly autoclave validation, require comprehensive risk assessment and failure mode analysis to ensure patient safety and regulatory compliance. The achievement of minimum load temperature represents a critical control point that demands systematic evaluation of potential failure modes and their consequences.
Risk assessment in autoclave sterilization begins with identifying critical process parameters that affect temperature achievement across the load. These include steam quality, chamber pressure, load configuration, and cycle parameters. Each parameter must be evaluated for potential failure modes using structured methodologies such as Failure Mode and Effects Analysis (FMEA) or Hazard Analysis and Critical Control Points (HACCP).
Temperature distribution failures represent a significant risk category, often resulting from improper loading patterns, inadequate steam penetration, or air entrapment. These failures can create cold spots where minimum temperature requirements are not met, potentially compromising sterility assurance. Risk assessment tools must quantify both the probability of occurrence and severity of consequences for each identified failure mode.
Validation protocols should incorporate worst-case scenarios that challenge the sterilization process, particularly focusing on difficult-to-sterilize locations within the load. Historical data analysis plays a crucial role in identifying recurring failure patterns and establishing risk priorities. This data-driven approach enables more targeted preventive measures and monitoring strategies.
Modern risk management approaches incorporate real-time monitoring systems with alert mechanisms for parameter deviations. These systems can detect early warning signs of potential failures before they impact sterility assurance levels. Implementation of redundant temperature monitoring at multiple load locations provides additional safeguards against undetected cold spots.
Regulatory frameworks, including ISO 17665 and FDA guidance documents, emphasize risk-based approaches to sterilization validation. These frameworks require documented evidence that all identified risks have been adequately addressed through appropriate design, monitoring, and control measures. Compliance with these requirements necessitates thorough documentation of risk assessment methodologies and mitigation strategies.
Personnel training represents another critical element in risk management for autoclave processes. Operators must understand the potential consequences of procedural deviations and recognize warning signs of process failures. Regular competency assessments and refresher training help minimize human error contributions to sterilization failures.
Continuous improvement processes should incorporate lessons learned from near-misses and actual failures, creating a feedback loop that enhances risk assessment accuracy over time. This evolutionary approach ensures that risk management strategies remain relevant as equipment ages and process knowledge expands.
Risk assessment in autoclave sterilization begins with identifying critical process parameters that affect temperature achievement across the load. These include steam quality, chamber pressure, load configuration, and cycle parameters. Each parameter must be evaluated for potential failure modes using structured methodologies such as Failure Mode and Effects Analysis (FMEA) or Hazard Analysis and Critical Control Points (HACCP).
Temperature distribution failures represent a significant risk category, often resulting from improper loading patterns, inadequate steam penetration, or air entrapment. These failures can create cold spots where minimum temperature requirements are not met, potentially compromising sterility assurance. Risk assessment tools must quantify both the probability of occurrence and severity of consequences for each identified failure mode.
Validation protocols should incorporate worst-case scenarios that challenge the sterilization process, particularly focusing on difficult-to-sterilize locations within the load. Historical data analysis plays a crucial role in identifying recurring failure patterns and establishing risk priorities. This data-driven approach enables more targeted preventive measures and monitoring strategies.
Modern risk management approaches incorporate real-time monitoring systems with alert mechanisms for parameter deviations. These systems can detect early warning signs of potential failures before they impact sterility assurance levels. Implementation of redundant temperature monitoring at multiple load locations provides additional safeguards against undetected cold spots.
Regulatory frameworks, including ISO 17665 and FDA guidance documents, emphasize risk-based approaches to sterilization validation. These frameworks require documented evidence that all identified risks have been adequately addressed through appropriate design, monitoring, and control measures. Compliance with these requirements necessitates thorough documentation of risk assessment methodologies and mitigation strategies.
Personnel training represents another critical element in risk management for autoclave processes. Operators must understand the potential consequences of procedural deviations and recognize warning signs of process failures. Regular competency assessments and refresher training help minimize human error contributions to sterilization failures.
Continuous improvement processes should incorporate lessons learned from near-misses and actual failures, creating a feedback loop that enhances risk assessment accuracy over time. This evolutionary approach ensures that risk management strategies remain relevant as equipment ages and process knowledge expands.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!