Autoclave vs. Conventional Ovens: Efficacy in Lab Settings
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sterilization Technology Evolution and Objectives
Sterilization technologies have undergone significant evolution since their inception in the late 19th century. The development trajectory began with basic heat-based methods, progressing through chemical sterilization approaches, and eventually incorporating advanced technologies like radiation and plasma sterilization. This evolution has been driven by the increasing demands for more efficient, reliable, and specialized sterilization methods across various laboratory and medical settings.
The autoclave, introduced in 1879 by Charles Chamberland, represented a revolutionary advancement in sterilization technology. Operating on the principle of pressurized steam, autoclaves quickly became the gold standard for laboratory sterilization due to their superior efficacy in eliminating microorganisms, including heat-resistant bacterial spores. The fundamental mechanism—utilizing saturated steam under pressure to achieve temperatures above 121°C—remains largely unchanged, though modern autoclaves incorporate sophisticated control systems and safety features.
Conventional ovens, by contrast, rely on dry heat sterilization, a method that predates autoclave technology. While less efficient in terms of heat transfer and requiring higher temperatures (typically 160-180°C) and longer exposure times, dry heat sterilization offers distinct advantages for certain applications, particularly for materials that may be damaged by moisture or for sterilizing powders, oils, and other hydrophobic substances.
The primary objective in sterilization technology development has consistently been the achievement of complete microbial inactivation while preserving the integrity of the sterilized materials. Secondary objectives include reducing cycle times, minimizing energy consumption, enhancing user safety, and developing more environmentally sustainable processes. These objectives have become increasingly important as laboratory practices have evolved and diversified.
Recent technological advancements have focused on improving the efficiency and versatility of both autoclave and conventional oven technologies. Modern autoclaves feature microprocessor controls, vacuum systems for improved steam penetration, and rapid cooling mechanisms. Similarly, contemporary laboratory ovens incorporate precise temperature control, uniform heat distribution systems, and programmable cycles tailored to specific applications.
The ongoing evolution of sterilization technologies aims to address emerging challenges, including the sterilization of complex medical devices, heat-sensitive materials, and the increasing concern regarding antibiotic-resistant microorganisms. Future development objectives include the integration of smart technologies for remote monitoring and validation, reduced environmental impact through lower water and energy consumption, and enhanced compatibility with a wider range of materials and applications.
The autoclave, introduced in 1879 by Charles Chamberland, represented a revolutionary advancement in sterilization technology. Operating on the principle of pressurized steam, autoclaves quickly became the gold standard for laboratory sterilization due to their superior efficacy in eliminating microorganisms, including heat-resistant bacterial spores. The fundamental mechanism—utilizing saturated steam under pressure to achieve temperatures above 121°C—remains largely unchanged, though modern autoclaves incorporate sophisticated control systems and safety features.
Conventional ovens, by contrast, rely on dry heat sterilization, a method that predates autoclave technology. While less efficient in terms of heat transfer and requiring higher temperatures (typically 160-180°C) and longer exposure times, dry heat sterilization offers distinct advantages for certain applications, particularly for materials that may be damaged by moisture or for sterilizing powders, oils, and other hydrophobic substances.
The primary objective in sterilization technology development has consistently been the achievement of complete microbial inactivation while preserving the integrity of the sterilized materials. Secondary objectives include reducing cycle times, minimizing energy consumption, enhancing user safety, and developing more environmentally sustainable processes. These objectives have become increasingly important as laboratory practices have evolved and diversified.
Recent technological advancements have focused on improving the efficiency and versatility of both autoclave and conventional oven technologies. Modern autoclaves feature microprocessor controls, vacuum systems for improved steam penetration, and rapid cooling mechanisms. Similarly, contemporary laboratory ovens incorporate precise temperature control, uniform heat distribution systems, and programmable cycles tailored to specific applications.
The ongoing evolution of sterilization technologies aims to address emerging challenges, including the sterilization of complex medical devices, heat-sensitive materials, and the increasing concern regarding antibiotic-resistant microorganisms. Future development objectives include the integration of smart technologies for remote monitoring and validation, reduced environmental impact through lower water and energy consumption, and enhanced compatibility with a wider range of materials and applications.
Market Analysis of Laboratory Sterilization Equipment
The laboratory sterilization equipment market has experienced significant growth over the past decade, driven by increasing demand for contamination-free environments in research, healthcare, and industrial settings. Currently valued at approximately 6.2 billion USD globally, this market is projected to grow at a compound annual growth rate (CAGR) of 7.8% through 2028, according to recent industry analyses.
Autoclaves dominate the sterilization equipment segment, accounting for roughly 45% of the total market share. This dominance stems from their superior efficacy in achieving complete sterilization through the combination of high pressure and steam, particularly crucial for microbiology and clinical laboratories. Conventional ovens, while representing a smaller market segment at approximately 20%, maintain steady demand due to their versatility and lower acquisition costs.
Regional analysis reveals North America as the largest market for laboratory sterilization equipment, holding approximately 35% of the global market share. This is attributed to stringent regulatory standards, well-established research infrastructure, and substantial healthcare expenditure. The Asia-Pacific region, however, is emerging as the fastest-growing market with an estimated CAGR of 9.3%, driven by expanding healthcare facilities, increasing research activities, and growing awareness about infection control protocols.
The competitive landscape features both established players and innovative entrants. Major manufacturers like Steris Corporation, Getinge Group, and Tuttnauer command significant market presence through comprehensive product portfolios and global distribution networks. Meanwhile, smaller specialized manufacturers are gaining traction by offering niche solutions with enhanced energy efficiency and digital integration capabilities.
Customer segmentation analysis indicates that academic and research laboratories constitute approximately 40% of end-users, followed by pharmaceutical and biotechnology companies (30%), hospitals and clinics (20%), and other industrial applications (10%). This distribution highlights the critical importance of sterilization equipment across diverse scientific and healthcare settings.
Price sensitivity varies significantly across market segments. While high-end research facilities prioritize precision and reliability over cost, smaller laboratories and educational institutions demonstrate greater price sensitivity, creating demand for more economical sterilization solutions. This market dynamic has spurred manufacturers to develop tiered product offerings that balance performance specifications with affordability.
The market is increasingly influenced by sustainability concerns, with growing demand for energy-efficient sterilization equipment that reduces operational costs and environmental impact. This trend is particularly evident in developed markets where carbon footprint reduction initiatives are gaining prominence in institutional procurement policies.
Autoclaves dominate the sterilization equipment segment, accounting for roughly 45% of the total market share. This dominance stems from their superior efficacy in achieving complete sterilization through the combination of high pressure and steam, particularly crucial for microbiology and clinical laboratories. Conventional ovens, while representing a smaller market segment at approximately 20%, maintain steady demand due to their versatility and lower acquisition costs.
Regional analysis reveals North America as the largest market for laboratory sterilization equipment, holding approximately 35% of the global market share. This is attributed to stringent regulatory standards, well-established research infrastructure, and substantial healthcare expenditure. The Asia-Pacific region, however, is emerging as the fastest-growing market with an estimated CAGR of 9.3%, driven by expanding healthcare facilities, increasing research activities, and growing awareness about infection control protocols.
The competitive landscape features both established players and innovative entrants. Major manufacturers like Steris Corporation, Getinge Group, and Tuttnauer command significant market presence through comprehensive product portfolios and global distribution networks. Meanwhile, smaller specialized manufacturers are gaining traction by offering niche solutions with enhanced energy efficiency and digital integration capabilities.
Customer segmentation analysis indicates that academic and research laboratories constitute approximately 40% of end-users, followed by pharmaceutical and biotechnology companies (30%), hospitals and clinics (20%), and other industrial applications (10%). This distribution highlights the critical importance of sterilization equipment across diverse scientific and healthcare settings.
Price sensitivity varies significantly across market segments. While high-end research facilities prioritize precision and reliability over cost, smaller laboratories and educational institutions demonstrate greater price sensitivity, creating demand for more economical sterilization solutions. This market dynamic has spurred manufacturers to develop tiered product offerings that balance performance specifications with affordability.
The market is increasingly influenced by sustainability concerns, with growing demand for energy-efficient sterilization equipment that reduces operational costs and environmental impact. This trend is particularly evident in developed markets where carbon footprint reduction initiatives are gaining prominence in institutional procurement policies.
Current Capabilities and Limitations of Sterilization Methods
Sterilization methods in laboratory settings have evolved significantly over the decades, with autoclaves and conventional ovens representing two primary approaches with distinct capabilities and limitations. Autoclaves utilize pressurized steam to achieve sterilization, typically operating at 121°C and 15 psi for 15-30 minutes. This method effectively penetrates materials and eliminates microorganisms through protein denaturation and coagulation, achieving a sterility assurance level (SAL) of 10^-6, which indicates a one-in-a-million probability of microbial survival.
Conventional ovens, employing dry heat sterilization, operate at higher temperatures (160-180°C) for longer durations (2-4 hours) to compensate for lower heat transfer efficiency. While less energy-efficient than autoclaves, they excel in sterilizing moisture-sensitive items, powders, oils, and certain glassware that might be damaged by steam.
The efficacy of autoclaves is particularly notable in their ability to rapidly penetrate porous materials and wrapped items, making them ideal for culture media, surgical instruments, and laboratory equipment. However, they present limitations when processing heat-sensitive materials, hydrophobic substances, and certain plastics that may deform under steam pressure. Additionally, autoclaves require regular validation through biological indicators and maintenance to prevent biofilm formation in water reservoirs.
Conventional ovens demonstrate superior performance with heat-stable, moisture-sensitive materials and non-aqueous substances. Their dry heat mechanism effectively destroys microorganisms through oxidation processes rather than protein denaturation. Nevertheless, they exhibit significant drawbacks including extended processing times, higher energy consumption, and uneven heat distribution that may create cold spots where sterilization remains incomplete.
Recent technological advancements have introduced hybrid systems combining features of both methods, such as vacuum-assisted ovens that improve heat penetration while maintaining a dry environment. Additionally, monitoring capabilities have evolved substantially, with modern autoclaves incorporating parametric release systems that validate sterilization cycles through critical parameter monitoring rather than relying solely on biological indicators.
Material compatibility remains a critical consideration in method selection. Certain polymers, electronics, and precision instruments tolerate dry heat better than steam, while porous materials and items with complex geometries benefit from steam's superior penetration capabilities. The choice between methods ultimately depends on specific laboratory requirements, material properties, and operational constraints including cycle time, energy efficiency, and validation protocols.
Conventional ovens, employing dry heat sterilization, operate at higher temperatures (160-180°C) for longer durations (2-4 hours) to compensate for lower heat transfer efficiency. While less energy-efficient than autoclaves, they excel in sterilizing moisture-sensitive items, powders, oils, and certain glassware that might be damaged by steam.
The efficacy of autoclaves is particularly notable in their ability to rapidly penetrate porous materials and wrapped items, making them ideal for culture media, surgical instruments, and laboratory equipment. However, they present limitations when processing heat-sensitive materials, hydrophobic substances, and certain plastics that may deform under steam pressure. Additionally, autoclaves require regular validation through biological indicators and maintenance to prevent biofilm formation in water reservoirs.
Conventional ovens demonstrate superior performance with heat-stable, moisture-sensitive materials and non-aqueous substances. Their dry heat mechanism effectively destroys microorganisms through oxidation processes rather than protein denaturation. Nevertheless, they exhibit significant drawbacks including extended processing times, higher energy consumption, and uneven heat distribution that may create cold spots where sterilization remains incomplete.
Recent technological advancements have introduced hybrid systems combining features of both methods, such as vacuum-assisted ovens that improve heat penetration while maintaining a dry environment. Additionally, monitoring capabilities have evolved substantially, with modern autoclaves incorporating parametric release systems that validate sterilization cycles through critical parameter monitoring rather than relying solely on biological indicators.
Material compatibility remains a critical consideration in method selection. Certain polymers, electronics, and precision instruments tolerate dry heat better than steam, while porous materials and items with complex geometries benefit from steam's superior penetration capabilities. The choice between methods ultimately depends on specific laboratory requirements, material properties, and operational constraints including cycle time, energy efficiency, and validation protocols.
Comparative Analysis of Autoclave and Conventional Oven Systems
01 Sterilization efficacy comparison between autoclaves and conventional ovens
Autoclaves generally provide superior sterilization efficacy compared to conventional ovens due to their use of pressurized steam. The combination of high temperature, pressure, and moisture in autoclaves enables more effective penetration into materials and elimination of microorganisms. Conventional ovens rely solely on dry heat, which typically requires longer exposure times and higher temperatures to achieve similar sterilization results.- Sterilization efficacy comparison between autoclaves and conventional ovens: Autoclaves generally provide superior sterilization efficacy compared to conventional ovens due to their ability to use pressurized steam. The combination of high temperature, pressure, and moisture in autoclaves enables more effective penetration into materials and elimination of microorganisms. Conventional ovens rely solely on dry heat, which requires higher temperatures and longer exposure times to achieve similar sterilization results.
- Temperature and pressure control mechanisms: Autoclaves feature sophisticated temperature and pressure control mechanisms that ensure consistent sterilization conditions throughout the chamber. These systems typically include pressure sensors, temperature probes, and automated control systems that maintain optimal sterilization parameters. Conventional ovens generally have simpler temperature control systems without pressure regulation capabilities, which can result in less uniform heating and reduced efficacy for certain applications.
- Material compatibility and processing considerations: Different materials respond differently to autoclave and conventional oven processing. Moisture-sensitive materials may be better suited for conventional oven treatment, while items requiring thorough sterilization often perform better in autoclaves. The selection between these technologies depends on material properties, heat sensitivity, moisture tolerance, and the required level of sterilization. Some specialized materials may require specific temperature profiles or processing conditions to maintain integrity while achieving desired efficacy.
- Energy efficiency and processing time comparison: Autoclaves typically achieve sterilization in shorter timeframes than conventional ovens due to the enhanced heat transfer properties of pressurized steam. This can result in energy savings despite the higher initial energy input required to generate steam and pressure. Conventional ovens generally require longer processing times at higher temperatures to achieve similar results, potentially leading to higher overall energy consumption for equivalent outcomes.
- Innovations in autoclave and oven technology: Recent innovations have improved the efficacy of both autoclave and conventional oven technologies. These advancements include better sealing mechanisms, more precise temperature control, improved air circulation systems, and enhanced monitoring capabilities. Some modern systems incorporate hybrid approaches that combine features of both technologies to optimize processing for specific applications. Digital controls and validation systems have also been developed to ensure consistent performance and documentation of sterilization parameters.
02 Temperature and pressure control mechanisms
Autoclaves feature sophisticated temperature and pressure control mechanisms that allow for precise sterilization parameters. These systems typically include pressure sensors, temperature probes, and automated control systems to maintain optimal conditions throughout the sterilization cycle. Conventional ovens generally have simpler temperature control systems without pressure regulation capabilities, which limits their effectiveness for certain applications requiring specific sterilization parameters.Expand Specific Solutions03 Applications in medical and laboratory settings
Autoclaves are widely used in medical and laboratory settings where complete sterilization is critical. They are particularly effective for sterilizing surgical instruments, laboratory equipment, and medical waste. Conventional ovens find more limited applications in these settings, typically used for dry heat sterilization of items that may be damaged by steam or for specific applications where dry heat is preferred, such as glassware or powders.Expand Specific Solutions04 Energy efficiency and operational costs
The energy efficiency and operational costs differ significantly between autoclaves and conventional ovens. Autoclaves typically achieve sterilization more quickly due to the enhanced heat transfer properties of steam, potentially reducing energy consumption despite requiring energy for both heating and pressurization. Conventional ovens generally operate for longer periods at higher temperatures, which can result in greater energy consumption for equivalent sterilization tasks.Expand Specific Solutions05 Material compatibility and limitations
Material compatibility is an important consideration when choosing between autoclaves and conventional ovens. Autoclaves may not be suitable for moisture-sensitive materials, heat-sensitive plastics, or items that can be damaged by rapid pressure changes. Conventional ovens provide an alternative for heat-stable, moisture-sensitive materials but may cause oxidation of certain metals and degradation of some polymers due to prolonged exposure to high temperatures in the presence of oxygen.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Sterilization
The autoclave versus conventional oven market in laboratory settings is currently in a growth phase, with increasing demand for sterilization and thermal processing equipment. The global laboratory equipment market is estimated to reach $5-7 billion by 2025, with autoclaves representing a significant segment. Technologically, autoclaves offer superior sterilization capabilities through pressurized steam, while conventional ovens provide more versatile heating applications. Leading players include established industrial equipment manufacturers like Siemens AG and 3D Systems, alongside specialized laboratory equipment providers such as Anton Paar GmbH. Medical technology companies including Fresenius Medical Care and Nakanishi are advancing autoclave technology for healthcare applications, while research institutions like Deutsches Zentrum für Luft- und Raumfahrt and Wuhan Institute of Virology drive innovation through scientific requirements for precise temperature control and sterilization protocols.
The Boeing Co.
Technical Solution: Boeing has developed specialized high-pressure autoclaves primarily for composite material curing in aerospace applications, which has applications in advanced laboratory settings. Their autoclave technology features precise temperature uniformity (±3°F across the working volume) and pressure control systems (up to 300 psi) that maintain consistent conditions throughout large processing volumes. Boeing's autoclaves incorporate advanced thermal management systems that provide rapid heating and cooling rates (up to 5°F/minute) while maintaining temperature uniformity. Their systems include sophisticated vacuum systems that remove volatiles during composite processing, which is critical for material research applications. Boeing has also implemented energy management systems in their autoclaves that optimize power consumption during different phases of the processing cycle.
Strengths: Exceptional temperature and pressure uniformity, advanced thermal management capabilities, and precise control systems. Weaknesses: Systems are primarily designed for industrial-scale composite processing rather than typical laboratory applications, and they require significant infrastructure and high capital investment.
Siemens AG
Technical Solution: Siemens has developed advanced laboratory autoclave systems that integrate digital monitoring capabilities with their HMI (Human Machine Interface) technology. Their autoclaves feature precise temperature and pressure control systems that maintain consistent sterilization conditions throughout the cycle. Siemens' laboratory autoclaves incorporate SIMATIC automation technology that allows for programmable sterilization cycles with validation protocols that meet international standards for laboratory applications. Their systems include data logging capabilities that create detailed records of each sterilization cycle for regulatory compliance. Siemens has also implemented energy recovery systems in their autoclaves that capture and reuse heat, reducing energy consumption by up to 30% compared to conventional systems.
Strengths: Superior automation capabilities, excellent data management for compliance, and energy efficiency features. Weaknesses: Higher initial investment cost compared to conventional ovens and requires specialized maintenance personnel.
Key Technical Innovations in Sterilization Processes
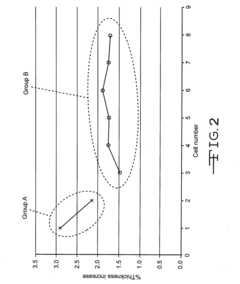
Autoclavable cfx cell
PatentInactiveUS20160359198A1
Innovation
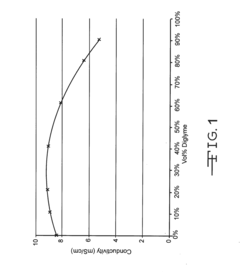
- A non-aqueous electrolyte formulation comprising a mixture of γ-butyrolactone (GBL) and diglyme with 1.0M LiBF4 salt, combined with a surfactant-free polymeric separator, to enhance the electrochemical cell's ability to withstand autoclave conditions without compromising electrical performance.
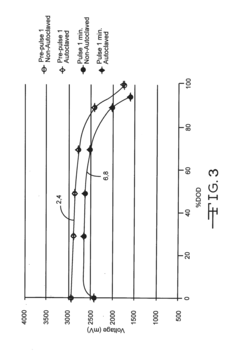
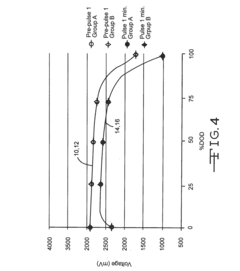
Sterilisation device and corresponding method
PatentInactiveEP2429594A1
Innovation
- A sterilization device using wave emission means, specifically microwaves, to achieve a controlled temperature variation process involving a first rise, decrease, and second rise in temperature to effectively sterilize products without the need for high pressures and temperatures, with automated control and monitoring.
Biosafety Standards and Regulatory Compliance
Laboratory sterilization processes are governed by stringent biosafety standards and regulatory frameworks that vary across regions but share common principles. For autoclaves and conventional ovens, compliance with these standards is essential to ensure laboratory safety and result validity. The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have established comprehensive guidelines for sterilization procedures in laboratory settings, with autoclaves being recognized as the gold standard for achieving Biosafety Level (BSL) requirements.
In the United States, the Occupational Safety and Health Administration (OSHA) mandates specific protocols for sterilization equipment operation, including regular validation testing and documentation. Autoclaves must meet standards set by the American Society for Testing and Materials (ASTM) and the Association for the Advancement of Medical Instrumentation (AAMI), particularly ANSI/AAMI ST79 which outlines steam sterilization requirements.
European laboratories follow the EN 285 standard for large steam sterilizers and EN 13060 for smaller units. These standards specify performance criteria including temperature uniformity, pressure parameters, and cycle validation methods. Conventional ovens, while useful for dry heat sterilization, must comply with different regulatory frameworks such as ISO 20857:2010 for dry heat sterilization processes.
Documentation requirements represent a significant aspect of regulatory compliance. Both autoclave and conventional oven users must maintain detailed logs of each sterilization cycle, including temperature profiles, duration, and load characteristics. For autoclaves, biological indicators containing Geobacillus stearothermophilus spores are typically required for validation, while Bacillus atrophaeus is standard for dry heat processes in conventional ovens.
Risk assessment frameworks differ substantially between the two technologies. Autoclaves present risks related to pressurized steam, requiring compliance with pressure vessel regulations and safety interlocks. Conventional ovens pose fire hazards and require adherence to electrical safety standards. Both systems must incorporate fail-safe mechanisms and emergency protocols as mandated by laboratory safety regulations.
Recent regulatory trends show increasing emphasis on energy efficiency and environmental impact. The Environmental Protection Agency (EPA) has introduced guidelines encouraging laboratories to adopt more sustainable sterilization practices, potentially influencing equipment selection between autoclaves and conventional ovens based on their respective energy consumption profiles and environmental footprints.
For laboratories seeking accreditation, compliance with ISO/IEC 17025 standards is essential, requiring demonstration of proper sterilization validation regardless of the technology employed. This includes establishing quality management systems that document equipment calibration, maintenance schedules, and operator training programs specific to either autoclave or conventional oven operation.
In the United States, the Occupational Safety and Health Administration (OSHA) mandates specific protocols for sterilization equipment operation, including regular validation testing and documentation. Autoclaves must meet standards set by the American Society for Testing and Materials (ASTM) and the Association for the Advancement of Medical Instrumentation (AAMI), particularly ANSI/AAMI ST79 which outlines steam sterilization requirements.
European laboratories follow the EN 285 standard for large steam sterilizers and EN 13060 for smaller units. These standards specify performance criteria including temperature uniformity, pressure parameters, and cycle validation methods. Conventional ovens, while useful for dry heat sterilization, must comply with different regulatory frameworks such as ISO 20857:2010 for dry heat sterilization processes.
Documentation requirements represent a significant aspect of regulatory compliance. Both autoclave and conventional oven users must maintain detailed logs of each sterilization cycle, including temperature profiles, duration, and load characteristics. For autoclaves, biological indicators containing Geobacillus stearothermophilus spores are typically required for validation, while Bacillus atrophaeus is standard for dry heat processes in conventional ovens.
Risk assessment frameworks differ substantially between the two technologies. Autoclaves present risks related to pressurized steam, requiring compliance with pressure vessel regulations and safety interlocks. Conventional ovens pose fire hazards and require adherence to electrical safety standards. Both systems must incorporate fail-safe mechanisms and emergency protocols as mandated by laboratory safety regulations.
Recent regulatory trends show increasing emphasis on energy efficiency and environmental impact. The Environmental Protection Agency (EPA) has introduced guidelines encouraging laboratories to adopt more sustainable sterilization practices, potentially influencing equipment selection between autoclaves and conventional ovens based on their respective energy consumption profiles and environmental footprints.
For laboratories seeking accreditation, compliance with ISO/IEC 17025 standards is essential, requiring demonstration of proper sterilization validation regardless of the technology employed. This includes establishing quality management systems that document equipment calibration, maintenance schedules, and operator training programs specific to either autoclave or conventional oven operation.
Energy Efficiency and Sustainability Considerations
Energy consumption represents a critical factor in laboratory operations, with significant implications for both operational costs and environmental impact. Autoclaves typically consume substantially more energy than conventional ovens due to their steam generation requirements and higher operating temperatures. A standard laboratory autoclave may consume between 3-7 kWh per cycle, while comparable conventional ovens typically operate at 1-2 kWh for similar duration cycles. This difference becomes particularly significant in facilities running multiple sterilization cycles daily.
Water consumption presents another sustainability concern unique to autoclaves. Standard models may use 50-150 gallons of water per cycle for steam generation and cooling processes. In contrast, conventional ovens require no water for operation, making them inherently more resource-efficient in water-stressed regions or facilities prioritizing water conservation.
Carbon footprint assessments reveal that autoclave operations generally produce higher greenhouse gas emissions compared to conventional ovens when considering both direct energy consumption and the embedded carbon in water usage. However, this calculation must be balanced against the efficacy of sterilization processes and the potential environmental costs of failed sterilization procedures.
Recent technological innovations have significantly improved the sustainability profile of both technologies. Modern autoclaves increasingly incorporate water recycling systems, reducing consumption by up to 90% compared to older models. Similarly, energy-efficient autoclaves with improved insulation and steam retention mechanisms have reduced energy requirements by 20-30%. Conventional ovens have likewise benefited from advances in insulation technology and precise digital temperature controls that minimize energy waste.
Life cycle assessment (LCA) studies indicate that while autoclaves have higher operational environmental impacts, their durability often exceeds that of conventional ovens, with typical service lives of 15-20 years versus 8-12 years for conventional ovens. This longevity factor partially offsets the higher operational resource consumption when considering total environmental impact over the equipment lifetime.
Laboratory certification programs increasingly incorporate sustainability metrics into their evaluation criteria. The implementation of energy management systems compliant with ISO 50001 standards has demonstrated potential energy savings of 10-15% in laboratory sterilization processes through systematic monitoring and optimization of equipment usage patterns, regardless of the specific technology employed.
Water consumption presents another sustainability concern unique to autoclaves. Standard models may use 50-150 gallons of water per cycle for steam generation and cooling processes. In contrast, conventional ovens require no water for operation, making them inherently more resource-efficient in water-stressed regions or facilities prioritizing water conservation.
Carbon footprint assessments reveal that autoclave operations generally produce higher greenhouse gas emissions compared to conventional ovens when considering both direct energy consumption and the embedded carbon in water usage. However, this calculation must be balanced against the efficacy of sterilization processes and the potential environmental costs of failed sterilization procedures.
Recent technological innovations have significantly improved the sustainability profile of both technologies. Modern autoclaves increasingly incorporate water recycling systems, reducing consumption by up to 90% compared to older models. Similarly, energy-efficient autoclaves with improved insulation and steam retention mechanisms have reduced energy requirements by 20-30%. Conventional ovens have likewise benefited from advances in insulation technology and precise digital temperature controls that minimize energy waste.
Life cycle assessment (LCA) studies indicate that while autoclaves have higher operational environmental impacts, their durability often exceeds that of conventional ovens, with typical service lives of 15-20 years versus 8-12 years for conventional ovens. This longevity factor partially offsets the higher operational resource consumption when considering total environmental impact over the equipment lifetime.
Laboratory certification programs increasingly incorporate sustainability metrics into their evaluation criteria. The implementation of energy management systems compliant with ISO 50001 standards has demonstrated potential energy savings of 10-15% in laboratory sterilization processes through systematic monitoring and optimization of equipment usage patterns, regardless of the specific technology employed.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!