Measure Autoclave Temperature Uniformity for Sterile Processing
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Temperature Uniformity Background and Objectives
Autoclave sterilization has been a cornerstone of infection control in healthcare settings since its development in the late 19th century. The technology has evolved from basic pressure cookers to sophisticated computerized systems, but the fundamental principle remains unchanged: using saturated steam under pressure to eliminate microorganisms. Temperature uniformity within autoclaves represents a critical parameter that directly impacts sterilization efficacy and patient safety.
The historical progression of autoclave technology reveals an increasing focus on temperature distribution. Early models often suffered from significant temperature variations, leading to potential sterilization failures in cooler zones. Modern autoclaves incorporate advanced engineering solutions, yet temperature uniformity remains a persistent challenge due to complex thermodynamic behaviors within the sterilization chamber.
Regulatory bodies worldwide, including the FDA, WHO, and ISO, have established stringent standards for autoclave performance. ISO 17665 specifically addresses temperature uniformity requirements, mandating that temperature variations should not exceed ±1°C during the sterilization cycle. These standards reflect the critical importance of uniform heat distribution in achieving sterility assurance levels (SAL) of 10^-6, the accepted benchmark for medical device sterilization.
Temperature uniformity challenges stem from multiple factors including chamber design, load configuration, steam quality, and air removal efficiency. Cold spots can form due to inadequate steam penetration, air entrapment, or thermal shadowing from dense materials. These variations compromise sterilization effectiveness and potentially endanger patients through inadequately processed medical devices.
The primary objective of temperature uniformity measurement is to identify and eliminate these cold spots through comprehensive mapping of thermal conditions throughout the autoclave chamber. This involves deploying multiple temperature sensors at strategic locations to capture spatial and temporal temperature variations during actual sterilization cycles.
Secondary objectives include validation of sterilization processes, compliance with regulatory requirements, optimization of cycle parameters, and development of loading patterns that minimize temperature variations. These measurements also support preventive maintenance programs by identifying deteriorating components before they lead to sterilization failures.
Recent technological advancements have introduced wireless temperature monitoring systems, real-time data analytics, and computational fluid dynamics modeling to enhance temperature uniformity assessment. These innovations enable more precise identification of problematic areas and facilitate evidence-based improvements to autoclave design and operation protocols.
The ultimate goal extends beyond regulatory compliance to establishing robust quality assurance systems that consistently deliver sterile medical devices, thereby protecting patients from healthcare-associated infections. As healthcare facilities increasingly process complex medical devices with challenging geometries, the importance of temperature uniformity measurement continues to grow as a critical safeguard in sterile processing operations.
The historical progression of autoclave technology reveals an increasing focus on temperature distribution. Early models often suffered from significant temperature variations, leading to potential sterilization failures in cooler zones. Modern autoclaves incorporate advanced engineering solutions, yet temperature uniformity remains a persistent challenge due to complex thermodynamic behaviors within the sterilization chamber.
Regulatory bodies worldwide, including the FDA, WHO, and ISO, have established stringent standards for autoclave performance. ISO 17665 specifically addresses temperature uniformity requirements, mandating that temperature variations should not exceed ±1°C during the sterilization cycle. These standards reflect the critical importance of uniform heat distribution in achieving sterility assurance levels (SAL) of 10^-6, the accepted benchmark for medical device sterilization.
Temperature uniformity challenges stem from multiple factors including chamber design, load configuration, steam quality, and air removal efficiency. Cold spots can form due to inadequate steam penetration, air entrapment, or thermal shadowing from dense materials. These variations compromise sterilization effectiveness and potentially endanger patients through inadequately processed medical devices.
The primary objective of temperature uniformity measurement is to identify and eliminate these cold spots through comprehensive mapping of thermal conditions throughout the autoclave chamber. This involves deploying multiple temperature sensors at strategic locations to capture spatial and temporal temperature variations during actual sterilization cycles.
Secondary objectives include validation of sterilization processes, compliance with regulatory requirements, optimization of cycle parameters, and development of loading patterns that minimize temperature variations. These measurements also support preventive maintenance programs by identifying deteriorating components before they lead to sterilization failures.
Recent technological advancements have introduced wireless temperature monitoring systems, real-time data analytics, and computational fluid dynamics modeling to enhance temperature uniformity assessment. These innovations enable more precise identification of problematic areas and facilitate evidence-based improvements to autoclave design and operation protocols.
The ultimate goal extends beyond regulatory compliance to establishing robust quality assurance systems that consistently deliver sterile medical devices, thereby protecting patients from healthcare-associated infections. As healthcare facilities increasingly process complex medical devices with challenging geometries, the importance of temperature uniformity measurement continues to grow as a critical safeguard in sterile processing operations.
Market Demand Analysis for Precise Sterilization Solutions
The global market for precise sterilization solutions is experiencing robust growth, driven primarily by heightened awareness of infection control protocols across healthcare facilities. The sterile processing market, valued at approximately $7.5 billion in 2022, is projected to reach $12.1 billion by 2028, growing at a CAGR of 8.3%. Within this segment, autoclave temperature uniformity measurement systems represent a critical component, as they directly impact sterilization efficacy and patient safety outcomes.
Healthcare-associated infections (HAIs) continue to pose significant challenges worldwide, with an estimated 1.7 million cases occurring annually in the United States alone, resulting in nearly 99,000 deaths and adding $20 billion in healthcare costs. This pressing concern has intensified regulatory scrutiny and compliance requirements for sterilization processes, creating substantial market demand for advanced temperature uniformity measurement solutions.
The COVID-19 pandemic has further accelerated this demand, as healthcare facilities worldwide have been compelled to reevaluate and strengthen their sterilization protocols. A survey conducted among 450 hospitals revealed that 78% planned to upgrade their sterilization monitoring systems within the next three years, with temperature uniformity measurement capabilities being a top priority for 65% of respondents.
Geographically, North America dominates the market with a 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The fastest growth is anticipated in emerging economies, particularly in India and China, where healthcare infrastructure development and increasing adoption of international sterilization standards are creating new market opportunities.
By end-user segment, hospitals constitute the largest market share at 56%, followed by pharmaceutical manufacturing (18%), medical device companies (14%), and research laboratories (12%). The hospital segment is expected to maintain its dominance due to the high volume of surgical procedures performed and stringent infection control requirements.
Key market drivers include the rising number of surgical procedures globally (increasing at 4.2% annually), growing prevalence of hospital-acquired infections, stringent regulatory frameworks such as the updated EU Medical Device Regulation, and technological advancements in sterilization monitoring systems. Additionally, the shift toward value-based healthcare models has intensified focus on preventing costly complications from inadequate sterilization.
Customer requirements are evolving toward integrated solutions that offer real-time monitoring, automated documentation, and predictive maintenance capabilities. According to industry surveys, 82% of healthcare facilities now prioritize digital connectivity features in their sterilization equipment purchases, representing a significant shift from traditional standalone systems.
Healthcare-associated infections (HAIs) continue to pose significant challenges worldwide, with an estimated 1.7 million cases occurring annually in the United States alone, resulting in nearly 99,000 deaths and adding $20 billion in healthcare costs. This pressing concern has intensified regulatory scrutiny and compliance requirements for sterilization processes, creating substantial market demand for advanced temperature uniformity measurement solutions.
The COVID-19 pandemic has further accelerated this demand, as healthcare facilities worldwide have been compelled to reevaluate and strengthen their sterilization protocols. A survey conducted among 450 hospitals revealed that 78% planned to upgrade their sterilization monitoring systems within the next three years, with temperature uniformity measurement capabilities being a top priority for 65% of respondents.
Geographically, North America dominates the market with a 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The fastest growth is anticipated in emerging economies, particularly in India and China, where healthcare infrastructure development and increasing adoption of international sterilization standards are creating new market opportunities.
By end-user segment, hospitals constitute the largest market share at 56%, followed by pharmaceutical manufacturing (18%), medical device companies (14%), and research laboratories (12%). The hospital segment is expected to maintain its dominance due to the high volume of surgical procedures performed and stringent infection control requirements.
Key market drivers include the rising number of surgical procedures globally (increasing at 4.2% annually), growing prevalence of hospital-acquired infections, stringent regulatory frameworks such as the updated EU Medical Device Regulation, and technological advancements in sterilization monitoring systems. Additionally, the shift toward value-based healthcare models has intensified focus on preventing costly complications from inadequate sterilization.
Customer requirements are evolving toward integrated solutions that offer real-time monitoring, automated documentation, and predictive maintenance capabilities. According to industry surveys, 82% of healthcare facilities now prioritize digital connectivity features in their sterilization equipment purchases, representing a significant shift from traditional standalone systems.
Current Challenges in Autoclave Temperature Measurement
Despite significant advancements in autoclave technology, temperature uniformity measurement in sterilization processes continues to present several critical challenges. The fundamental issue lies in achieving consistent temperature distribution throughout the autoclave chamber, particularly in densely loaded configurations. Current measurement systems often fail to detect cold spots where sterilization parameters may not be met, potentially compromising patient safety in healthcare settings.
Traditional temperature monitoring relies on fixed sensors at predetermined locations, which cannot account for the dynamic thermal behavior within complex loads. These systems typically utilize 3-6 measurement points, insufficient for comprehensive spatial temperature mapping in modern autoclaves that can exceed 1000 liters in capacity. This sampling limitation creates significant blind spots in temperature verification protocols.
The physical properties of different materials being sterilized further complicate measurement accuracy. Dense surgical instruments, porous textiles, and hollow devices each present unique thermal conductivity challenges, creating micro-environments with potentially significant temperature variations. Current measurement technologies struggle to account for these material-specific heat transfer characteristics.
Sensor technology itself presents limitations. Conventional thermocouples and resistance temperature detectors (RTDs) have response times that may miss transient temperature fluctuations during critical sterilization phases. Additionally, wired sensors create practical challenges in placement and may disrupt normal steam flow patterns, potentially altering the very conditions they aim to measure.
Validation protocols present another significant challenge. The industry lacks standardized methodologies for determining optimal sensor placement that would ensure detection of worst-case scenarios. This creates inconsistencies in how temperature uniformity is verified across different facilities and equipment models.
Data interpretation complexities further compound these issues. Current systems generate substantial measurement data but often lack sophisticated analysis tools to translate this information into actionable insights about sterilization efficacy. Many facilities struggle to establish meaningful acceptance criteria beyond simple minimum temperature thresholds.
Regulatory requirements add another layer of complexity, with different standards across regions creating confusion about compliance requirements. FDA, ISO, and regional health authorities maintain different specifications for temperature uniformity, creating challenges for global manufacturers and healthcare facilities operating across multiple jurisdictions.
The economic constraints of implementing comprehensive temperature mapping systems present practical barriers to adoption. Advanced multi-sensor systems with wireless capabilities and real-time monitoring features represent significant capital investments that many facilities find difficult to justify despite their technical advantages.
Traditional temperature monitoring relies on fixed sensors at predetermined locations, which cannot account for the dynamic thermal behavior within complex loads. These systems typically utilize 3-6 measurement points, insufficient for comprehensive spatial temperature mapping in modern autoclaves that can exceed 1000 liters in capacity. This sampling limitation creates significant blind spots in temperature verification protocols.
The physical properties of different materials being sterilized further complicate measurement accuracy. Dense surgical instruments, porous textiles, and hollow devices each present unique thermal conductivity challenges, creating micro-environments with potentially significant temperature variations. Current measurement technologies struggle to account for these material-specific heat transfer characteristics.
Sensor technology itself presents limitations. Conventional thermocouples and resistance temperature detectors (RTDs) have response times that may miss transient temperature fluctuations during critical sterilization phases. Additionally, wired sensors create practical challenges in placement and may disrupt normal steam flow patterns, potentially altering the very conditions they aim to measure.
Validation protocols present another significant challenge. The industry lacks standardized methodologies for determining optimal sensor placement that would ensure detection of worst-case scenarios. This creates inconsistencies in how temperature uniformity is verified across different facilities and equipment models.
Data interpretation complexities further compound these issues. Current systems generate substantial measurement data but often lack sophisticated analysis tools to translate this information into actionable insights about sterilization efficacy. Many facilities struggle to establish meaningful acceptance criteria beyond simple minimum temperature thresholds.
Regulatory requirements add another layer of complexity, with different standards across regions creating confusion about compliance requirements. FDA, ISO, and regional health authorities maintain different specifications for temperature uniformity, creating challenges for global manufacturers and healthcare facilities operating across multiple jurisdictions.
The economic constraints of implementing comprehensive temperature mapping systems present practical barriers to adoption. Advanced multi-sensor systems with wireless capabilities and real-time monitoring features represent significant capital investments that many facilities find difficult to justify despite their technical advantages.
Current Methodologies for Temperature Uniformity Assessment
01 Temperature sensor placement for uniform measurement
Strategic placement of temperature sensors within autoclaves is crucial for ensuring accurate temperature uniformity measurement. Multiple sensors positioned at different locations can monitor temperature variations throughout the chamber. This approach helps identify cold spots and ensures that sterilization parameters are met consistently across the entire autoclave volume, improving process validation and reliability.- Temperature sensor placement for uniform measurement: Strategic placement of temperature sensors within autoclaves is crucial for ensuring accurate temperature uniformity measurement. Multiple sensors positioned at different locations can monitor temperature variations throughout the chamber. This approach helps identify cold spots or areas with temperature fluctuations, ensuring sterilization effectiveness across the entire autoclave chamber. Advanced sensor arrays can create thermal mapping of the autoclave interior, providing comprehensive data on temperature distribution.
- Real-time temperature monitoring systems: Real-time temperature monitoring systems utilize advanced sensors and data acquisition technology to continuously track temperature conditions inside autoclaves. These systems provide immediate feedback on temperature variations, allowing operators to make adjustments during the sterilization cycle. Wireless temperature monitoring solutions enable remote observation of autoclave performance without disrupting the sterilization process. The integration of digital displays and alert mechanisms ensures that temperature uniformity is maintained within specified parameters.
- Temperature uniformity validation methods: Various validation methods are employed to verify temperature uniformity in autoclaves. These include thermal mapping procedures that document temperature distribution throughout the chamber, qualification protocols that establish acceptable temperature ranges, and challenge tests using biological or chemical indicators placed at critical points. Regular validation ensures that all areas within the autoclave reach and maintain the required sterilization temperature. Documentation of these validation processes is essential for regulatory compliance and quality assurance.
- Heating element design for temperature uniformity: The design and arrangement of heating elements significantly impact temperature uniformity in autoclaves. Innovative heating element configurations can eliminate cold spots and ensure even heat distribution throughout the chamber. Some designs incorporate multiple heating zones that can be independently controlled to maintain consistent temperatures across different areas. Circulation systems that actively move heated air or steam throughout the chamber further enhance temperature uniformity. Advanced materials used in heating elements can improve heat transfer efficiency and temperature stability.
- Computational modeling for temperature distribution analysis: Computational fluid dynamics and thermal modeling techniques are increasingly used to analyze and predict temperature distribution within autoclaves. These advanced simulation methods help identify potential areas of temperature non-uniformity before physical testing. Engineers can optimize autoclave design parameters based on simulation results, improving overall temperature uniformity. The models account for factors such as load configuration, steam flow patterns, and chamber geometry that affect heat distribution. This approach reduces development time and enhances the effectiveness of autoclave sterilization processes.
02 Real-time temperature monitoring systems
Advanced real-time monitoring systems enable continuous tracking of temperature conditions within autoclaves. These systems utilize digital sensors and data acquisition technology to provide immediate feedback on temperature uniformity. Real-time monitoring allows operators to detect deviations promptly and make necessary adjustments during the sterilization cycle, enhancing process control and product safety.Expand Specific Solutions03 Thermal mapping and validation techniques
Thermal mapping involves comprehensive temperature profiling throughout the autoclave chamber to validate temperature uniformity. This technique uses multiple temperature probes to create a three-dimensional temperature map, identifying potential cold spots or areas of temperature variation. Regular validation studies ensure that sterilization processes consistently meet required parameters across the entire load, supporting regulatory compliance and process optimization.Expand Specific Solutions04 Improved heating element design and distribution
Innovative heating element designs and distribution patterns can significantly enhance temperature uniformity in autoclaves. Strategic placement of heating elements around the chamber, combined with optimized steam distribution systems, reduces temperature gradients. These design improvements ensure more consistent heat transfer throughout the chamber, minimizing cold spots and improving sterilization efficacy across various load configurations.Expand Specific Solutions05 Computational modeling for temperature uniformity prediction
Computational fluid dynamics (CFD) and thermal modeling techniques are employed to predict and optimize temperature uniformity in autoclaves. These advanced simulation methods analyze heat transfer patterns, steam flow, and potential temperature variations before physical testing. By identifying potential uniformity issues during the design phase, manufacturers can implement corrective measures earlier, resulting in more efficient autoclave designs with superior temperature uniformity characteristics.Expand Specific Solutions
Leading Manufacturers and Solution Providers
The autoclave temperature uniformity measurement market for sterile processing is in a growth phase, driven by increasing regulatory requirements in healthcare and pharmaceutical sectors. The market size is expanding due to rising demand for precise sterilization validation in medical facilities worldwide. Technologically, the field shows varying maturity levels with established players like Fedegari Autoclavi SpA offering advanced solutions alongside newer entrants. Companies such as EBRO Electronic GmbH & Co. KG and Truking Technology Ltd. are developing specialized temperature monitoring systems, while pharmaceutical equipment manufacturers like Fresenius Kabi Deutschland GmbH integrate these capabilities into their sterilization equipment. Research institutions including Zhejiang University contribute to technological advancements, creating a competitive landscape balanced between specialized measurement device manufacturers and comprehensive sterilization solution providers.
Fedegari Autoclavi SpA
Technical Solution: Fedegari has developed advanced temperature mapping systems for autoclaves using wireless data loggers with high-precision platinum RTD sensors (PT100) strategically positioned throughout the chamber. Their proprietary Thema4 process controller integrates with up to 96 temperature probes to create detailed 3D thermal maps of the autoclave chamber. The system employs sophisticated algorithms to analyze temperature distribution patterns and automatically identifies cold spots where sterilization might be compromised. Fedegari's solution includes validation software that complies with FDA 21 CFR Part 11 requirements, enabling real-time monitoring and automated documentation of temperature uniformity tests. Their technology incorporates adaptive PID control systems that continuously adjust heating elements to maintain temperature uniformity within ±0.5°C across the entire chamber, significantly exceeding industry standards.
Strengths: Industry-leading precision with temperature uniformity control within ±0.5°C; comprehensive validation documentation system; wireless technology eliminates cable-related contamination risks. Weaknesses: Higher initial investment compared to conventional systems; requires specialized training for operators to utilize advanced features effectively.
Truking Technology Ltd.
Technical Solution: Truking Technology has engineered a multi-point temperature measurement system for pharmaceutical autoclaves that employs a network of distributed thermal sensors connected to a central monitoring unit. Their solution features high-precision platinum resistance temperature detectors (RTDs) with accuracy of ±0.1°C, positioned according to a mathematical model that optimizes sensor placement based on chamber geometry. The system incorporates real-time data acquisition at 1-second intervals and applies statistical process control methods to analyze temperature variations. Truking's proprietary algorithm creates heat distribution maps that identify potential cold spots and temperature gradients within the chamber. Their technology includes automatic calibration verification and sensor redundancy to ensure measurement reliability. The system integrates with manufacturing execution systems (MES) to provide comprehensive batch documentation and traceability for regulatory compliance.
Strengths: Excellent integration with manufacturing execution systems; high sampling rate captures transient temperature fluctuations; automated calibration verification reduces maintenance requirements. Weaknesses: System complexity requires significant technical support; limited flexibility for retrofitting to non-Truking autoclave models.
Key Technical Innovations in Thermal Mapping Systems
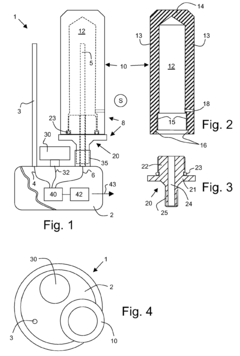
Measuring device for checking the effectiveness of a steam sterilisation process
PatentActiveEP1850102A1
Innovation
- A measuring device with a reversibly connected measuring chamber that can be easily detached from the base body for cleaning and replacement, featuring a hollow body made of high-temperature resistant materials like PEEK or PTFE, allowing for separate access to the temperature sensor and easy exchange of components, along with a pressure sensor for vacuum verification.
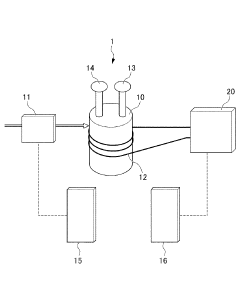
Autoclave device
PatentInactiveJP2018168419A
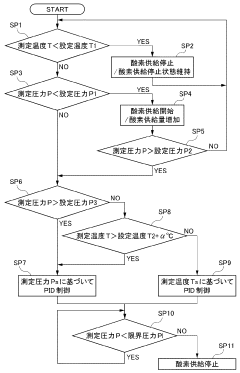
Innovation
- An autoclave apparatus with integrated temperature and pressure measurement units, oxygen flow control, and external heater control, allowing for automatic adjustment of internal conditions based on measured values.
Regulatory Standards and Compliance Requirements
Autoclave sterilization processes are governed by stringent regulatory frameworks designed to ensure patient safety and infection control. The FDA's Quality System Regulation (21 CFR Part 820) establishes comprehensive requirements for medical device manufacturers, including specific provisions for equipment validation and process monitoring. For healthcare facilities, compliance with standards set by organizations such as the Association for the Advancement of Medical Instrumentation (AAMI) is mandatory, particularly AAMI ST79 which provides detailed guidelines for steam sterilization and sterility assurance.
The Joint Commission (TJC) enforces strict compliance with temperature uniformity standards through regular audits and inspections. Healthcare facilities must demonstrate consistent adherence to these standards, with documentation showing that autoclaves maintain specified temperatures throughout the chamber during operation. Non-compliance can result in citations, penalties, or even loss of accreditation, significantly impacting a facility's operations and reputation.
International standards further shape compliance requirements, with ISO 17665 establishing parameters for moist heat sterilization validation and routine control. This standard specifies that temperature variations within autoclave chambers must not exceed ±1°C during the sterilization phase, requiring precise measurement methodologies and calibrated instrumentation. The European Medical Device Regulation (MDR) imposes additional requirements for manufacturers selling equipment in European markets.
Documentation requirements represent a critical aspect of regulatory compliance. Facilities must maintain detailed records of temperature mapping studies, routine monitoring data, calibration certificates, and validation protocols. These records must be readily accessible during regulatory inspections and demonstrate a consistent pattern of compliance over time. The FDA's guidance on process validation emphasizes the need for statistical analysis of temperature uniformity data to establish process capability and reliability.
Risk-based approaches to compliance have gained prominence in recent regulatory frameworks. Facilities must conduct thorough risk assessments to identify potential failure modes in temperature uniformity and implement appropriate monitoring and control strategies. This approach allows for more targeted compliance efforts focused on critical parameters that directly impact sterilization efficacy and patient safety.
Emerging regulatory trends indicate increasing scrutiny of real-time monitoring capabilities and automated documentation systems. Regulatory bodies are placing greater emphasis on continuous monitoring rather than periodic validation, driving innovation in sensor technology and data management systems for autoclave temperature uniformity measurement.
The Joint Commission (TJC) enforces strict compliance with temperature uniformity standards through regular audits and inspections. Healthcare facilities must demonstrate consistent adherence to these standards, with documentation showing that autoclaves maintain specified temperatures throughout the chamber during operation. Non-compliance can result in citations, penalties, or even loss of accreditation, significantly impacting a facility's operations and reputation.
International standards further shape compliance requirements, with ISO 17665 establishing parameters for moist heat sterilization validation and routine control. This standard specifies that temperature variations within autoclave chambers must not exceed ±1°C during the sterilization phase, requiring precise measurement methodologies and calibrated instrumentation. The European Medical Device Regulation (MDR) imposes additional requirements for manufacturers selling equipment in European markets.
Documentation requirements represent a critical aspect of regulatory compliance. Facilities must maintain detailed records of temperature mapping studies, routine monitoring data, calibration certificates, and validation protocols. These records must be readily accessible during regulatory inspections and demonstrate a consistent pattern of compliance over time. The FDA's guidance on process validation emphasizes the need for statistical analysis of temperature uniformity data to establish process capability and reliability.
Risk-based approaches to compliance have gained prominence in recent regulatory frameworks. Facilities must conduct thorough risk assessments to identify potential failure modes in temperature uniformity and implement appropriate monitoring and control strategies. This approach allows for more targeted compliance efforts focused on critical parameters that directly impact sterilization efficacy and patient safety.
Emerging regulatory trends indicate increasing scrutiny of real-time monitoring capabilities and automated documentation systems. Regulatory bodies are placing greater emphasis on continuous monitoring rather than periodic validation, driving innovation in sensor technology and data management systems for autoclave temperature uniformity measurement.
Risk Management in Sterile Processing Validation
Risk management is a critical component of sterile processing validation, particularly when considering temperature uniformity in autoclaves. Effective risk management frameworks must be established to identify, assess, and mitigate potential hazards that could compromise sterilization efficacy. The primary risks associated with autoclave temperature non-uniformity include inadequate microbial inactivation, potential for contaminated instruments, and subsequent patient infections.
A comprehensive risk assessment methodology should follow established standards such as ISO 14971 and AAMI TIR34, which provide guidance specifically for healthcare facilities. These frameworks recommend a systematic approach beginning with hazard identification through techniques like Failure Mode and Effects Analysis (FMEA) or Hazard Analysis and Critical Control Points (HACCP). For autoclave temperature uniformity, critical failure modes include sensor malfunction, steam distribution issues, loading configuration problems, and calibration drift.
Risk evaluation must quantify both the probability and severity of each identified risk. Temperature non-uniformity presents particularly high-risk scenarios when cold spots remain below the minimum sterilization temperature (typically 121°C for gravity displacement or 132°C for pre-vacuum cycles). Statistical process control methods can help establish acceptable variability thresholds based on validated sterilization parameters.
Implementation of risk control measures should follow a hierarchical approach. Engineering controls such as improved autoclave design with better steam distribution systems offer the highest level of protection. Administrative controls include development of standardized loading patterns, regular preventive maintenance schedules, and comprehensive staff training programs. Detection mechanisms like independent temperature monitoring systems provide additional safeguards.
Ongoing risk monitoring requires establishment of key performance indicators (KPIs) that track temperature uniformity over time. These may include maximum temperature differentials between monitoring points, cycle failure rates, and biological indicator test results. Regular review of these metrics enables continuous improvement of the sterilization process.
Documentation plays a crucial role in risk management, with all assessments, control measures, and monitoring results requiring thorough recording. This documentation serves both regulatory compliance purposes and provides evidence for quality assurance reviews. Many healthcare facilities now implement electronic tracking systems that integrate risk management data with equipment maintenance records and sterilization cycle parameters.
A comprehensive risk assessment methodology should follow established standards such as ISO 14971 and AAMI TIR34, which provide guidance specifically for healthcare facilities. These frameworks recommend a systematic approach beginning with hazard identification through techniques like Failure Mode and Effects Analysis (FMEA) or Hazard Analysis and Critical Control Points (HACCP). For autoclave temperature uniformity, critical failure modes include sensor malfunction, steam distribution issues, loading configuration problems, and calibration drift.
Risk evaluation must quantify both the probability and severity of each identified risk. Temperature non-uniformity presents particularly high-risk scenarios when cold spots remain below the minimum sterilization temperature (typically 121°C for gravity displacement or 132°C for pre-vacuum cycles). Statistical process control methods can help establish acceptable variability thresholds based on validated sterilization parameters.
Implementation of risk control measures should follow a hierarchical approach. Engineering controls such as improved autoclave design with better steam distribution systems offer the highest level of protection. Administrative controls include development of standardized loading patterns, regular preventive maintenance schedules, and comprehensive staff training programs. Detection mechanisms like independent temperature monitoring systems provide additional safeguards.
Ongoing risk monitoring requires establishment of key performance indicators (KPIs) that track temperature uniformity over time. These may include maximum temperature differentials between monitoring points, cycle failure rates, and biological indicator test results. Regular review of these metrics enables continuous improvement of the sterilization process.
Documentation plays a crucial role in risk management, with all assessments, control measures, and monitoring results requiring thorough recording. This documentation serves both regulatory compliance purposes and provides evidence for quality assurance reviews. Many healthcare facilities now implement electronic tracking systems that integrate risk management data with equipment maintenance records and sterilization cycle parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!