Autoclave Pressure Settings: Adjustments for Optimal Sterilization
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Technology Background and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This technology leverages the principles of moist heat under pressure to eliminate microorganisms through protein denaturation and coagulation. The historical progression of autoclave technology demonstrates a continuous refinement in pressure control mechanisms, from manual gauges to sophisticated digital systems that offer precise adjustments and monitoring capabilities.
The current technological landscape of autoclave sterilization encompasses various types of systems, including gravity displacement, pre-vacuum, and steam-flush pressure-pulse autoclaves, each employing different pressure profiles to achieve sterilization. Modern autoclaves incorporate advanced pressure sensors, microprocessor controls, and automated validation systems that ensure consistent sterilization outcomes across diverse load configurations and material types.
Recent advancements in pressure management algorithms have significantly enhanced the efficiency and reliability of autoclave operations. These innovations allow for dynamic pressure adjustments during the sterilization cycle, responding to variables such as load density, moisture content, and thermal conductivity of materials being sterilized. Such adaptability represents a substantial improvement over traditional fixed-parameter approaches.
The primary objective of optimizing autoclave pressure settings is to achieve complete sterilization while minimizing cycle time, energy consumption, and potential damage to sterilized items. This optimization requires a delicate balance between pressure, temperature, and time parameters, as these factors collectively determine the efficacy of microbial inactivation. Research indicates that even minor deviations in pressure settings can significantly impact sterilization outcomes, particularly for challenging loads or specialized materials.
Global healthcare standards, including those established by organizations such as the World Health Organization (WHO) and the Association for the Advancement of Medical Instrumentation (AAMI), have increasingly emphasized the importance of precise pressure control in autoclave operations. These standards reflect growing recognition that optimal pressure settings must be tailored to specific sterilization requirements rather than applying universal parameters across all scenarios.
The trajectory of autoclave technology development points toward increasingly intelligent systems capable of real-time pressure adjustments based on continuous monitoring of critical parameters. This evolution aligns with broader trends in medical technology, including the integration of Internet of Things (IoT) capabilities, artificial intelligence for predictive maintenance, and enhanced data analytics for process optimization and validation.
The current technological landscape of autoclave sterilization encompasses various types of systems, including gravity displacement, pre-vacuum, and steam-flush pressure-pulse autoclaves, each employing different pressure profiles to achieve sterilization. Modern autoclaves incorporate advanced pressure sensors, microprocessor controls, and automated validation systems that ensure consistent sterilization outcomes across diverse load configurations and material types.
Recent advancements in pressure management algorithms have significantly enhanced the efficiency and reliability of autoclave operations. These innovations allow for dynamic pressure adjustments during the sterilization cycle, responding to variables such as load density, moisture content, and thermal conductivity of materials being sterilized. Such adaptability represents a substantial improvement over traditional fixed-parameter approaches.
The primary objective of optimizing autoclave pressure settings is to achieve complete sterilization while minimizing cycle time, energy consumption, and potential damage to sterilized items. This optimization requires a delicate balance between pressure, temperature, and time parameters, as these factors collectively determine the efficacy of microbial inactivation. Research indicates that even minor deviations in pressure settings can significantly impact sterilization outcomes, particularly for challenging loads or specialized materials.
Global healthcare standards, including those established by organizations such as the World Health Organization (WHO) and the Association for the Advancement of Medical Instrumentation (AAMI), have increasingly emphasized the importance of precise pressure control in autoclave operations. These standards reflect growing recognition that optimal pressure settings must be tailored to specific sterilization requirements rather than applying universal parameters across all scenarios.
The trajectory of autoclave technology development points toward increasingly intelligent systems capable of real-time pressure adjustments based on continuous monitoring of critical parameters. This evolution aligns with broader trends in medical technology, including the integration of Internet of Things (IoT) capabilities, artificial intelligence for predictive maintenance, and enhanced data analytics for process optimization and validation.
Market Demand Analysis for Advanced Sterilization Solutions
The global sterilization market is experiencing robust growth, driven by increasing healthcare expenditures, rising surgical procedures, and growing awareness of infection control protocols. The market for advanced sterilization solutions was valued at approximately 6.7 billion USD in 2022 and is projected to reach 10.5 billion USD by 2028, representing a compound annual growth rate of 7.8% during the forecast period.
Healthcare facilities constitute the largest segment of end-users, with hospitals accounting for over 40% of the market share. The COVID-19 pandemic has significantly accelerated demand for effective sterilization technologies, particularly those offering improved efficiency and reliability in pressure management systems for autoclaves.
Pharmaceutical and biotechnology companies represent the fastest-growing segment, with increasing requirements for precise sterilization parameters to ensure product integrity and compliance with stringent regulatory standards. These industries demand autoclave systems with advanced pressure control mechanisms that can be validated and documented for regulatory submissions.
Regional analysis indicates North America currently holds the largest market share at 35%, followed by Europe at 28% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the highest growth rate due to expanding healthcare infrastructure, increasing surgical procedures, and growing awareness about hospital-acquired infections in emerging economies like China and India.
The demand for autoclave pressure optimization solutions is particularly strong in ambulatory surgical centers and clinics, which are increasingly adopting outpatient surgical procedures. These facilities require efficient, reliable sterilization systems with precise pressure control to maintain quick turnaround times while ensuring complete sterilization.
Market research indicates that end-users are increasingly prioritizing autoclave systems with intelligent pressure management features, including automated pressure adjustments based on load type, real-time monitoring capabilities, and data logging for compliance documentation. The willingness to pay premium prices for these advanced features has increased by 15% over the past three years.
Consumer behavior analysis reveals growing preference for sterilization equipment with reduced cycle times achieved through optimized pressure profiles. Healthcare facilities are increasingly recognizing that precise pressure control not only improves sterilization efficacy but also extends equipment lifespan and reduces operational costs through energy efficiency.
Market forecasts suggest that demand for retrofit pressure optimization systems for existing autoclaves will grow significantly, as healthcare facilities seek cost-effective solutions to upgrade their sterilization capabilities without complete system replacement. This represents a substantial market opportunity for pressure control technology providers.
Healthcare facilities constitute the largest segment of end-users, with hospitals accounting for over 40% of the market share. The COVID-19 pandemic has significantly accelerated demand for effective sterilization technologies, particularly those offering improved efficiency and reliability in pressure management systems for autoclaves.
Pharmaceutical and biotechnology companies represent the fastest-growing segment, with increasing requirements for precise sterilization parameters to ensure product integrity and compliance with stringent regulatory standards. These industries demand autoclave systems with advanced pressure control mechanisms that can be validated and documented for regulatory submissions.
Regional analysis indicates North America currently holds the largest market share at 35%, followed by Europe at 28% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the highest growth rate due to expanding healthcare infrastructure, increasing surgical procedures, and growing awareness about hospital-acquired infections in emerging economies like China and India.
The demand for autoclave pressure optimization solutions is particularly strong in ambulatory surgical centers and clinics, which are increasingly adopting outpatient surgical procedures. These facilities require efficient, reliable sterilization systems with precise pressure control to maintain quick turnaround times while ensuring complete sterilization.
Market research indicates that end-users are increasingly prioritizing autoclave systems with intelligent pressure management features, including automated pressure adjustments based on load type, real-time monitoring capabilities, and data logging for compliance documentation. The willingness to pay premium prices for these advanced features has increased by 15% over the past three years.
Consumer behavior analysis reveals growing preference for sterilization equipment with reduced cycle times achieved through optimized pressure profiles. Healthcare facilities are increasingly recognizing that precise pressure control not only improves sterilization efficacy but also extends equipment lifespan and reduces operational costs through energy efficiency.
Market forecasts suggest that demand for retrofit pressure optimization systems for existing autoclaves will grow significantly, as healthcare facilities seek cost-effective solutions to upgrade their sterilization capabilities without complete system replacement. This represents a substantial market opportunity for pressure control technology providers.
Current Autoclave Pressure Technology Challenges
Despite significant advancements in autoclave technology, several critical challenges persist in pressure management systems that impact sterilization efficacy. The primary technical obstacle involves achieving and maintaining precise pressure levels throughout the sterilization cycle. Current pressure control mechanisms often exhibit fluctuations of ±0.2 bar, which can compromise sterilization outcomes for sensitive medical instruments and complex laboratory equipment. These variations become particularly problematic when processing dense loads or porous materials that require consistent pressure penetration.
Another significant challenge is the energy inefficiency of conventional pressure generation systems. Most commercial autoclaves utilize steam generators that consume substantial electrical power, with typical industrial models requiring 15-20 kW/hour during operation. This high energy consumption translates to increased operational costs and environmental impact, creating a technological bottleneck for facilities seeking sustainable sterilization solutions.
The integration of pressure monitoring with other critical parameters presents additional complications. Current systems struggle to synchronize pressure adjustments with temperature variations and humidity levels in real-time. This limitation results in suboptimal sterilization cycles, particularly for specialized applications like pharmaceutical production or advanced biomaterial processing, where precise environmental control is essential for product integrity.
Material constraints further compound pressure technology challenges. Pressure chamber components experience accelerated degradation due to repeated exposure to high-pressure steam environments, leading to maintenance issues and potential safety concerns. The average service life of pressure seals and gaskets remains limited to approximately 500-700 cycles before requiring replacement, creating reliability issues for high-throughput operations.
Geographical disparities in autoclave pressure technology adoption are evident globally. While North American and European facilities predominantly utilize advanced digital pressure control systems with ±0.1 bar precision, developing regions often rely on analog pressure management with wider tolerance ranges of ±0.5 bar. This technological gap impacts global standardization efforts and creates inconsistencies in sterilization protocols across international healthcare networks.
The miniaturization trend in medical devices has introduced new pressure-related challenges. Smaller autoclave systems designed for point-of-care applications struggle to maintain stable pressure profiles in compact chambers, with pressure uniformity deviations reaching up to 15% in some portable units. This limitation restricts the deployment of sterilization capabilities in resource-constrained or field environments where conventional infrastructure is unavailable.
Another significant challenge is the energy inefficiency of conventional pressure generation systems. Most commercial autoclaves utilize steam generators that consume substantial electrical power, with typical industrial models requiring 15-20 kW/hour during operation. This high energy consumption translates to increased operational costs and environmental impact, creating a technological bottleneck for facilities seeking sustainable sterilization solutions.
The integration of pressure monitoring with other critical parameters presents additional complications. Current systems struggle to synchronize pressure adjustments with temperature variations and humidity levels in real-time. This limitation results in suboptimal sterilization cycles, particularly for specialized applications like pharmaceutical production or advanced biomaterial processing, where precise environmental control is essential for product integrity.
Material constraints further compound pressure technology challenges. Pressure chamber components experience accelerated degradation due to repeated exposure to high-pressure steam environments, leading to maintenance issues and potential safety concerns. The average service life of pressure seals and gaskets remains limited to approximately 500-700 cycles before requiring replacement, creating reliability issues for high-throughput operations.
Geographical disparities in autoclave pressure technology adoption are evident globally. While North American and European facilities predominantly utilize advanced digital pressure control systems with ±0.1 bar precision, developing regions often rely on analog pressure management with wider tolerance ranges of ±0.5 bar. This technological gap impacts global standardization efforts and creates inconsistencies in sterilization protocols across international healthcare networks.
The miniaturization trend in medical devices has introduced new pressure-related challenges. Smaller autoclave systems designed for point-of-care applications struggle to maintain stable pressure profiles in compact chambers, with pressure uniformity deviations reaching up to 15% in some portable units. This limitation restricts the deployment of sterilization capabilities in resource-constrained or field environments where conventional infrastructure is unavailable.
Current Pressure Setting Optimization Methodologies
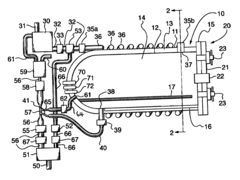
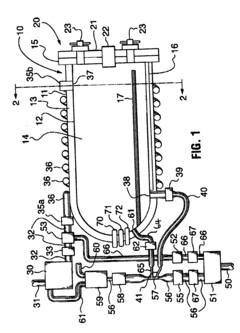
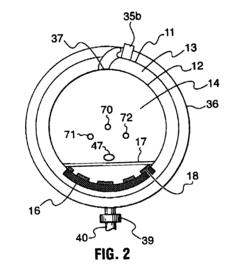
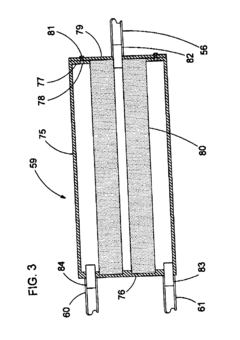
01 Pressure settings for sterilization efficiency
Autoclave pressure settings are critical for ensuring effective sterilization of medical instruments and materials. The pressure typically ranges from 15-30 psi (103-207 kPa) and works in conjunction with temperature (usually 121-134°C) to kill microorganisms. Higher pressure settings allow for higher temperatures, which reduce the time required for complete sterilization. The relationship between pressure, temperature, and time must be carefully calibrated to achieve sterility assurance levels required for medical applications.- Standard pressure settings for different autoclave applications: Autoclaves operate at specific pressure settings depending on the application. For medical sterilization, pressures typically range from 15-30 psi (103-207 kPa), while industrial applications may require higher pressures of 30-60 psi (207-414 kPa). The pressure settings are critical for achieving the required temperature for effective sterilization or processing. Different materials and items being sterilized may require specific pressure settings to ensure complete elimination of microorganisms without damaging the items.
- Pressure control mechanisms in modern autoclaves: Modern autoclaves incorporate sophisticated pressure control mechanisms to maintain precise pressure levels throughout the sterilization cycle. These mechanisms include pressure sensors, relief valves, and electronic control systems that continuously monitor and adjust pressure. Some advanced systems feature programmable pressure profiles that can automatically adjust pressure settings based on the load type and sterilization requirements. These control mechanisms ensure safety and effectiveness by preventing over-pressurization while maintaining the necessary conditions for sterilization.
- Relationship between pressure and temperature in autoclave operation: The relationship between pressure and temperature in autoclaves follows the principles of thermodynamics, where increased pressure allows water to reach higher temperatures before boiling. This relationship is crucial for achieving sterilization, as most autoclaves operate at 121-134°C, which requires pressures of 15-30 psi above atmospheric pressure. The precise control of this pressure-temperature relationship ensures that the autoclave reaches and maintains the conditions necessary for effective sterilization while preventing damage to sensitive materials.
- Pressure cycling and pulsing techniques: Advanced autoclave systems employ pressure cycling or pulsing techniques to enhance sterilization effectiveness. These techniques involve alternating between high and low pressure states to improve steam penetration into complex items. Pre-vacuum cycles reduce air pockets by creating negative pressure before steam introduction, while post-vacuum cycles help with drying. Pressure pulsing can significantly reduce sterilization time while ensuring complete elimination of microorganisms, particularly in loads with complex geometries or dense materials.
- Safety features related to pressure management: Autoclaves incorporate multiple safety features to manage pressure-related risks. These include pressure relief valves that automatically release excess pressure, redundant pressure monitoring systems, and emergency shutdown mechanisms. Door interlock systems prevent opening while the chamber is pressurized, and some models feature dual pressure control systems for additional safety. Regular calibration and maintenance of these safety features are essential to prevent accidents and ensure reliable operation of the autoclave under various pressure conditions.
02 Pressure control systems and monitoring
Modern autoclaves incorporate sophisticated pressure control systems that maintain optimal pressure throughout the sterilization cycle. These systems include pressure sensors, regulators, safety valves, and electronic controllers that continuously monitor and adjust pressure levels. Some advanced systems feature automated pressure profiling capabilities that can adjust pressure settings based on load type and sterilization requirements. Real-time monitoring and data logging of pressure parameters ensure compliance with sterilization protocols and regulatory standards.Expand Specific Solutions03 Pressure settings for different materials and applications
Different materials and applications require specific autoclave pressure settings. Delicate instruments may require lower pressure settings to prevent damage, while dense materials might need higher pressure for effective sterilization. Industrial applications often use higher pressures than medical applications. Specialized pressure cycles have been developed for porous loads, liquids, and heat-sensitive materials. The composition, density, and thermal conductivity of the materials being sterilized directly influence the optimal pressure settings required.Expand Specific Solutions04 Safety mechanisms for pressure regulation
Autoclaves incorporate multiple safety mechanisms to prevent pressure-related accidents. These include pressure relief valves that automatically release excess pressure, redundant pressure monitoring systems, door interlocking mechanisms that prevent opening under pressure, and emergency shutdown systems. Modern autoclaves also feature alarms that alert operators to pressure anomalies and automated systems that can safely abort cycles if pressure parameters exceed safe limits. These safety features are designed to protect both equipment and operators from potential hazards associated with high-pressure steam environments.Expand Specific Solutions05 Innovative pressure cycling techniques
Recent innovations in autoclave technology include advanced pressure cycling techniques that enhance sterilization efficiency. These include pulsed pressure profiles, pressure-vacuum alternating cycles, and variable pressure patterns that improve steam penetration into complex instruments. Some systems employ rapid pressure changes to create turbulence that dislodges air pockets and ensures complete steam contact with all surfaces. These innovative approaches can reduce cycle times, improve energy efficiency, and enhance sterilization effectiveness for challenging loads such as long-lumen instruments and porous materials.Expand Specific Solutions
Leading Manufacturers and Competitive Landscape
The autoclave pressure settings market is in a mature growth phase with a global market size estimated at $2-3 billion annually. The technology has reached high maturity levels, with incremental innovations focusing on optimization rather than disruptive changes. Key players demonstrate varying technological capabilities: Fedegari Autoclavi SpA leads with advanced pressure control systems for pharmaceutical applications, while Shinva Medical and Truking Technology dominate in Asia with cost-effective solutions. Olympus and MEDIVATORS focus on specialized medical sterilization, and Straumann and Dentsply target dental applications. The competitive landscape shows regional specialization with European companies (Fedegari) emphasizing precision engineering, Asian manufacturers (Shinva) prioritizing scale, and North American firms focusing on specialized healthcare applications.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva Medical has developed advanced pressure control systems for their autoclaves featuring dynamic pressure adjustment technology. Their solution incorporates multi-parameter monitoring with real-time feedback loops that continuously adjust pressure settings based on load characteristics and sterilization phase. The system employs proprietary algorithms that analyze temperature distribution, moisture content, and air removal efficiency to optimize pressure profiles throughout the sterilization cycle. Shinva's pressure management system includes automatic compensation mechanisms that account for altitude variations and different load densities, ensuring consistent sterilization efficacy across diverse operating environments. Their latest models feature precision pressure sensors with accuracy within ±0.01 bar and rapid response times under 0.5 seconds, enabling precise control during critical transition phases.
Strengths: Superior pressure stability with minimal fluctuations (±0.01 bar) enabling consistent sterilization results across varied load types. Advanced algorithm-based pressure profiling optimizes cycle times while maintaining sterilization efficacy. Weaknesses: Higher initial investment compared to basic systems, and requires more technical expertise for maintenance and calibration of the sophisticated pressure control components.
Olympus Corp.
Technical Solution: Olympus has developed a specialized pressure management system for their medical autoclaves focused on endoscope reprocessing and delicate instrument sterilization. Their solution features a gentle pressure ramping technology that carefully controls pressure increase and decrease rates to protect sensitive instruments while ensuring sterilization efficacy. The system employs dual-sensor verification where independent pressure sensors cross-validate readings to prevent over-pressurization events that could damage delicate medical devices. Olympus' technology includes specialized pressure profiles optimized for hollow lumen instruments, with precisely timed pressure pulses that enhance steam penetration into challenging geometries. Their pressure control system incorporates instrument-specific pressure limits stored in a database that automatically applies the appropriate pressure parameters based on the identified load contents.
Strengths: Specialized pressure profiles optimized for delicate and complex medical instruments, particularly endoscopes with challenging geometries. Excellent protection against pressure-related instrument damage through sophisticated safety systems. Weaknesses: More focused on specialized medical applications rather than general-purpose sterilization, and limited capacity compared to industrial-scale systems.
Key Technical Innovations in Pressure Control Systems
Method and system for steam sterilization of endoscopes
PatentInactiveEP3915459A1
Innovation
- A method and apparatus utilizing superheated steam delivered through pressure-resistant fittings and controlled by a microprocessor, with optional suction mechanisms and temperature/pressure sensors, to ensure thorough sterilization of endoscope channels while minimizing damage to the equipment.
Fixed vacuum-insulated saturated steam autoclave
PatentInactiveUS20060057021A1
Innovation
- A double-walled vacuum-sealed vessel with a self-contained water supply and heat-conductive metal tubing, combined with a PID temperature controller and PIC microprocessor, allows for precise temperature control and rapid steam generation and removal, using concurrent positive and negative air pressures to ensure thorough sterilization and efficient operation.
Material Compatibility and Load Configuration Considerations
The effectiveness of autoclave sterilization is significantly influenced by the compatibility of materials being processed and the configuration of the load within the chamber. Different materials respond uniquely to pressure and temperature combinations, necessitating careful consideration when establishing autoclave protocols. Materials such as stainless steel, glass, and certain heat-resistant plastics can withstand standard autoclave conditions, while others may deform, melt, or release harmful substances when exposed to high pressure and temperature.
For metal instruments, higher pressure settings can be utilized without compromising structural integrity, allowing for more aggressive sterilization cycles. However, delicate instruments with complex mechanisms may require modified pressure settings to prevent damage to moving parts or seals. Similarly, porous materials like textiles and paper wrappings demand specific pressure considerations to ensure steam penetration without causing excessive moisture retention or degradation.
Load configuration within the autoclave chamber directly impacts pressure distribution and steam penetration. Overcrowded chambers create "shadowing" effects where items shield others from direct steam contact, resulting in incomplete sterilization despite appropriate pressure readings. Strategic spacing between items allows for optimal steam circulation and uniform pressure application throughout the load. Vertical positioning of containers with openings facing downward facilitates air displacement and prevents condensate pooling, which can interfere with sterilization efficacy.
The relationship between load density and pressure requirements follows a generally proportional pattern. Denser loads with higher thermal mass require sustained pressure to achieve temperature equilibrium throughout all items. Conversely, lighter loads may achieve sterilization with shorter pressure holding times. This relationship must be quantified through validation studies specific to common load configurations used in the facility.
Packaging materials introduce additional variables affecting pressure requirements. Wrapped instruments require higher pressure differentials to force steam through barrier materials compared to unwrapped items. The permeability characteristics of different wrapping materials (paper, non-woven fabrics, rigid containers) necessitate adjustments to pressure parameters to ensure adequate steam penetration while maintaining package integrity.
Standardized loading patterns based on material compatibility groups can optimize autoclave efficiency while ensuring sterilization efficacy. These patterns should account for thermal conductivity differences between materials, preventing situations where items requiring different pressure-temperature exposures are processed together. Implementation of such standardized approaches reduces cycle failures and extends the operational life of both the autoclave and the processed items.
For metal instruments, higher pressure settings can be utilized without compromising structural integrity, allowing for more aggressive sterilization cycles. However, delicate instruments with complex mechanisms may require modified pressure settings to prevent damage to moving parts or seals. Similarly, porous materials like textiles and paper wrappings demand specific pressure considerations to ensure steam penetration without causing excessive moisture retention or degradation.
Load configuration within the autoclave chamber directly impacts pressure distribution and steam penetration. Overcrowded chambers create "shadowing" effects where items shield others from direct steam contact, resulting in incomplete sterilization despite appropriate pressure readings. Strategic spacing between items allows for optimal steam circulation and uniform pressure application throughout the load. Vertical positioning of containers with openings facing downward facilitates air displacement and prevents condensate pooling, which can interfere with sterilization efficacy.
The relationship between load density and pressure requirements follows a generally proportional pattern. Denser loads with higher thermal mass require sustained pressure to achieve temperature equilibrium throughout all items. Conversely, lighter loads may achieve sterilization with shorter pressure holding times. This relationship must be quantified through validation studies specific to common load configurations used in the facility.
Packaging materials introduce additional variables affecting pressure requirements. Wrapped instruments require higher pressure differentials to force steam through barrier materials compared to unwrapped items. The permeability characteristics of different wrapping materials (paper, non-woven fabrics, rigid containers) necessitate adjustments to pressure parameters to ensure adequate steam penetration while maintaining package integrity.
Standardized loading patterns based on material compatibility groups can optimize autoclave efficiency while ensuring sterilization efficacy. These patterns should account for thermal conductivity differences between materials, preventing situations where items requiring different pressure-temperature exposures are processed together. Implementation of such standardized approaches reduces cycle failures and extends the operational life of both the autoclave and the processed items.
Energy Efficiency and Sustainability in Autoclave Operations
The optimization of energy consumption in autoclave operations represents a critical frontier in sustainable sterilization practices. Modern healthcare and industrial facilities face mounting pressure to reduce their environmental footprint while maintaining rigorous sterilization standards. Autoclave pressure settings directly impact energy consumption, with higher pressures typically requiring greater energy inputs to achieve and maintain temperature thresholds.
Recent advancements in autoclave technology have introduced variable pressure algorithms that dynamically adjust pressure settings based on load characteristics, resulting in energy savings of 15-25% compared to traditional fixed-pressure cycles. These smart systems incorporate load sensors and predictive modeling to determine the minimum effective pressure required for complete sterilization, eliminating wasteful energy expenditure.
Heat recovery systems represent another significant advancement in autoclave sustainability. By capturing and repurposing waste heat from exhaust steam, facilities can reduce overall energy consumption by up to 30%. Integrated heat exchangers transfer thermal energy to incoming water or adjacent systems, creating a closed-loop efficiency improvement that reduces both energy costs and environmental impact.
Water conservation technologies complement pressure optimization strategies in modern autoclave design. Vacuum-assisted systems achieve sterilization temperatures at lower pressures, reducing both energy and water requirements. Advanced water reclamation systems can recover up to 80% of water used in autoclave operations, significantly reducing resource consumption in water-stressed regions.
The implementation of preventive maintenance protocols specifically targeting pressure components has demonstrated substantial energy efficiency improvements. Regular calibration of pressure sensors, inspection of gaskets and seals, and optimization of steam delivery systems can prevent pressure fluctuations that lead to extended cycle times and increased energy usage. Studies indicate that well-maintained autoclaves consume 10-15% less energy than those operating with suboptimal pressure control systems.
Carbon footprint analyses of autoclave operations reveal that pressure optimization represents the single most impactful intervention for sustainability improvement. By implementing pressure-optimized sterilization cycles, healthcare facilities can reduce associated carbon emissions by 20-40% depending on energy source and autoclave design. This reduction becomes increasingly significant as facilities transition to renewable energy sources, creating multiplicative sustainability benefits.
Future developments in pressure-optimized autoclave technology point toward AI-driven systems capable of continuous learning and adaptation. These systems will analyze historical performance data to further refine pressure settings based on specific load characteristics, ambient conditions, and sterilization requirements, potentially unlocking additional energy savings of 5-10% beyond current optimization techniques.
Recent advancements in autoclave technology have introduced variable pressure algorithms that dynamically adjust pressure settings based on load characteristics, resulting in energy savings of 15-25% compared to traditional fixed-pressure cycles. These smart systems incorporate load sensors and predictive modeling to determine the minimum effective pressure required for complete sterilization, eliminating wasteful energy expenditure.
Heat recovery systems represent another significant advancement in autoclave sustainability. By capturing and repurposing waste heat from exhaust steam, facilities can reduce overall energy consumption by up to 30%. Integrated heat exchangers transfer thermal energy to incoming water or adjacent systems, creating a closed-loop efficiency improvement that reduces both energy costs and environmental impact.
Water conservation technologies complement pressure optimization strategies in modern autoclave design. Vacuum-assisted systems achieve sterilization temperatures at lower pressures, reducing both energy and water requirements. Advanced water reclamation systems can recover up to 80% of water used in autoclave operations, significantly reducing resource consumption in water-stressed regions.
The implementation of preventive maintenance protocols specifically targeting pressure components has demonstrated substantial energy efficiency improvements. Regular calibration of pressure sensors, inspection of gaskets and seals, and optimization of steam delivery systems can prevent pressure fluctuations that lead to extended cycle times and increased energy usage. Studies indicate that well-maintained autoclaves consume 10-15% less energy than those operating with suboptimal pressure control systems.
Carbon footprint analyses of autoclave operations reveal that pressure optimization represents the single most impactful intervention for sustainability improvement. By implementing pressure-optimized sterilization cycles, healthcare facilities can reduce associated carbon emissions by 20-40% depending on energy source and autoclave design. This reduction becomes increasingly significant as facilities transition to renewable energy sources, creating multiplicative sustainability benefits.
Future developments in pressure-optimized autoclave technology point toward AI-driven systems capable of continuous learning and adaptation. These systems will analyze historical performance data to further refine pressure settings based on specific load characteristics, ambient conditions, and sterilization requirements, potentially unlocking additional energy savings of 5-10% beyond current optimization techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!