How to Manage Autoclave Inventory for Operational Efficiency
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Inventory Management Background and Objectives
Autoclave sterilization has been a cornerstone of infection control and material processing across healthcare, laboratory, and industrial settings for over a century. The evolution of autoclave technology has progressed from basic pressure cookers to sophisticated computerized systems capable of precise sterilization cycles. Despite technological advancements, inventory management for autoclave supplies and materials remains a persistent operational challenge that impacts efficiency, cost control, and regulatory compliance.
The primary objective of effective autoclave inventory management is to ensure continuous operational readiness while optimizing resource utilization. This involves maintaining appropriate stock levels of sterilization pouches, biological indicators, chemical indicators, wrapping materials, and replacement parts without excessive capital tied up in inventory. Historical approaches to autoclave inventory management have largely relied on manual tracking systems, which are prone to human error and inefficiencies.
Current industry data indicates that healthcare facilities typically experience 15-20% wastage in autoclave supplies due to improper inventory management, with an estimated 30% of autoclave downtime attributed to unavailability of necessary consumables or replacement components. These inefficiencies translate to significant operational costs and potential compliance risks, particularly in highly regulated environments such as hospitals and pharmaceutical manufacturing.
The technological landscape for autoclave inventory management has evolved from paper-based systems to barcode tracking, and more recently to RFID-enabled solutions and IoT-connected inventory systems. This progression reflects the growing recognition of inventory management as a critical factor in operational excellence rather than merely an administrative function.
Regulatory frameworks, including those from organizations such as the FDA, CDC, and ISO, have increasingly emphasized the importance of traceability and documentation in sterilization processes, further driving the need for sophisticated inventory management solutions. These regulations require detailed records of sterilization cycles, including the specific materials and supplies used, creating additional complexity in inventory tracking requirements.
The convergence of lean management principles with digital transformation initiatives has created new opportunities for optimizing autoclave inventory management. The goal is to develop systems that not only track inventory levels but also predict usage patterns, automate reordering processes, and integrate with broader facility management systems to create a holistic approach to operational efficiency.
This technical research aims to comprehensively examine current approaches to autoclave inventory management, identify key challenges and bottlenecks, and explore emerging technologies and methodologies that can significantly enhance operational efficiency in this critical area.
The primary objective of effective autoclave inventory management is to ensure continuous operational readiness while optimizing resource utilization. This involves maintaining appropriate stock levels of sterilization pouches, biological indicators, chemical indicators, wrapping materials, and replacement parts without excessive capital tied up in inventory. Historical approaches to autoclave inventory management have largely relied on manual tracking systems, which are prone to human error and inefficiencies.
Current industry data indicates that healthcare facilities typically experience 15-20% wastage in autoclave supplies due to improper inventory management, with an estimated 30% of autoclave downtime attributed to unavailability of necessary consumables or replacement components. These inefficiencies translate to significant operational costs and potential compliance risks, particularly in highly regulated environments such as hospitals and pharmaceutical manufacturing.
The technological landscape for autoclave inventory management has evolved from paper-based systems to barcode tracking, and more recently to RFID-enabled solutions and IoT-connected inventory systems. This progression reflects the growing recognition of inventory management as a critical factor in operational excellence rather than merely an administrative function.
Regulatory frameworks, including those from organizations such as the FDA, CDC, and ISO, have increasingly emphasized the importance of traceability and documentation in sterilization processes, further driving the need for sophisticated inventory management solutions. These regulations require detailed records of sterilization cycles, including the specific materials and supplies used, creating additional complexity in inventory tracking requirements.
The convergence of lean management principles with digital transformation initiatives has created new opportunities for optimizing autoclave inventory management. The goal is to develop systems that not only track inventory levels but also predict usage patterns, automate reordering processes, and integrate with broader facility management systems to create a holistic approach to operational efficiency.
This technical research aims to comprehensively examine current approaches to autoclave inventory management, identify key challenges and bottlenecks, and explore emerging technologies and methodologies that can significantly enhance operational efficiency in this critical area.
Market Demand Analysis for Efficient Sterilization Workflows
The global sterilization equipment market is experiencing significant growth, with the autoclave segment maintaining a dominant position due to its reliability and effectiveness in healthcare settings. Market research indicates the global medical sterilization equipment market was valued at approximately $7.5 billion in 2022 and is projected to reach $12.1 billion by 2028, growing at a CAGR of 8.3%. This growth is primarily driven by increasing surgical procedures, rising hospital-acquired infections, and stringent regulatory requirements for sterilization.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, represent the largest market segment for autoclave inventory management solutions. A survey of 500 healthcare facilities revealed that 78% reported challenges with their current sterilization workflows, citing inefficiencies in tracking, scheduling, and maintenance of autoclave equipment and supplies. These inefficiencies directly impact operational costs and patient care quality.
The COVID-19 pandemic has significantly accelerated demand for efficient sterilization workflows. Healthcare facilities reported a 35% increase in sterilization requirements during peak pandemic periods, highlighting the critical need for optimized inventory management systems. This surge has created a sustained market opportunity as facilities seek to maintain higher sterilization standards even post-pandemic.
Dental practices represent another rapidly growing market segment, with over 200,000 dental offices in the United States alone requiring regular sterilization of instruments. Research indicates that dental practices lose an average of 4.2 productive hours weekly due to inefficient sterilization workflows, translating to approximately $24,000 in annual revenue loss per practice.
Pharmaceutical and biotechnology companies constitute a premium market segment with specialized requirements for autoclave inventory management. These organizations prioritize validation documentation, precise cycle parameters, and integration with quality management systems. The pharmaceutical sterilization equipment market is growing at 9.7% annually, outpacing the overall market.
Emerging economies present substantial growth opportunities, with healthcare infrastructure development in Asia-Pacific and Latin America regions driving demand for modern sterilization equipment and management systems. China and India are experiencing particularly rapid growth rates of 12.4% and 11.8% respectively in the medical sterilization equipment market.
Customer pain points consistently identified across market segments include inventory stockouts (reported by 67% of facilities), difficulty tracking sterilization status (62%), maintenance scheduling challenges (58%), and lack of data-driven optimization (71%). These challenges represent specific market needs that effective autoclave inventory management solutions must address to capture market share.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, represent the largest market segment for autoclave inventory management solutions. A survey of 500 healthcare facilities revealed that 78% reported challenges with their current sterilization workflows, citing inefficiencies in tracking, scheduling, and maintenance of autoclave equipment and supplies. These inefficiencies directly impact operational costs and patient care quality.
The COVID-19 pandemic has significantly accelerated demand for efficient sterilization workflows. Healthcare facilities reported a 35% increase in sterilization requirements during peak pandemic periods, highlighting the critical need for optimized inventory management systems. This surge has created a sustained market opportunity as facilities seek to maintain higher sterilization standards even post-pandemic.
Dental practices represent another rapidly growing market segment, with over 200,000 dental offices in the United States alone requiring regular sterilization of instruments. Research indicates that dental practices lose an average of 4.2 productive hours weekly due to inefficient sterilization workflows, translating to approximately $24,000 in annual revenue loss per practice.
Pharmaceutical and biotechnology companies constitute a premium market segment with specialized requirements for autoclave inventory management. These organizations prioritize validation documentation, precise cycle parameters, and integration with quality management systems. The pharmaceutical sterilization equipment market is growing at 9.7% annually, outpacing the overall market.
Emerging economies present substantial growth opportunities, with healthcare infrastructure development in Asia-Pacific and Latin America regions driving demand for modern sterilization equipment and management systems. China and India are experiencing particularly rapid growth rates of 12.4% and 11.8% respectively in the medical sterilization equipment market.
Customer pain points consistently identified across market segments include inventory stockouts (reported by 67% of facilities), difficulty tracking sterilization status (62%), maintenance scheduling challenges (58%), and lack of data-driven optimization (71%). These challenges represent specific market needs that effective autoclave inventory management solutions must address to capture market share.
Current Challenges in Autoclave Inventory Control Systems
The current landscape of autoclave inventory control systems reveals several significant challenges that impede operational efficiency in healthcare facilities, laboratories, and manufacturing environments. Traditional inventory management approaches often rely on manual tracking methods, including paper-based logs and spreadsheets, which are inherently prone to human error and inconsistency. These methods frequently result in documentation discrepancies, missing items, and inefficient resource allocation.
Real-time inventory visibility remains a persistent challenge, as many facilities lack systems capable of providing immediate insights into autoclave supply levels, usage patterns, and reprocessing status. This limitation creates bottlenecks in workflow, particularly during high-demand periods when critical supplies may be unexpectedly depleted, leading to operational disruptions and potential delays in patient care or production processes.
Integration issues between inventory management systems and other operational software platforms further complicate efficient autoclave inventory control. Many facilities operate with disparate systems that do not communicate effectively, creating information silos that prevent comprehensive data analysis and informed decision-making. This fragmentation often necessitates duplicate data entry, increasing administrative burden and error potential.
Forecasting and demand planning present additional challenges, as many current systems lack sophisticated predictive analytics capabilities. Without accurate forecasting tools, facilities struggle to anticipate usage patterns, seasonal fluctuations, and exceptional demand scenarios, resulting in either overstocking (tying up capital and storage space) or understocking (causing operational delays).
Compliance tracking and documentation requirements add another layer of complexity to autoclave inventory management. Healthcare facilities must maintain detailed records for regulatory purposes, including sterilization parameters, maintenance schedules, and usage history. Current systems often fail to automate these documentation processes, creating compliance risks and increasing administrative workload.
Cost control and optimization remain elusive goals within existing inventory management frameworks. Many facilities lack visibility into the true costs associated with autoclave operations, including procurement, maintenance, energy consumption, and labor. Without this financial transparency, organizations struggle to identify cost-saving opportunities and optimize resource allocation.
Lastly, staff training and system adoption present ongoing challenges. Complex inventory management systems often face resistance from staff accustomed to traditional methods, while inadequate training programs can limit the effectiveness of even the most sophisticated technological solutions. This human factor significantly impacts the successful implementation of improved inventory control systems.
Real-time inventory visibility remains a persistent challenge, as many facilities lack systems capable of providing immediate insights into autoclave supply levels, usage patterns, and reprocessing status. This limitation creates bottlenecks in workflow, particularly during high-demand periods when critical supplies may be unexpectedly depleted, leading to operational disruptions and potential delays in patient care or production processes.
Integration issues between inventory management systems and other operational software platforms further complicate efficient autoclave inventory control. Many facilities operate with disparate systems that do not communicate effectively, creating information silos that prevent comprehensive data analysis and informed decision-making. This fragmentation often necessitates duplicate data entry, increasing administrative burden and error potential.
Forecasting and demand planning present additional challenges, as many current systems lack sophisticated predictive analytics capabilities. Without accurate forecasting tools, facilities struggle to anticipate usage patterns, seasonal fluctuations, and exceptional demand scenarios, resulting in either overstocking (tying up capital and storage space) or understocking (causing operational delays).
Compliance tracking and documentation requirements add another layer of complexity to autoclave inventory management. Healthcare facilities must maintain detailed records for regulatory purposes, including sterilization parameters, maintenance schedules, and usage history. Current systems often fail to automate these documentation processes, creating compliance risks and increasing administrative workload.
Cost control and optimization remain elusive goals within existing inventory management frameworks. Many facilities lack visibility into the true costs associated with autoclave operations, including procurement, maintenance, energy consumption, and labor. Without this financial transparency, organizations struggle to identify cost-saving opportunities and optimize resource allocation.
Lastly, staff training and system adoption present ongoing challenges. Complex inventory management systems often face resistance from staff accustomed to traditional methods, while inadequate training programs can limit the effectiveness of even the most sophisticated technological solutions. This human factor significantly impacts the successful implementation of improved inventory control systems.
Current Technological Solutions for Autoclave Tracking
01 Automated inventory tracking systems for autoclaves
Automated systems can be implemented to track inventory within autoclaves, ensuring efficient use of space and processing time. These systems use sensors and RFID technology to monitor items being sterilized, their location within the autoclave, and their processing status. This automation reduces manual tracking errors, improves throughput, and provides real-time visibility of sterilization processes, ultimately enhancing operational efficiency in medical and laboratory settings.- Automated inventory tracking systems for autoclaves: Automated systems can be implemented to track inventory in autoclave environments, enhancing operational efficiency. These systems utilize RFID tags, barcodes, or other identification technologies to monitor sterilization equipment and supplies in real-time. The automation reduces manual tracking errors, ensures proper stock levels, and provides accurate data on equipment usage and maintenance needs, ultimately improving workflow in medical and laboratory settings.
- Real-time monitoring and management of autoclave operations: Real-time monitoring systems for autoclaves enable efficient management of sterilization processes. These solutions incorporate sensors and IoT technology to track operational parameters, cycle completion, and equipment status. By providing immediate feedback on autoclave performance, these systems help maintain quality control, reduce downtime, and optimize resource allocation, leading to improved operational efficiency and compliance with sterilization protocols.
- Supply chain optimization for autoclave consumables: Supply chain management systems specifically designed for autoclave consumables help optimize inventory levels and reduce costs. These systems implement just-in-time ordering, vendor-managed inventory, and predictive analytics to forecast usage patterns. By streamlining the procurement process for sterilization supplies, healthcare facilities and laboratories can minimize storage requirements, prevent stockouts, and reduce waste from expired materials.
- Integration of autoclave management with facility-wide systems: Integrated management systems connect autoclave operations with broader facility management platforms. These solutions synchronize sterilization equipment data with enterprise resource planning, maintenance management, and quality assurance systems. The integration enables comprehensive visibility across departments, facilitates resource allocation, and supports data-driven decision making, resulting in improved operational efficiency throughout the organization.
- Mobile applications for autoclave inventory management: Mobile applications provide flexible solutions for managing autoclave inventory and operations. These apps allow staff to scan, track, and update sterilization equipment status from anywhere in the facility using smartphones or tablets. Features typically include push notifications for cycle completion, maintenance alerts, and inventory level warnings. The mobility aspect reduces response time, improves staff productivity, and enhances overall operational efficiency in sterilization departments.
02 Predictive maintenance and monitoring for autoclave systems
Implementing predictive maintenance strategies for autoclave equipment can significantly improve operational efficiency. By continuously monitoring key parameters such as temperature, pressure, and cycle times, potential issues can be identified before they cause equipment failure. These systems analyze performance data to schedule maintenance during non-peak periods, reducing unexpected downtime and extending equipment lifespan while ensuring sterilization quality remains consistent.Expand Specific Solutions03 Supply chain integration for autoclave consumables
Integrating autoclave inventory management with broader supply chain systems enables just-in-time delivery of consumables and replacement parts. These integrated systems automatically trigger reordering when supplies reach predetermined thresholds, preventing stockouts while minimizing excess inventory. By connecting autoclave usage data with procurement systems, organizations can optimize their purchasing patterns, reduce carrying costs, and ensure continuous operation without interruptions due to supply shortages.Expand Specific Solutions04 Workflow optimization through load planning and scheduling
Advanced scheduling and load planning systems can optimize autoclave utilization by efficiently grouping items with similar sterilization requirements. These systems analyze incoming sterilization needs, equipment availability, and processing parameters to create optimal loading configurations and processing schedules. By maximizing the items processed in each cycle while maintaining sterilization standards, facilities can increase throughput, reduce energy consumption, and improve overall operational efficiency.Expand Specific Solutions05 Digital twin technology for autoclave process simulation
Digital twin technology creates virtual models of autoclave systems to simulate and optimize sterilization processes before implementation. These digital replicas allow operators to test different loading configurations, cycle parameters, and maintenance schedules without disrupting actual operations. By identifying inefficiencies and bottlenecks in a virtual environment, organizations can implement data-driven improvements to their physical autoclave systems, resulting in enhanced operational efficiency, reduced resource consumption, and improved sterilization outcomes.Expand Specific Solutions
Leading Providers in Autoclave Inventory Management Solutions
The autoclave inventory management market is currently in a growth phase, with increasing demand for operational efficiency solutions across industries. The market size is expanding due to the rising adoption of automated inventory systems in manufacturing, healthcare, and aerospace sectors. Technologically, the field is evolving from manual tracking to sophisticated digital solutions, with varying levels of maturity. Companies like Chengdu Haike Machinery and Inner Mongolia Xiongyuan Technology lead in manufacturing specialized autoclave equipment, while technology giants such as IBM, Siemens, and Hitachi are developing advanced inventory management systems. Software providers like ZAICO and Vimaan Robotics are introducing AI and computer vision solutions specifically for inventory tracking, creating a competitive landscape where traditional manufacturing expertise meets cutting-edge digital innovation.
Vimaan Robotics, Inc.
Technical Solution: Vimaan Robotics has pioneered an advanced computer vision and drone-based solution specifically for autoclave inventory management. Their system employs autonomous drones equipped with high-resolution cameras and thermal sensors to conduct regular inventory audits of autoclave equipment without disrupting operations. The drones capture detailed visual data of autoclave positioning, status indicators, and maintenance tags, while their proprietary computer vision algorithms automatically identify and catalog each piece of equipment. The system integrates with digital twins of the facility to track autoclave movement and utilization in real-time. Vimaan's cloud platform provides analytics on autoclave cycle efficiency, space utilization, and workflow optimization, with reported inventory accuracy improvements of over 99% and labor cost reductions of up to 80% compared to manual inventory processes.
Strengths: Non-intrusive inventory monitoring without operational disruption; highly accurate spatial tracking capabilities; reduced human error in inventory counts. Weaknesses: Higher initial capital investment; requires appropriate facility conditions for drone operation; potential regulatory hurdles in certain industries.
ZAICO, Inc.
Technical Solution: ZAICO has developed a specialized cloud-based inventory management system that addresses the unique challenges of autoclave equipment tracking. Their solution features QR code and barcode scanning capabilities specifically optimized for high-temperature, high-pressure environments. The platform includes specialized modules for tracking autoclave cycles, maintenance schedules, and sterilization validation. ZAICO's mobile-first approach allows technicians to update inventory status in real-time from the production floor, while their API-driven architecture enables integration with existing ERP and CMMS systems. The company has implemented machine learning algorithms that analyze historical usage patterns to optimize autoclave scheduling and predict maintenance needs, resulting in reported efficiency improvements of up to 30% for clients in healthcare and manufacturing sectors.
Strengths: Purpose-built for inventory tracking in challenging industrial environments; intuitive mobile interface reduces training requirements; flexible deployment options including cloud and on-premises. Weaknesses: More limited enterprise-level integration capabilities compared to larger vendors; smaller support network for global operations; fewer advanced analytics features.
Key Innovations in Sterilization Process Monitoring
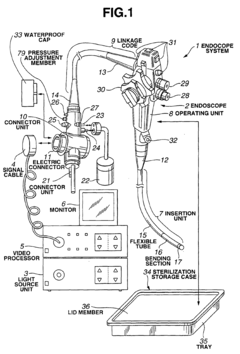
Storage device for steam sterilization
PatentInactiveEP1566185A1
Innovation
- A steam sterilization storing device comprising cooling holding means, drying holding means, and storage means, which includes fans and air channels to efficiently cool and dry the equipment, allowing for rapid reuse by integrating cooling and drying functions within a single unit.
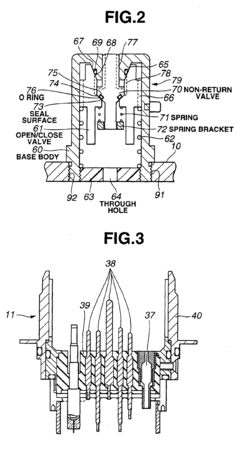
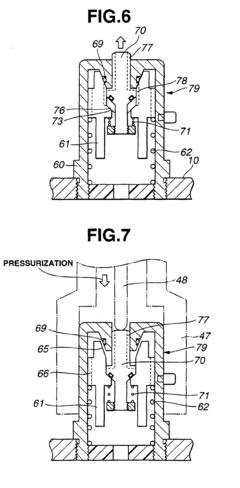
High-pressure steam sterilization system for medical equipment, and device and method for sterilizing the medical equipment
PatentInactiveEP1452186A1
Innovation
- A medical equipment autoclaving system with a communication vent and pressure-adjusting check valve that opens only when internal pressure exceeds external pressure, allowing controlled depressurization and pressurization processes to prevent damage and ensure drying, while maintaining a lower pressure during the dry phase to prevent moisture accumulation.
Regulatory Compliance in Medical Device Sterilization
Medical device sterilization is subject to stringent regulatory frameworks that ensure patient safety and infection control. For autoclave inventory management, compliance with regulations such as FDA's Quality System Regulation (21 CFR Part 820), ISO 13485 for medical device quality management systems, and specific sterilization standards like ISO 17665 for moist heat sterilization is mandatory. These regulations establish requirements for validation, monitoring, documentation, and traceability throughout the sterilization process.
Healthcare facilities must maintain comprehensive records of sterilization cycles, including parameters like temperature, pressure, and exposure time for each batch of processed instruments. This documentation serves as evidence of compliance during regulatory inspections and audits. Failure to meet these requirements can result in severe consequences, including facility closure, product recalls, and legal liability.
Effective autoclave inventory management systems must incorporate regulatory compliance features that automatically track sterilization parameters and maintain electronic records that satisfy regulatory requirements. These systems should generate alerts for cycle failures, parameter deviations, or when instruments approach their sterilization expiration dates.
The regulatory landscape continues to evolve, with increasing emphasis on unique device identification (UDI) requirements that enhance traceability throughout the medical device lifecycle. Modern inventory management solutions must accommodate these requirements by incorporating barcode or RFID technology that links each instrument to its complete sterilization history.
Risk management is another critical aspect of regulatory compliance in sterilization processes. Healthcare facilities must implement systems that identify potential failure modes, assess their impact on patient safety, and establish mitigation measures. This includes regular calibration and maintenance of autoclaves, staff training programs, and validation protocols for new instrument types.
International harmonization efforts are underway to standardize sterilization requirements across different regulatory jurisdictions, though significant regional variations remain. Organizations operating globally must navigate these differences while maintaining consistent quality standards. Cloud-based inventory management systems can help by incorporating region-specific compliance modules that adapt to local regulatory requirements.
Regulatory bodies increasingly focus on environmental considerations in sterilization processes, including energy efficiency and waste management. Advanced autoclave inventory systems can optimize load configurations and cycle scheduling to reduce resource consumption while maintaining compliance with sterilization standards.
Healthcare facilities must maintain comprehensive records of sterilization cycles, including parameters like temperature, pressure, and exposure time for each batch of processed instruments. This documentation serves as evidence of compliance during regulatory inspections and audits. Failure to meet these requirements can result in severe consequences, including facility closure, product recalls, and legal liability.
Effective autoclave inventory management systems must incorporate regulatory compliance features that automatically track sterilization parameters and maintain electronic records that satisfy regulatory requirements. These systems should generate alerts for cycle failures, parameter deviations, or when instruments approach their sterilization expiration dates.
The regulatory landscape continues to evolve, with increasing emphasis on unique device identification (UDI) requirements that enhance traceability throughout the medical device lifecycle. Modern inventory management solutions must accommodate these requirements by incorporating barcode or RFID technology that links each instrument to its complete sterilization history.
Risk management is another critical aspect of regulatory compliance in sterilization processes. Healthcare facilities must implement systems that identify potential failure modes, assess their impact on patient safety, and establish mitigation measures. This includes regular calibration and maintenance of autoclaves, staff training programs, and validation protocols for new instrument types.
International harmonization efforts are underway to standardize sterilization requirements across different regulatory jurisdictions, though significant regional variations remain. Organizations operating globally must navigate these differences while maintaining consistent quality standards. Cloud-based inventory management systems can help by incorporating region-specific compliance modules that adapt to local regulatory requirements.
Regulatory bodies increasingly focus on environmental considerations in sterilization processes, including energy efficiency and waste management. Advanced autoclave inventory systems can optimize load configurations and cycle scheduling to reduce resource consumption while maintaining compliance with sterilization standards.
Cost-Benefit Analysis of Automated Inventory Solutions
Implementing automated inventory management solutions for autoclave operations requires careful financial analysis to justify the investment. Initial implementation costs for automated systems typically range from $50,000 to $250,000 depending on facility size and complexity. This includes hardware components (RFID readers, barcode scanners, sensors), software platforms, integration services, and staff training. While substantial, these costs should be evaluated against the long-term operational benefits.
The return on investment timeline generally spans 18-36 months, with larger facilities achieving faster payback periods due to economies of scale. Labor cost reduction represents one of the most significant benefits, with automated systems reducing inventory management time by 65-80%. This translates to approximately 15-25 hours saved weekly for medium-sized facilities, allowing staff to focus on higher-value activities.
Inventory carrying cost reduction provides another substantial benefit. Automated systems typically reduce excess inventory by 20-30% through improved visibility and demand forecasting. For facilities with $500,000 in autoclave supplies, this represents $100,000-150,000 in freed capital. Additionally, automated solutions minimize emergency orders and rush shipping fees, which often carry 25-40% premiums over standard procurement.
Error reduction capabilities of automated systems deliver both direct and indirect cost benefits. Manual inventory processes have error rates of 3-5%, while automated systems reduce this to below 0.5%. Each error avoided saves not only the cost of the item but also prevents potential procedure delays, which can cost $2,000-5,000 per hour in operating room settings.
Maintenance and ongoing costs must be factored into the analysis. Annual software licensing typically runs 15-20% of initial software costs, while hardware maintenance averages 5-10% of equipment value. Cloud-based solutions may reduce these costs but introduce subscription fees ranging from $500-2,500 monthly depending on facility size and transaction volume.
Scalability considerations affect long-term value. Systems with modular designs allow for incremental expansion without complete replacement, providing better lifetime value despite potentially higher initial costs. The most cost-effective solutions offer integration capabilities with existing hospital management systems, avoiding duplicate data entry and maximizing workflow efficiency.
The return on investment timeline generally spans 18-36 months, with larger facilities achieving faster payback periods due to economies of scale. Labor cost reduction represents one of the most significant benefits, with automated systems reducing inventory management time by 65-80%. This translates to approximately 15-25 hours saved weekly for medium-sized facilities, allowing staff to focus on higher-value activities.
Inventory carrying cost reduction provides another substantial benefit. Automated systems typically reduce excess inventory by 20-30% through improved visibility and demand forecasting. For facilities with $500,000 in autoclave supplies, this represents $100,000-150,000 in freed capital. Additionally, automated solutions minimize emergency orders and rush shipping fees, which often carry 25-40% premiums over standard procurement.
Error reduction capabilities of automated systems deliver both direct and indirect cost benefits. Manual inventory processes have error rates of 3-5%, while automated systems reduce this to below 0.5%. Each error avoided saves not only the cost of the item but also prevents potential procedure delays, which can cost $2,000-5,000 per hour in operating room settings.
Maintenance and ongoing costs must be factored into the analysis. Annual software licensing typically runs 15-20% of initial software costs, while hardware maintenance averages 5-10% of equipment value. Cloud-based solutions may reduce these costs but introduce subscription fees ranging from $500-2,500 monthly depending on facility size and transaction volume.
Scalability considerations affect long-term value. Systems with modular designs allow for incremental expansion without complete replacement, providing better lifetime value despite potentially higher initial costs. The most cost-effective solutions offer integration capabilities with existing hospital management systems, avoiding duplicate data entry and maximizing workflow efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!