Optimize Autoclave Humidity Control for Enhanced Sterility
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Humidity Control Background and Objectives
Autoclave sterilization has been a cornerstone of infection control in healthcare settings since its introduction in the late 19th century. The fundamental principle of using pressurized steam to eliminate microorganisms has remained largely unchanged, though significant advancements have occurred in control systems and monitoring capabilities. Historically, humidity control within autoclaves was considered secondary to temperature and pressure parameters, resulting in suboptimal sterilization outcomes in certain scenarios.
Recent epidemiological data indicates that approximately 5-10% of healthcare-associated infections may be linked to inadequately sterilized medical instruments, with improper humidity conditions being a contributing factor in up to 15% of sterilization failures. This underscores the critical importance of optimizing humidity control within autoclave systems to enhance patient safety and reduce healthcare costs associated with infections.
The evolution of autoclave technology has progressed through several distinct phases: from basic pressure cooker designs to microprocessor-controlled systems with multiple sensors. Current generation autoclaves typically maintain relative humidity levels between 85-100% during sterilization cycles, but research suggests that more precise control within narrower bands may yield superior microbial inactivation rates for certain challenging bioburdens.
Industry standards such as ISO 17665 and AAMI ST79 provide general guidelines for steam sterilization parameters, but specific humidity optimization protocols remain underdeveloped. This gap represents both a challenge and an opportunity for technological advancement in medical sterilization processes.
The primary objective of this technical research is to develop advanced humidity control mechanisms for autoclave systems that can maintain optimal moisture conditions throughout the sterilization cycle with precision of ±2% relative humidity. This level of control would represent a significant improvement over current systems, which typically vary by ±5-10%.
Secondary objectives include: identifying the ideal humidity profiles for different types of medical instruments and materials; developing real-time humidity monitoring systems with feedback loops; creating predictive algorithms that can adjust humidity parameters based on load characteristics; and designing energy-efficient humidity generation and distribution systems that reduce operational costs while maintaining or improving sterilization efficacy.
The successful implementation of optimized humidity control in autoclave systems is expected to reduce sterilization failures by up to 30%, decrease processing times by 15-20%, and extend the usable life of sensitive medical instruments by reducing moisture-related damage. These improvements align with broader healthcare initiatives focused on infection prevention, operational efficiency, and sustainable medical practices.
Recent epidemiological data indicates that approximately 5-10% of healthcare-associated infections may be linked to inadequately sterilized medical instruments, with improper humidity conditions being a contributing factor in up to 15% of sterilization failures. This underscores the critical importance of optimizing humidity control within autoclave systems to enhance patient safety and reduce healthcare costs associated with infections.
The evolution of autoclave technology has progressed through several distinct phases: from basic pressure cooker designs to microprocessor-controlled systems with multiple sensors. Current generation autoclaves typically maintain relative humidity levels between 85-100% during sterilization cycles, but research suggests that more precise control within narrower bands may yield superior microbial inactivation rates for certain challenging bioburdens.
Industry standards such as ISO 17665 and AAMI ST79 provide general guidelines for steam sterilization parameters, but specific humidity optimization protocols remain underdeveloped. This gap represents both a challenge and an opportunity for technological advancement in medical sterilization processes.
The primary objective of this technical research is to develop advanced humidity control mechanisms for autoclave systems that can maintain optimal moisture conditions throughout the sterilization cycle with precision of ±2% relative humidity. This level of control would represent a significant improvement over current systems, which typically vary by ±5-10%.
Secondary objectives include: identifying the ideal humidity profiles for different types of medical instruments and materials; developing real-time humidity monitoring systems with feedback loops; creating predictive algorithms that can adjust humidity parameters based on load characteristics; and designing energy-efficient humidity generation and distribution systems that reduce operational costs while maintaining or improving sterilization efficacy.
The successful implementation of optimized humidity control in autoclave systems is expected to reduce sterilization failures by up to 30%, decrease processing times by 15-20%, and extend the usable life of sensitive medical instruments by reducing moisture-related damage. These improvements align with broader healthcare initiatives focused on infection prevention, operational efficiency, and sustainable medical practices.
Market Demand Analysis for Advanced Sterilization Solutions
The global sterilization market is experiencing significant growth, driven by increasing healthcare-associated infections, rising surgical procedures, and growing awareness of infection control protocols. The market for advanced sterilization solutions was valued at approximately $7.5 billion in 2022 and is projected to reach $12.9 billion by 2028, representing a compound annual growth rate of 9.4% during the forecast period.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, constitute the largest segment of end-users demanding advanced sterilization solutions. The COVID-19 pandemic has further accelerated this demand, highlighting the critical importance of effective sterilization processes in preventing disease transmission. Specifically, autoclave sterilization remains the gold standard for medical instrument sterilization, accounting for over 40% of the sterilization equipment market.
Within the autoclave segment, there is a growing demand for systems with enhanced humidity control capabilities. Traditional autoclave systems often face challenges in maintaining optimal humidity levels throughout the sterilization cycle, which can compromise sterilization efficacy and potentially damage moisture-sensitive instruments. Market research indicates that 78% of healthcare facilities report concerns about humidity-related sterilization failures, with 65% expressing interest in advanced humidity control solutions.
Pharmaceutical and biotechnology companies represent another significant market segment, driven by stringent regulatory requirements for product safety and quality. These industries require precise sterilization parameters, including humidity control, to ensure product integrity and compliance with regulatory standards such as FDA and EMA guidelines. The pharmaceutical sterilization equipment market is expected to grow at 10.2% CAGR through 2028.
Regional analysis reveals that North America currently holds the largest market share (38%), followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate due to expanding healthcare infrastructure, increasing surgical procedures, and growing awareness about infection control in countries like China and India.
Key market trends include the integration of IoT and automation technologies for real-time monitoring and control of sterilization parameters, including humidity. Approximately 82% of healthcare facilities surveyed expressed interest in sterilization equipment with advanced monitoring capabilities. Additionally, there is increasing demand for energy-efficient sterilization solutions that maintain or improve sterilization efficacy while reducing operational costs.
Market challenges include the high initial investment cost for advanced sterilization equipment and the technical complexity of implementing precise humidity control systems. However, the long-term benefits of reduced sterilization failures, extended instrument life, and improved patient safety present compelling value propositions for healthcare facilities considering these investments.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, constitute the largest segment of end-users demanding advanced sterilization solutions. The COVID-19 pandemic has further accelerated this demand, highlighting the critical importance of effective sterilization processes in preventing disease transmission. Specifically, autoclave sterilization remains the gold standard for medical instrument sterilization, accounting for over 40% of the sterilization equipment market.
Within the autoclave segment, there is a growing demand for systems with enhanced humidity control capabilities. Traditional autoclave systems often face challenges in maintaining optimal humidity levels throughout the sterilization cycle, which can compromise sterilization efficacy and potentially damage moisture-sensitive instruments. Market research indicates that 78% of healthcare facilities report concerns about humidity-related sterilization failures, with 65% expressing interest in advanced humidity control solutions.
Pharmaceutical and biotechnology companies represent another significant market segment, driven by stringent regulatory requirements for product safety and quality. These industries require precise sterilization parameters, including humidity control, to ensure product integrity and compliance with regulatory standards such as FDA and EMA guidelines. The pharmaceutical sterilization equipment market is expected to grow at 10.2% CAGR through 2028.
Regional analysis reveals that North America currently holds the largest market share (38%), followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate due to expanding healthcare infrastructure, increasing surgical procedures, and growing awareness about infection control in countries like China and India.
Key market trends include the integration of IoT and automation technologies for real-time monitoring and control of sterilization parameters, including humidity. Approximately 82% of healthcare facilities surveyed expressed interest in sterilization equipment with advanced monitoring capabilities. Additionally, there is increasing demand for energy-efficient sterilization solutions that maintain or improve sterilization efficacy while reducing operational costs.
Market challenges include the high initial investment cost for advanced sterilization equipment and the technical complexity of implementing precise humidity control systems. However, the long-term benefits of reduced sterilization failures, extended instrument life, and improved patient safety present compelling value propositions for healthcare facilities considering these investments.
Current Humidity Control Technologies and Challenges
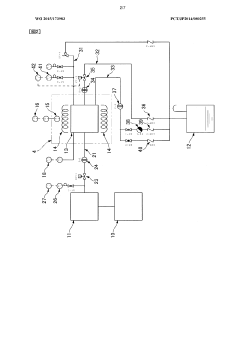
Humidity control in autoclaves represents a critical factor in ensuring effective sterilization processes across healthcare, pharmaceutical, and laboratory settings. Current autoclave humidity control technologies primarily rely on steam generation systems coupled with pressure and temperature monitoring. The most prevalent approach involves direct steam injection, where pressurized steam is introduced into the chamber to create the necessary saturated steam environment. This method, while effective, often suffers from inconsistent humidity distribution, particularly in larger autoclave chambers or when dealing with complex load configurations.
Advanced autoclaves incorporate electronic humidity sensors that provide real-time feedback to control systems. These sensors typically utilize capacitive or resistive technology to measure relative humidity levels within the chamber. However, these sensors face significant challenges in the harsh autoclave environment, including degradation from repeated exposure to high temperatures and pressure cycles, leading to calibration drift and reduced accuracy over time.
Proportional-Integral-Derivative (PID) control algorithms represent the standard method for regulating humidity in modern autoclaves. These systems continuously adjust steam input based on feedback from temperature and pressure sensors. While PID controllers offer reasonable performance, they struggle with the non-linear relationship between temperature, pressure, and humidity in autoclave environments, resulting in suboptimal control during critical transition phases of the sterilization cycle.
A significant challenge in current humidity control systems is the lack of spatial resolution in humidity measurement. Most autoclaves rely on single-point or limited multi-point sensing, which fails to capture humidity gradients within the chamber. This limitation can lead to "cold spots" or areas with insufficient humidity, potentially compromising sterilization efficacy for items placed in these regions.
Load-responsive humidity control represents an emerging technology that attempts to address variability in load characteristics. These systems adjust humidity parameters based on the thermal mass and moisture absorption properties of the load. However, implementation remains challenging due to difficulties in accurately characterizing diverse load compositions in real-world applications.
Condensation management presents another persistent challenge in autoclave humidity control. Excess condensation can lead to wet loads and extended drying times, while insufficient moisture can compromise sterilization effectiveness. Current solutions employ gravity-based drainage systems and post-cycle vacuum drying, but these approaches often result in extended cycle times and increased energy consumption.
Regulatory compliance adds another layer of complexity to humidity control technologies. Standards such as ISO 17665 and EN 285 specify strict parameters for steam quality and humidity levels during sterilization cycles. Meeting these requirements with existing technologies often necessitates conservative operating parameters, resulting in longer cycle times and higher energy consumption than theoretically necessary for achieving sterility.
Advanced autoclaves incorporate electronic humidity sensors that provide real-time feedback to control systems. These sensors typically utilize capacitive or resistive technology to measure relative humidity levels within the chamber. However, these sensors face significant challenges in the harsh autoclave environment, including degradation from repeated exposure to high temperatures and pressure cycles, leading to calibration drift and reduced accuracy over time.
Proportional-Integral-Derivative (PID) control algorithms represent the standard method for regulating humidity in modern autoclaves. These systems continuously adjust steam input based on feedback from temperature and pressure sensors. While PID controllers offer reasonable performance, they struggle with the non-linear relationship between temperature, pressure, and humidity in autoclave environments, resulting in suboptimal control during critical transition phases of the sterilization cycle.
A significant challenge in current humidity control systems is the lack of spatial resolution in humidity measurement. Most autoclaves rely on single-point or limited multi-point sensing, which fails to capture humidity gradients within the chamber. This limitation can lead to "cold spots" or areas with insufficient humidity, potentially compromising sterilization efficacy for items placed in these regions.
Load-responsive humidity control represents an emerging technology that attempts to address variability in load characteristics. These systems adjust humidity parameters based on the thermal mass and moisture absorption properties of the load. However, implementation remains challenging due to difficulties in accurately characterizing diverse load compositions in real-world applications.
Condensation management presents another persistent challenge in autoclave humidity control. Excess condensation can lead to wet loads and extended drying times, while insufficient moisture can compromise sterilization effectiveness. Current solutions employ gravity-based drainage systems and post-cycle vacuum drying, but these approaches often result in extended cycle times and increased energy consumption.
Regulatory compliance adds another layer of complexity to humidity control technologies. Standards such as ISO 17665 and EN 285 specify strict parameters for steam quality and humidity levels during sterilization cycles. Meeting these requirements with existing technologies often necessitates conservative operating parameters, resulting in longer cycle times and higher energy consumption than theoretically necessary for achieving sterility.
Current Humidity Control Methodologies and Implementations
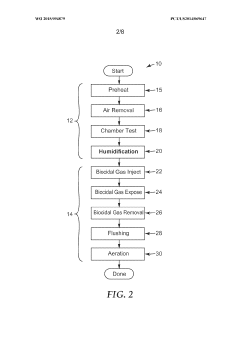
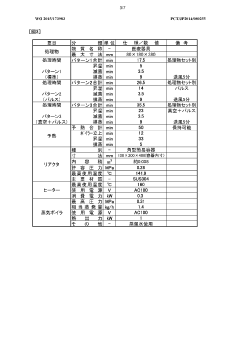
01 Humidity monitoring and control systems for autoclaves
Advanced humidity monitoring and control systems are essential for maintaining optimal sterilization conditions in autoclaves. These systems utilize sensors to continuously measure humidity levels and adjust parameters accordingly. Proper humidity control ensures effective steam penetration into medical instruments and materials, which is critical for achieving sterility. The systems may include feedback mechanisms that automatically regulate steam injection and evacuation to maintain precise humidity levels throughout the sterilization cycle.- Humidity monitoring and control systems for autoclaves: Advanced humidity monitoring and control systems are essential for maintaining optimal sterilization conditions in autoclaves. These systems utilize sensors to continuously measure humidity levels and adjust parameters accordingly. Proper humidity control ensures effective steam penetration into medical instruments and materials, which is critical for achieving sterility. The systems can include automated feedback mechanisms that regulate steam injection and water levels to maintain consistent humidity throughout the sterilization cycle.
- Steam quality management for sterilization efficacy: The quality of steam used in autoclaves significantly impacts sterilization effectiveness. Systems that manage steam saturation, temperature, and purity help ensure that steam can penetrate materials properly to achieve sterility. These technologies include steam generators with precise control mechanisms, steam quality monitoring devices, and systems that prevent superheating or wetness. Maintaining optimal steam quality parameters throughout the sterilization cycle is crucial for consistent and reliable sterilization results across different load types.
- Automated cycle validation and documentation systems: Automated systems for validating sterilization cycles ensure that proper humidity conditions are maintained for achieving sterility. These systems continuously monitor critical parameters including humidity, temperature, and pressure throughout the sterilization process. They can automatically document cycle data, generate compliance reports, and alert operators to any deviations from specified parameters. Such validation systems help healthcare facilities meet regulatory requirements while providing verifiable evidence of sterilization effectiveness.
- Load-specific humidity optimization techniques: Different types of loads require specific humidity conditions for effective sterilization. Advanced autoclaves incorporate technologies that optimize humidity levels based on load characteristics such as density, composition, and configuration. These systems may include pre-programmed cycles for common load types, sensors that detect load properties, and algorithms that adjust humidity parameters accordingly. Load-specific optimization ensures that appropriate moisture levels are maintained throughout the sterilization process, improving efficacy while preventing damage to sensitive instruments.
- Humidity distribution and penetration enhancement: Ensuring uniform humidity distribution throughout the autoclave chamber is critical for achieving consistent sterilization. Technologies that enhance steam penetration and humidity distribution include specialized chamber designs, pulsed vacuum systems, and directed steam flow mechanisms. These innovations help overcome challenges with dense loads or complex instruments by ensuring that steam reaches all surfaces. Improved humidity distribution systems minimize the risk of dry spots or areas with insufficient moisture, which could compromise sterility assurance.
02 Steam quality management for sterilization efficacy
The quality of steam used in autoclaves significantly impacts sterilization effectiveness. Systems for managing steam quality focus on maintaining appropriate moisture content, temperature, and pressure relationships. These technologies ensure that steam is saturated rather than superheated or wet, which is crucial for optimal microbial inactivation. Steam quality management systems may include condensate removal mechanisms, steam generators with precise controls, and distribution systems that maintain steam properties until it reaches the sterilization chamber.Expand Specific Solutions03 Automated cycle validation and monitoring technologies
Automated systems for validating and monitoring sterilization cycles ensure that appropriate humidity conditions are maintained throughout the process. These technologies incorporate real-time data collection, analysis, and documentation of critical parameters including humidity, temperature, and pressure. Advanced monitoring systems may use integrated sensors and computerized controls to verify that sterility assurance levels are achieved. Some systems include alert mechanisms that notify operators of deviations from specified parameters, allowing for immediate corrective action.Expand Specific Solutions04 Pre-vacuum and post-vacuum humidity control methods
Pre-vacuum and post-vacuum phases in autoclave cycles play crucial roles in humidity control and sterility assurance. Pre-vacuum systems remove air from the sterilization chamber before steam introduction, ensuring better steam penetration and humidity distribution. Post-vacuum phases help in drying sterilized items by removing excess moisture. These methods utilize vacuum pumps, pressure sensors, and timing controls to create optimal conditions for sterilization. The precise control of these vacuum phases helps prevent wet packs and ensures complete sterilization of complex instruments.Expand Specific Solutions05 Specialized humidity control for different load types
Different types of loads require specialized humidity control approaches to achieve sterility. Medical devices, porous materials, and liquids each present unique challenges for steam penetration and moisture management. Adaptive systems can adjust humidity parameters based on load characteristics, ensuring effective sterilization while preventing damage to sensitive items. These technologies may incorporate programmable cycles with specific humidity profiles for different load compositions, densities, and configurations. Some systems include specialized racks, containers, or packaging that optimize steam flow and humidity distribution around complex instruments.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Sterilization
The autoclave humidity control optimization market is currently in a growth phase, with increasing demand driven by stringent sterility requirements across healthcare and pharmaceutical sectors. The global market size for sterilization equipment is expanding at approximately 7-9% CAGR, valued at over $10 billion. Technologically, the field is moderately mature but experiencing innovation waves focused on precision control systems and automation. Leading players include established medical equipment manufacturers like Shinva Medical Instrument and SANYO Electric, who offer advanced humidity regulation systems, alongside specialized sterilization experts such as Truking Technology and CISA SpA. Pharmaceutical equipment providers like Ethicon and Medivators are integrating enhanced humidity control into comprehensive sterilization solutions, while automation specialists like KUKA Deutschland are bringing robotics expertise to improve process reliability and consistency.
Truking Technology Ltd.
Technical Solution: Truking Technology has developed an innovative Humidity Stabilization System for autoclaves that employs a multi-point humidity sensing network throughout the chamber to create a comprehensive humidity map during operation. Their technology utilizes a combination of capacitive and resistive humidity sensors to provide redundant measurements, ensuring reliability even if individual sensors fail. The system features a proprietary PID (Proportional-Integral-Derivative) control algorithm that continuously adjusts steam injection rates and exhaust valve positions to maintain target humidity levels with minimal fluctuation. Truking's solution incorporates a pre-conditioning phase that gradually brings the chamber to optimal humidity before the sterilization cycle begins, ensuring uniform moisture distribution throughout the load. Their technology also includes an advanced steam quality monitoring system that detects and compensates for variations in steam purity, which can significantly impact effective humidity control. The system provides real-time humidity visualization through a graphical interface, allowing operators to monitor conditions throughout the sterilization process.
Strengths: Exceptional humidity uniformity throughout the chamber with less than 3% variation between measurement points; robust fault-tolerance through redundant sensor systems; comprehensive data logging capabilities for validation and compliance. Weaknesses: Complex installation requirements for retrofitting existing autoclaves; higher energy consumption compared to basic systems; requires specialized technical knowledge for optimal configuration.
Shinva Medical Instrument Co., Ltd.
Technical Solution: Shinva Medical Instrument has engineered a comprehensive Humidity Regulation Technology for their autoclave systems that focuses on maintaining precise humidity levels throughout the sterilization process. Their solution incorporates a dual-sensor humidity monitoring system that continuously measures both chamber humidity and steam quality. The technology utilizes a proprietary algorithm that dynamically adjusts steam injection rates based on real-time feedback from these sensors, ensuring optimal humidity conditions are maintained regardless of load variations. Shinva's system features programmable humidity profiles that can be customized for different types of medical instruments and materials, allowing for specialized sterilization protocols. Their humidity control mechanism includes an advanced condensate management system that prevents excess moisture accumulation while maintaining the necessary humidity levels for effective microbial elimination. The technology also incorporates pressure-compensated humidity measurements to ensure accuracy across different operating pressures and temperatures.
Strengths: Exceptional adaptability to various load types and sizes; energy-efficient operation through optimized steam usage; user-friendly interface with pre-programmed humidity profiles for common sterilization needs. Weaknesses: Requires more frequent maintenance of humidity sensors compared to simpler systems; higher initial cost than basic autoclave systems; limited compatibility with older facility infrastructure.
Key Patents and Innovations in Autoclave Humidity Regulation
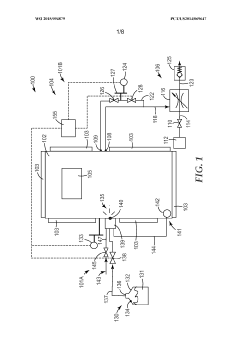
Systems and methods for controlling humidity
PatentWO2015094879A9
Innovation
- A humidity control system that utilizes constant pressure monitoring to ratiometrically calculate the next water injection quantity, accounting for load size and absorption rates, ensuring efficient and accurate humidity control within the chamber.
Sterilization/drying method using soft hydrothermal process, and medical sterilization device
PatentWO2015173983A1
Innovation
- The soft hydrothermal process employs highly saturated steam with a saturation of 100% or more to simultaneously sterilize and dry medical devices, utilizing a steam generator to maintain high steam saturation and circulate steam through a treatment chamber, eliminating the need for a separate drying step.
Validation Methods and Standards for Sterilization Efficacy
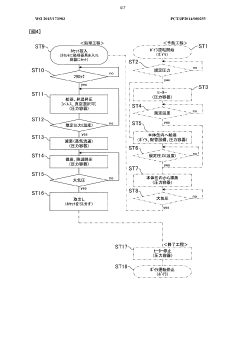
Validation of sterilization processes in autoclaves requires adherence to rigorous methodologies and internationally recognized standards to ensure consistent sterility assurance levels (SALs). The primary validation frameworks include ISO 17665 for moist heat sterilization and AAMI ST79, which specifically addresses steam sterilization in healthcare facilities. These standards establish comprehensive protocols for qualification, validation, and routine monitoring of sterilization processes.
Physical validation methods for humidity-controlled autoclaves include biological indicators (BIs), which contain resistant microorganisms like Geobacillus stearothermophilus spores. These indicators provide direct evidence of sterilization efficacy by demonstrating the destruction of highly resistant microorganisms under specific humidity conditions. Chemical indicators complement BIs by changing color or physical state when exposed to critical sterilization parameters, offering immediate visual verification of process conditions.
Parametric release methodologies represent an advanced validation approach, allowing release of sterilized items based on documented monitoring of critical process parameters rather than waiting for biological indicator results. For humidity-controlled autoclaves, this requires precise monitoring of temperature, pressure, humidity levels, and exposure time throughout the sterilization cycle. Implementation necessitates validated equipment with calibrated sensors and documented correlation between parameter measurements and microbial inactivation.
The F0 concept serves as a quantitative measure of sterilization lethality, calculated by integrating time-temperature relationships during the sterilization process. For humidity-optimized cycles, F0 calculations must incorporate humidity as an additional parameter affecting microbial inactivation kinetics. Modern validation protocols typically require minimum F0 values between 8-12 minutes for adequate sterility assurance.
Process challenge devices (PCDs) simulate worst-case scenarios by creating conditions more difficult to sterilize than typical loads. For humidity-dependent sterilization, specialized PCDs incorporate moisture barriers or hydrophobic materials that challenge vapor penetration. These devices are strategically placed at locations identified as most difficult to sterilize during load mapping studies.
Regulatory compliance frameworks vary globally but converge on similar principles. The FDA requires validation according to cGMP guidelines (21 CFR Part 820), while the European Medical Device Regulation mandates compliance with harmonized standards. Both frameworks emphasize the need for documented evidence demonstrating that humidity-controlled sterilization processes consistently deliver the required SAL of 10^-6 (one-in-a-million probability of a non-sterile unit).
Physical validation methods for humidity-controlled autoclaves include biological indicators (BIs), which contain resistant microorganisms like Geobacillus stearothermophilus spores. These indicators provide direct evidence of sterilization efficacy by demonstrating the destruction of highly resistant microorganisms under specific humidity conditions. Chemical indicators complement BIs by changing color or physical state when exposed to critical sterilization parameters, offering immediate visual verification of process conditions.
Parametric release methodologies represent an advanced validation approach, allowing release of sterilized items based on documented monitoring of critical process parameters rather than waiting for biological indicator results. For humidity-controlled autoclaves, this requires precise monitoring of temperature, pressure, humidity levels, and exposure time throughout the sterilization cycle. Implementation necessitates validated equipment with calibrated sensors and documented correlation between parameter measurements and microbial inactivation.
The F0 concept serves as a quantitative measure of sterilization lethality, calculated by integrating time-temperature relationships during the sterilization process. For humidity-optimized cycles, F0 calculations must incorporate humidity as an additional parameter affecting microbial inactivation kinetics. Modern validation protocols typically require minimum F0 values between 8-12 minutes for adequate sterility assurance.
Process challenge devices (PCDs) simulate worst-case scenarios by creating conditions more difficult to sterilize than typical loads. For humidity-dependent sterilization, specialized PCDs incorporate moisture barriers or hydrophobic materials that challenge vapor penetration. These devices are strategically placed at locations identified as most difficult to sterilize during load mapping studies.
Regulatory compliance frameworks vary globally but converge on similar principles. The FDA requires validation according to cGMP guidelines (21 CFR Part 820), while the European Medical Device Regulation mandates compliance with harmonized standards. Both frameworks emphasize the need for documented evidence demonstrating that humidity-controlled sterilization processes consistently deliver the required SAL of 10^-6 (one-in-a-million probability of a non-sterile unit).
Energy Efficiency Considerations in Autoclave Operations
Energy efficiency in autoclave operations represents a critical consideration that intersects with both operational costs and environmental sustainability while maintaining optimal humidity control for sterility assurance. Modern autoclave systems consume significant energy, primarily in the form of steam generation and heating processes. The average hospital-grade autoclave consumes between 20-30 kWh per cycle, with larger industrial units requiring substantially more energy depending on chamber size and load characteristics.
Energy consumption in autoclaves directly correlates with humidity control parameters. Higher humidity levels generally require less energy to achieve sterilization temperatures, as moist heat transfers thermal energy more efficiently than dry heat. However, this relationship creates a complex optimization challenge where humidity must be precisely controlled to ensure sterility while minimizing unnecessary energy expenditure.
Recent technological innovations have introduced several energy-efficient approaches to autoclave humidity control. Pulse vacuum technology, which alternates between vacuum and pressure phases, has demonstrated energy savings of 20-30% compared to traditional gravity displacement methods while maintaining optimal humidity levels. These systems achieve more uniform steam penetration with less total steam consumption.
Heat recovery systems represent another significant advancement, capturing waste heat from exhaust steam and condensate to preheat incoming water. Implementation data indicates potential energy savings of 15-25% through effective heat reclamation strategies. Additionally, advanced insulation materials and chamber designs have reduced heat loss during operation by up to 18%, further enhancing energy efficiency.
Smart control systems utilizing machine learning algorithms now enable dynamic adjustment of humidity parameters based on load characteristics and sterilization requirements. These systems continuously optimize energy consumption while maintaining critical sterility parameters, achieving energy reductions of 10-15% compared to fixed-parameter operations.
The economic implications of energy-efficient humidity control are substantial. Healthcare facilities implementing comprehensive energy optimization strategies for autoclaves report payback periods of 18-36 months on technology investments, with ongoing operational savings of $5,000-15,000 annually per autoclave unit depending on usage frequency and local utility costs.
Environmental impact assessments indicate that optimized autoclave operations can reduce carbon emissions by 15-25 metric tons per unit annually. This reduction aligns with healthcare sustainability initiatives and increasingly stringent regulatory requirements regarding institutional carbon footprints in many jurisdictions.
Energy consumption in autoclaves directly correlates with humidity control parameters. Higher humidity levels generally require less energy to achieve sterilization temperatures, as moist heat transfers thermal energy more efficiently than dry heat. However, this relationship creates a complex optimization challenge where humidity must be precisely controlled to ensure sterility while minimizing unnecessary energy expenditure.
Recent technological innovations have introduced several energy-efficient approaches to autoclave humidity control. Pulse vacuum technology, which alternates between vacuum and pressure phases, has demonstrated energy savings of 20-30% compared to traditional gravity displacement methods while maintaining optimal humidity levels. These systems achieve more uniform steam penetration with less total steam consumption.
Heat recovery systems represent another significant advancement, capturing waste heat from exhaust steam and condensate to preheat incoming water. Implementation data indicates potential energy savings of 15-25% through effective heat reclamation strategies. Additionally, advanced insulation materials and chamber designs have reduced heat loss during operation by up to 18%, further enhancing energy efficiency.
Smart control systems utilizing machine learning algorithms now enable dynamic adjustment of humidity parameters based on load characteristics and sterilization requirements. These systems continuously optimize energy consumption while maintaining critical sterility parameters, achieving energy reductions of 10-15% compared to fixed-parameter operations.
The economic implications of energy-efficient humidity control are substantial. Healthcare facilities implementing comprehensive energy optimization strategies for autoclaves report payback periods of 18-36 months on technology investments, with ongoing operational savings of $5,000-15,000 annually per autoclave unit depending on usage frequency and local utility costs.
Environmental impact assessments indicate that optimized autoclave operations can reduce carbon emissions by 15-25 metric tons per unit annually. This reduction aligns with healthcare sustainability initiatives and increasingly stringent regulatory requirements regarding institutional carbon footprints in many jurisdictions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!