Autoclave vs. Dry Heat: Best Sterilization Method for Lab Equipment
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sterilization Technology Evolution and Objectives
Sterilization methods have evolved significantly over centuries, from basic heat applications in ancient civilizations to sophisticated technologies in modern laboratories. The historical trajectory began with fire-based sterilization in prehistoric times, progressing through boiling water techniques in the Middle Ages. The 19th century marked a pivotal turning point with Louis Pasteur's germ theory and the subsequent development of steam sterilization principles by Charles Chamberland in 1879, which laid the foundation for modern autoclave technology.
The autoclave emerged as a revolutionary advancement, utilizing pressurized steam to achieve higher temperatures than boiling water alone, enabling more effective microbial elimination. Concurrently, dry heat sterilization methods were refined, offering alternative approaches particularly suitable for materials sensitive to moisture. The 20th century witnessed significant technological refinements in both methods, with automated controls, improved chamber designs, and enhanced validation protocols.
Recent decades have seen the integration of digital monitoring systems, programmable cycles, and advanced materials in sterilization equipment. Modern autoclaves feature sophisticated pressure and temperature control mechanisms, while dry heat sterilizers have evolved to incorporate forced air circulation and precise temperature distribution technologies. These advancements have substantially improved efficiency, reliability, and process validation capabilities.
The primary objective of sterilization technology development remains the complete elimination of all microorganisms, including highly resistant bacterial spores, while maintaining the integrity and functionality of the sterilized items. Secondary objectives include reducing cycle times, minimizing energy consumption, extending equipment lifespan, and ensuring operator safety. Environmental considerations have also become increasingly important, with newer designs focusing on reducing water usage and minimizing chemical emissions.
Current technological trajectories point toward further automation, improved energy efficiency, and enhanced process monitoring capabilities. The integration of IoT (Internet of Things) technologies enables remote monitoring and predictive maintenance, while machine learning algorithms optimize sterilization parameters based on load characteristics. Research efforts are increasingly focused on developing sterilization methods that operate at lower temperatures and pressures while maintaining efficacy, particularly for heat-sensitive materials and complex instruments.
The selection between autoclave and dry heat sterilization methods represents a critical decision point for laboratory operations, with implications for equipment longevity, operational efficiency, and experimental validity. Understanding the evolutionary context and technological objectives of these sterilization methods provides essential context for evaluating their respective advantages and limitations in contemporary laboratory settings.
The autoclave emerged as a revolutionary advancement, utilizing pressurized steam to achieve higher temperatures than boiling water alone, enabling more effective microbial elimination. Concurrently, dry heat sterilization methods were refined, offering alternative approaches particularly suitable for materials sensitive to moisture. The 20th century witnessed significant technological refinements in both methods, with automated controls, improved chamber designs, and enhanced validation protocols.
Recent decades have seen the integration of digital monitoring systems, programmable cycles, and advanced materials in sterilization equipment. Modern autoclaves feature sophisticated pressure and temperature control mechanisms, while dry heat sterilizers have evolved to incorporate forced air circulation and precise temperature distribution technologies. These advancements have substantially improved efficiency, reliability, and process validation capabilities.
The primary objective of sterilization technology development remains the complete elimination of all microorganisms, including highly resistant bacterial spores, while maintaining the integrity and functionality of the sterilized items. Secondary objectives include reducing cycle times, minimizing energy consumption, extending equipment lifespan, and ensuring operator safety. Environmental considerations have also become increasingly important, with newer designs focusing on reducing water usage and minimizing chemical emissions.
Current technological trajectories point toward further automation, improved energy efficiency, and enhanced process monitoring capabilities. The integration of IoT (Internet of Things) technologies enables remote monitoring and predictive maintenance, while machine learning algorithms optimize sterilization parameters based on load characteristics. Research efforts are increasingly focused on developing sterilization methods that operate at lower temperatures and pressures while maintaining efficacy, particularly for heat-sensitive materials and complex instruments.
The selection between autoclave and dry heat sterilization methods represents a critical decision point for laboratory operations, with implications for equipment longevity, operational efficiency, and experimental validity. Understanding the evolutionary context and technological objectives of these sterilization methods provides essential context for evaluating their respective advantages and limitations in contemporary laboratory settings.
Laboratory Equipment Sterilization Market Analysis
The laboratory equipment sterilization market has witnessed significant growth in recent years, driven by increasing awareness of infection control and stringent regulatory requirements across healthcare and research facilities. The global market for laboratory sterilization equipment was valued at approximately $1.8 billion in 2022 and is projected to reach $2.5 billion by 2027, growing at a CAGR of 6.8% during the forecast period.
Autoclave sterilization dominates the market with nearly 65% market share due to its reliability, effectiveness against a wide range of microorganisms, and established protocols. The dry heat sterilization segment holds about 15% of the market, with the remaining 20% distributed among other methods including radiation, chemical, and filtration sterilization techniques.
Geographically, North America leads the market with approximately 38% share, followed by Europe (30%) and Asia-Pacific (22%). The Asia-Pacific region is expected to witness the highest growth rate during the forecast period due to expanding healthcare infrastructure, increasing research activities, and growing awareness about sterilization standards.
By end-user segment, hospitals and clinical laboratories constitute the largest market share (45%), followed by pharmaceutical and biotechnology companies (30%), academic and research institutes (15%), and others (10%). The pharmaceutical and biotechnology segment is projected to grow at the highest rate due to increasing R&D activities and stringent regulatory requirements.
Key market drivers include rising incidence of hospital-acquired infections, growing number of surgical procedures, expanding pharmaceutical and biotechnology sectors, and increasing focus on research and development activities. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of proper sterilization protocols.
Market restraints include high equipment costs, especially for advanced autoclave systems, and operational challenges in low-resource settings. Additionally, concerns regarding the environmental impact of certain sterilization methods are influencing market dynamics and driving innovation toward more sustainable solutions.
The competitive landscape features established players like Steris Corporation, Getinge AB, 3M Company, and Belimed AG, alongside emerging companies introducing innovative technologies. Recent market trends indicate a shift toward more energy-efficient, environmentally friendly sterilization methods and the integration of automation and digital monitoring capabilities in sterilization equipment.
Autoclave sterilization dominates the market with nearly 65% market share due to its reliability, effectiveness against a wide range of microorganisms, and established protocols. The dry heat sterilization segment holds about 15% of the market, with the remaining 20% distributed among other methods including radiation, chemical, and filtration sterilization techniques.
Geographically, North America leads the market with approximately 38% share, followed by Europe (30%) and Asia-Pacific (22%). The Asia-Pacific region is expected to witness the highest growth rate during the forecast period due to expanding healthcare infrastructure, increasing research activities, and growing awareness about sterilization standards.
By end-user segment, hospitals and clinical laboratories constitute the largest market share (45%), followed by pharmaceutical and biotechnology companies (30%), academic and research institutes (15%), and others (10%). The pharmaceutical and biotechnology segment is projected to grow at the highest rate due to increasing R&D activities and stringent regulatory requirements.
Key market drivers include rising incidence of hospital-acquired infections, growing number of surgical procedures, expanding pharmaceutical and biotechnology sectors, and increasing focus on research and development activities. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of proper sterilization protocols.
Market restraints include high equipment costs, especially for advanced autoclave systems, and operational challenges in low-resource settings. Additionally, concerns regarding the environmental impact of certain sterilization methods are influencing market dynamics and driving innovation toward more sustainable solutions.
The competitive landscape features established players like Steris Corporation, Getinge AB, 3M Company, and Belimed AG, alongside emerging companies introducing innovative technologies. Recent market trends indicate a shift toward more energy-efficient, environmentally friendly sterilization methods and the integration of automation and digital monitoring capabilities in sterilization equipment.
Current Sterilization Methods and Technical Barriers
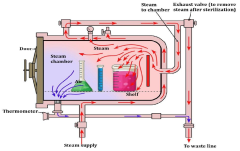
Laboratory sterilization methods have evolved significantly over the decades, with autoclave and dry heat sterilization emerging as two predominant techniques. Currently, autoclaves utilize saturated steam under pressure (typically 121°C at 15 psi) to achieve sterilization through protein denaturation and coagulation. This method has become the gold standard in many laboratory settings due to its reliability and efficiency, with cycle times typically ranging from 15-30 minutes for most applications.
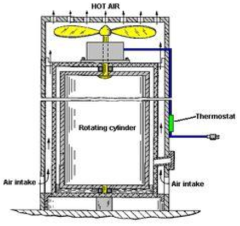
Dry heat sterilization, by contrast, operates through oxidative destruction of microbial cells at temperatures ranging from 160-180°C, with exposure times between 2-4 hours depending on temperature selection. This method functions through direct conduction of heat to materials, making it particularly suitable for moisture-sensitive items, powders, oils, and certain glassware.
Despite their widespread adoption, both methods face significant technical barriers. Autoclaves struggle with penetration issues in dense materials and can damage heat-sensitive components. The high moisture environment can accelerate corrosion in certain metals and may compromise the integrity of some polymers through hydrolysis reactions. Additionally, incomplete air removal from chambers can create "cold spots" where sterilization fails to occur.
Dry heat systems face efficiency challenges due to longer cycle times and higher energy consumption. Temperature distribution uniformity remains problematic in many units, creating potential sterilization failures in areas receiving insufficient thermal exposure. The method's inability to penetrate packaging effectively also limits its application scope.
Material compatibility represents a critical constraint for both technologies. Autoclave processes can damage electronics, sharp instruments, and certain plastics, while dry heat's extreme temperatures restrict its use with many modern laboratory polymers and composite materials. Neither method is suitable for all biological materials, particularly those containing volatile compounds.
Validation protocols present another significant barrier, with biological indicators showing variable reliability across different load configurations. Real-time monitoring capabilities remain limited, particularly for dry heat systems, creating uncertainty about cycle effectiveness until post-process testing is completed.
Energy efficiency concerns have become increasingly prominent, with traditional autoclaves consuming substantial water resources and both methods requiring significant energy inputs. This has driven research toward more sustainable alternatives and optimization of existing technologies through improved insulation, cycle programming, and resource recovery systems.
Cross-contamination risks persist in both methods, particularly in facilities processing diverse materials. Biofilm formation in autoclave plumbing systems and particulate redistribution in dry heat chambers represent ongoing challenges requiring specialized maintenance protocols and design considerations.
Dry heat sterilization, by contrast, operates through oxidative destruction of microbial cells at temperatures ranging from 160-180°C, with exposure times between 2-4 hours depending on temperature selection. This method functions through direct conduction of heat to materials, making it particularly suitable for moisture-sensitive items, powders, oils, and certain glassware.
Despite their widespread adoption, both methods face significant technical barriers. Autoclaves struggle with penetration issues in dense materials and can damage heat-sensitive components. The high moisture environment can accelerate corrosion in certain metals and may compromise the integrity of some polymers through hydrolysis reactions. Additionally, incomplete air removal from chambers can create "cold spots" where sterilization fails to occur.
Dry heat systems face efficiency challenges due to longer cycle times and higher energy consumption. Temperature distribution uniformity remains problematic in many units, creating potential sterilization failures in areas receiving insufficient thermal exposure. The method's inability to penetrate packaging effectively also limits its application scope.
Material compatibility represents a critical constraint for both technologies. Autoclave processes can damage electronics, sharp instruments, and certain plastics, while dry heat's extreme temperatures restrict its use with many modern laboratory polymers and composite materials. Neither method is suitable for all biological materials, particularly those containing volatile compounds.
Validation protocols present another significant barrier, with biological indicators showing variable reliability across different load configurations. Real-time monitoring capabilities remain limited, particularly for dry heat systems, creating uncertainty about cycle effectiveness until post-process testing is completed.
Energy efficiency concerns have become increasingly prominent, with traditional autoclaves consuming substantial water resources and both methods requiring significant energy inputs. This has driven research toward more sustainable alternatives and optimization of existing technologies through improved insulation, cycle programming, and resource recovery systems.
Cross-contamination risks persist in both methods, particularly in facilities processing diverse materials. Biofilm formation in autoclave plumbing systems and particulate redistribution in dry heat chambers represent ongoing challenges requiring specialized maintenance protocols and design considerations.
Comparative Analysis of Autoclave and Dry Heat Methods
01 Autoclave sterilization parameters and effectiveness
Autoclave sterilization uses saturated steam under pressure to achieve effective microbial inactivation. The effectiveness depends on critical parameters including temperature (typically 121-134°C), pressure (15-30 psi), and exposure time (15-30 minutes). This method is particularly effective against bacteria, viruses, fungi, and spores due to the combined effect of heat and moisture, which denatures proteins and disrupts cell membranes. The steam penetration ensures uniform heat distribution throughout the load, making it suitable for heat-stable medical devices, surgical instruments, and laboratory equipment.- Autoclave sterilization effectiveness: Autoclave sterilization uses saturated steam under pressure to achieve high-temperature sterilization. This method is highly effective for killing microorganisms, including bacteria, viruses, fungi, and spores. The effectiveness of autoclave sterilization depends on several factors including temperature (typically 121-134°C), pressure (15-30 psi), exposure time (usually 15-30 minutes), and proper loading of materials. Autoclaves are particularly suitable for heat-resistant materials like surgical instruments, laboratory equipment, and certain medical devices.
- Dry heat sterilization parameters and applications: Dry heat sterilization involves exposing materials to high temperatures in the absence of moisture. This method typically requires higher temperatures (160-180°C) and longer exposure times (1-2 hours) compared to autoclave sterilization. Dry heat works through oxidation processes to destroy microorganisms. It is particularly useful for sterilizing materials that can be damaged by moisture, such as powders, oils, glassware, and metal instruments. The effectiveness depends on proper temperature distribution, exposure duration, and loading configuration within the sterilization chamber.
- Comparative effectiveness of sterilization methods: When comparing autoclave and dry heat sterilization, autoclave is generally more efficient and requires less time due to the enhanced heat penetration provided by steam. Autoclave sterilization achieves sterility at lower temperatures (121°C) compared to dry heat (160-180°C). However, dry heat provides better penetration for dense materials and leaves no moisture residue. The choice between methods depends on material compatibility, required sterility assurance level, processing time constraints, and the nature of the items being sterilized.
- Monitoring and validation of sterilization processes: Effective sterilization requires proper monitoring and validation to ensure that the required sterility assurance level is achieved. This includes the use of biological indicators containing resistant bacterial spores, chemical indicators that change color when exposed to sterilization conditions, and physical monitors that track temperature, pressure, and time parameters. Regular validation protocols, including installation qualification, operational qualification, and performance qualification, are essential to verify that sterilization equipment consistently delivers effective microbial inactivation across all load items.
- Innovations in sterilization equipment and processes: Recent innovations in sterilization technology have improved the effectiveness and efficiency of both autoclave and dry heat methods. These include advanced control systems for precise parameter management, improved chamber designs for better heat distribution, energy-efficient models, rapid cooling systems, and integrated monitoring capabilities. Some modern systems combine multiple sterilization principles or incorporate pre-vacuum phases to enhance steam penetration. Additionally, specialized cycles have been developed for specific materials and applications, optimizing sterilization effectiveness while minimizing damage to sensitive items.
02 Dry heat sterilization mechanisms and applications
Dry heat sterilization operates through oxidation, protein denaturation, and dehydration of microorganisms. It typically requires higher temperatures (160-180°C) and longer exposure times (1-2 hours) compared to autoclave methods. This method is particularly suitable for heat-stable materials that are sensitive to moisture, such as powders, oils, and certain metal instruments. While less efficient than steam sterilization in terms of time and energy consumption, dry heat offers advantages for moisture-sensitive items and leaves no residue, making it valuable for specific applications in pharmaceutical, laboratory, and medical settings.Expand Specific Solutions03 Comparative effectiveness of sterilization methods
Studies comparing autoclave and dry heat sterilization show distinct effectiveness profiles. Autoclave sterilization achieves faster microbial inactivation due to the superior heat transfer properties of steam and its ability to penetrate materials. Dry heat requires longer exposure times but may provide more complete sterilization for certain materials. The choice between methods depends on material compatibility, required sterility assurance level, and processing constraints. Validation studies indicate that both methods can achieve complete sterilization when properly implemented according to established parameters, though autoclave sterilization generally offers greater efficiency for compatible materials.Expand Specific Solutions04 Innovations in sterilization equipment and monitoring
Recent innovations in sterilization technology include advanced monitoring systems, improved chamber designs, and automated validation processes. Modern autoclaves feature precise digital controls, rapid cooling systems, and efficient steam generation. Dry heat sterilizers have evolved with better insulation, more uniform heat distribution, and reduced energy consumption. Both systems now commonly incorporate real-time monitoring with biological and chemical indicators to verify sterilization effectiveness. These technological advancements have improved reliability, reduced cycle times, and enhanced the ability to document sterilization processes for regulatory compliance.Expand Specific Solutions05 Material compatibility and sterilization method selection
Material compatibility is a critical factor in selecting between autoclave and dry heat sterilization. Heat-sensitive polymers, electronics, and certain medical devices may be damaged by the high temperatures of either method. Moisture-sensitive materials, including powders, oils, and certain metals prone to corrosion, are better suited for dry heat sterilization. Conversely, dense materials benefit from steam's superior heat transfer properties. The selection process should consider the physical and chemical properties of the materials, required sterility assurance level, processing time constraints, and potential impact on material functionality and longevity.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The sterilization technology market for laboratory equipment is in a mature growth phase, with a significant market size driven by healthcare and research sectors. Autoclave sterilization dominates due to its reliability and effectiveness for most applications, while dry heat serves specialized niches. Leading players include established medical technology companies like Stryker Corp., Olympus Corp., and Becton, Dickinson & Co., who offer comprehensive sterilization solutions. Specialized sterilization equipment manufacturers such as Fedegari Autoclavi SpA and Turbett Surgical are advancing technological innovations in both methods. The competitive landscape shows a balance between large medical device conglomerates and specialized sterilization equipment providers, with increasing focus on energy efficiency, automation, and validation protocols.
Turbett Surgical, Inc.
Technical Solution: Turbett Surgical has developed an innovative containerized sterilization system specifically designed to address the limitations of traditional autoclave methods for surgical and laboratory equipment. Their patented Wagner System utilizes a specialized container that allows for direct steam penetration while maintaining instrument organization and sterility post-processing. The technology employs precisely controlled steam dynamics that achieve sterilization at 132-135°C with optimized pressure cycles that reduce cycle times by up to 50% compared to conventional wrapped tray methods[9]. A key innovation is their condensate management system that actively removes moisture during the sterilization process, addressing one of the primary drawbacks of autoclave sterilization. The containers feature RFID-enabled tracking technology that integrates with hospital inventory systems, providing complete documentation of sterilization parameters and equipment history. Turbett's system also incorporates specialized materials that withstand repeated sterilization cycles while maintaining structural integrity, extending the lifespan of both the containers and the instruments they protect.
Strengths: Significantly reduced cycle times improving laboratory throughput; superior moisture management compared to traditional autoclaves; maintains organization of instrument sets throughout the process; reduced consumable waste from elimination of sterilization wraps; comprehensive tracking capabilities. Weaknesses: Higher initial investment than conventional autoclave accessories; requires specific training for optimal use; limited application for certain specialized equipment; system is optimized for steam rather than offering dry heat alternatives; requires compatible autoclave systems with specific cycle parameters.
Fedegari Autoclavi SpA
Technical Solution: Fedegari has developed advanced autoclave sterilization systems that utilize saturated steam under pressure (typically 121-134°C at 15-30 psi) to achieve sterility assurance levels (SAL) of 10^-6. Their FOF series autoclaves incorporate patented SuperHeat technology that combines steam with additional heating elements to create superheated steam, eliminating the need for post-sterilization drying cycles[1]. This approach maintains the penetrative advantages of traditional steam while addressing moisture concerns. Fedegari's systems feature precise control over temperature gradients and pressure fluctuations through their Thema4 process controller, allowing customized cycles for different laboratory equipment with validation according to ISO 17665 standards[3]. Their autoclaves also implement resource optimization algorithms that reduce water consumption by up to 90% compared to conventional systems.
Strengths: Superior penetration capability for complex instruments with lumens or porous materials; faster cycle times than traditional autoclaves; reduced water and energy consumption; comprehensive validation protocols. Weaknesses: Higher initial investment costs; requires steam generation infrastructure; potential for moisture-sensitive materials damage despite technological improvements; more complex maintenance requirements than dry heat systems.
Critical Patents and Research in Sterilization Science
Modular dry heat sterilizer
PatentInactiveUS20120251384A1
Innovation
- A modular dry heat sterilizer using a housing with air inlets and outlets for dry heat and filtered air, allowing for efficient sterilization and cooling of animal cages without dedicated plumbing or water usage, with a mobile and modular design that fits through standard doorways and requires minimal maintenance.
An apparatus for sterilization and incubation
PatentActiveIN201811017743A
Innovation
- A combined apparatus capable of performing dry heat, wet heat, and chemical sterilization, along with a water bath for incubation, integrating steam generation, heating elements, and chemical injection systems within a single unit.
Regulatory Standards and Compliance Requirements
Sterilization methods for laboratory equipment must adhere to stringent regulatory standards established by various international and national bodies. The FDA (Food and Drug Administration) in the United States has established specific guidelines under 21 CFR Part 211 for pharmaceutical manufacturing and 21 CFR Part 820 for medical devices, both of which include requirements for sterilization validation. These regulations mandate documented evidence that sterilization processes consistently achieve a Sterility Assurance Level (SAL) of 10^-6, meaning a probability of not more than one viable microorganism in one million sterilized items.
The International Organization for Standardization (ISO) provides comprehensive frameworks through ISO 17665 for moist heat sterilization and ISO 20857 for dry heat sterilization. These standards outline the development, validation, and routine control requirements for each sterilization method. Compliance with these standards requires extensive documentation, including installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) protocols.
European regulations, particularly the Medical Device Regulation (MDR 2017/745), impose additional requirements for sterilization processes used in medical device manufacturing. The European standard EN 285 specifically addresses large steam sterilizers, while EN 13060 covers small steam sterilizers, both providing technical specifications that must be met for compliance.
For laboratory settings, the Clinical Laboratory Improvement Amendments (CLIA) in the US and equivalent regulations in other regions establish quality standards for all laboratory testing. These regulations include requirements for equipment maintenance and sterilization to ensure accurate and reliable test results.
Compliance with these regulations necessitates comprehensive validation protocols. For autoclaves, this includes biological indicators containing Geobacillus stearothermophilus spores, which are particularly resistant to moist heat. For dry heat sterilization, Bacillus atrophaeus serves as the standard biological indicator. Chemical indicators that change color or physical state when exposed to specific sterilization parameters are also required for routine monitoring.
Documentation requirements are extensive and include sterilization cycle parameters, maintenance records, calibration certificates, validation reports, and routine monitoring results. Regular audits by regulatory bodies may review these records to ensure ongoing compliance. Non-compliance can result in significant penalties, product recalls, and damage to institutional reputation.
The choice between autoclave and dry heat sterilization must therefore consider not only technical efficacy but also the ability to consistently meet these regulatory requirements, including the necessary validation protocols and documentation systems to demonstrate compliance.
The International Organization for Standardization (ISO) provides comprehensive frameworks through ISO 17665 for moist heat sterilization and ISO 20857 for dry heat sterilization. These standards outline the development, validation, and routine control requirements for each sterilization method. Compliance with these standards requires extensive documentation, including installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) protocols.
European regulations, particularly the Medical Device Regulation (MDR 2017/745), impose additional requirements for sterilization processes used in medical device manufacturing. The European standard EN 285 specifically addresses large steam sterilizers, while EN 13060 covers small steam sterilizers, both providing technical specifications that must be met for compliance.
For laboratory settings, the Clinical Laboratory Improvement Amendments (CLIA) in the US and equivalent regulations in other regions establish quality standards for all laboratory testing. These regulations include requirements for equipment maintenance and sterilization to ensure accurate and reliable test results.
Compliance with these regulations necessitates comprehensive validation protocols. For autoclaves, this includes biological indicators containing Geobacillus stearothermophilus spores, which are particularly resistant to moist heat. For dry heat sterilization, Bacillus atrophaeus serves as the standard biological indicator. Chemical indicators that change color or physical state when exposed to specific sterilization parameters are also required for routine monitoring.
Documentation requirements are extensive and include sterilization cycle parameters, maintenance records, calibration certificates, validation reports, and routine monitoring results. Regular audits by regulatory bodies may review these records to ensure ongoing compliance. Non-compliance can result in significant penalties, product recalls, and damage to institutional reputation.
The choice between autoclave and dry heat sterilization must therefore consider not only technical efficacy but also the ability to consistently meet these regulatory requirements, including the necessary validation protocols and documentation systems to demonstrate compliance.
Environmental Impact and Sustainability Considerations
The environmental impact of sterilization methods represents a critical consideration in laboratory operations, particularly as sustainability becomes increasingly prioritized across scientific and medical sectors. Autoclave sterilization, while highly effective, consumes significant energy during its operation cycle, requiring sustained high temperatures and pressure for extended periods. A standard autoclave cycle typically consumes between 1.5-3 kWh of electricity, with larger industrial models consuming substantially more.
Water usage presents another environmental concern with autoclave sterilization. The steam generation process requires considerable water resources, with estimates suggesting that a medium-sized laboratory autoclave may consume 50-100 gallons of water per day under regular operation. Additionally, the water discharged after sterilization cycles often contains chemical additives and potentially hazardous residues that require proper treatment before release into wastewater systems.
Dry heat sterilization, by comparison, demonstrates different environmental characteristics. While it typically requires longer operation times and higher temperatures than autoclaves, dry heat systems generally consume less water resources. However, the extended operation time translates to potentially higher cumulative energy consumption, particularly for older models lacking advanced insulation or energy recovery systems.
Carbon footprint analysis reveals that both methods contribute significantly to a laboratory's environmental impact. Recent studies indicate that sterilization processes may account for 5-10% of a typical research laboratory's total energy consumption. The carbon emissions associated with these processes vary considerably depending on regional energy sources, with laboratories powered by renewable energy demonstrating substantially lower environmental impacts.
Waste generation differs markedly between the two methods. Autoclave sterilization often requires specialized packaging materials that can withstand steam penetration, while dry heat sterilization may allow for reusable containers with longer lifespans. The disposal of single-use sterilization indicators and validation materials contributes to the waste stream of both methods, though innovations in biodegradable indicators are emerging.
Technological advancements are progressively addressing these environmental concerns. Modern autoclave systems increasingly incorporate water recycling capabilities, reducing consumption by up to 90% compared to older models. Similarly, energy-efficient dry heat sterilizers with improved insulation and programmable cycles minimize unnecessary energy expenditure. The integration of renewable energy sources and heat recovery systems represents a promising direction for reducing the environmental footprint of both sterilization methods in laboratory settings.
Water usage presents another environmental concern with autoclave sterilization. The steam generation process requires considerable water resources, with estimates suggesting that a medium-sized laboratory autoclave may consume 50-100 gallons of water per day under regular operation. Additionally, the water discharged after sterilization cycles often contains chemical additives and potentially hazardous residues that require proper treatment before release into wastewater systems.
Dry heat sterilization, by comparison, demonstrates different environmental characteristics. While it typically requires longer operation times and higher temperatures than autoclaves, dry heat systems generally consume less water resources. However, the extended operation time translates to potentially higher cumulative energy consumption, particularly for older models lacking advanced insulation or energy recovery systems.
Carbon footprint analysis reveals that both methods contribute significantly to a laboratory's environmental impact. Recent studies indicate that sterilization processes may account for 5-10% of a typical research laboratory's total energy consumption. The carbon emissions associated with these processes vary considerably depending on regional energy sources, with laboratories powered by renewable energy demonstrating substantially lower environmental impacts.
Waste generation differs markedly between the two methods. Autoclave sterilization often requires specialized packaging materials that can withstand steam penetration, while dry heat sterilization may allow for reusable containers with longer lifespans. The disposal of single-use sterilization indicators and validation materials contributes to the waste stream of both methods, though innovations in biodegradable indicators are emerging.
Technological advancements are progressively addressing these environmental concerns. Modern autoclave systems increasingly incorporate water recycling capabilities, reducing consumption by up to 90% compared to older models. Similarly, energy-efficient dry heat sterilizers with improved insulation and programmable cycles minimize unnecessary energy expenditure. The integration of renewable energy sources and heat recovery systems represents a promising direction for reducing the environmental footprint of both sterilization methods in laboratory settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!