Autoclave Performance Metrics: Improving Sterilization Protocols
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Evolution and Objectives
Autoclave sterilization technology has evolved significantly since its inception in the late 19th century when Charles Chamberland developed the first pressure steam sterilizer. This evolution has been driven by the fundamental need for reliable sterilization in healthcare, laboratory, and industrial settings. Early autoclaves relied on simple pressure and temperature mechanisms, while modern systems incorporate sophisticated electronic controls, validation protocols, and efficiency features.
The progression of autoclave technology can be traced through several key developmental phases. The initial mechanical era (1880s-1950s) featured basic pressure vessels with manual controls. This was followed by the electromechanical period (1950s-1980s) that introduced automated timing and temperature regulation. The digital revolution (1980s-2000s) brought computerized control systems and data logging capabilities, while the current integration phase (2000s-present) features network connectivity, remote monitoring, and advanced validation protocols.
Current technological objectives in autoclave sterilization focus on several critical areas. Energy efficiency has become paramount as institutions seek to reduce operational costs and environmental impact. Modern autoclaves aim to minimize water and electricity consumption while maintaining sterilization efficacy. Cycle time optimization represents another key objective, with research directed toward achieving reliable sterilization in shorter timeframes without compromising safety or effectiveness.
Validation and monitoring capabilities continue to advance, with emphasis on real-time parameter tracking, automated documentation, and integration with quality management systems. These developments support regulatory compliance while enhancing process reliability. Additionally, there is growing interest in specialized protocols for novel materials and devices, particularly in the medical and biotechnology sectors where sensitive components require effective sterilization without damage.
The emergence of Industry 4.0 principles has influenced autoclave technology development, with objectives now including IoT integration, predictive maintenance capabilities, and data analytics for process optimization. These features allow for more proactive management of sterilization infrastructure and better resource allocation.
Looking forward, the field is moving toward greater sustainability, with research into alternative sterilization methods that complement traditional autoclave processes. This includes exploration of lower-temperature steam processes, combination technologies, and specialized cycle parameters tailored to specific load types. The ultimate goal remains consistent: to ensure complete sterilization while optimizing resource utilization, minimizing cycle times, and providing robust validation mechanisms.
The progression of autoclave technology can be traced through several key developmental phases. The initial mechanical era (1880s-1950s) featured basic pressure vessels with manual controls. This was followed by the electromechanical period (1950s-1980s) that introduced automated timing and temperature regulation. The digital revolution (1980s-2000s) brought computerized control systems and data logging capabilities, while the current integration phase (2000s-present) features network connectivity, remote monitoring, and advanced validation protocols.
Current technological objectives in autoclave sterilization focus on several critical areas. Energy efficiency has become paramount as institutions seek to reduce operational costs and environmental impact. Modern autoclaves aim to minimize water and electricity consumption while maintaining sterilization efficacy. Cycle time optimization represents another key objective, with research directed toward achieving reliable sterilization in shorter timeframes without compromising safety or effectiveness.
Validation and monitoring capabilities continue to advance, with emphasis on real-time parameter tracking, automated documentation, and integration with quality management systems. These developments support regulatory compliance while enhancing process reliability. Additionally, there is growing interest in specialized protocols for novel materials and devices, particularly in the medical and biotechnology sectors where sensitive components require effective sterilization without damage.
The emergence of Industry 4.0 principles has influenced autoclave technology development, with objectives now including IoT integration, predictive maintenance capabilities, and data analytics for process optimization. These features allow for more proactive management of sterilization infrastructure and better resource allocation.
Looking forward, the field is moving toward greater sustainability, with research into alternative sterilization methods that complement traditional autoclave processes. This includes exploration of lower-temperature steam processes, combination technologies, and specialized cycle parameters tailored to specific load types. The ultimate goal remains consistent: to ensure complete sterilization while optimizing resource utilization, minimizing cycle times, and providing robust validation mechanisms.
Market Analysis of Advanced Sterilization Solutions
The global sterilization solutions market is experiencing robust growth, driven by increasing healthcare expenditures, rising surgical procedures, and heightened awareness of infection control protocols. Currently valued at approximately 7.1 billion USD, the market is projected to reach 12.5 billion USD by 2028, representing a compound annual growth rate of 8.3% during the forecast period.
Healthcare facilities remain the dominant end-users, accounting for nearly 65% of market share, with pharmaceutical and biotechnology companies following at 20%. Hospitals specifically are investing heavily in advanced sterilization technologies to combat healthcare-associated infections (HAIs), which affect millions of patients annually and result in substantial financial burdens on healthcare systems worldwide.
Regionally, North America leads the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 22%. The Asia-Pacific region is expected to witness the fastest growth rate of 10.2% through 2028, primarily due to expanding healthcare infrastructure, increasing medical tourism, and growing awareness about sterilization standards in developing economies like China and India.
By technology segment, steam sterilization (autoclave) continues to dominate with 40% market share due to its reliability, cost-effectiveness, and broad application range. However, low-temperature sterilization methods including hydrogen peroxide gas plasma, ethylene oxide, and vaporized hydrogen peroxide systems are gaining significant traction, collectively growing at 9.7% annually.
Consumer demand is increasingly focused on sterilization solutions that offer shorter cycle times, improved energy efficiency, enhanced monitoring capabilities, and reduced environmental impact. Healthcare facilities are particularly interested in systems that provide comprehensive data logging and validation features to meet stringent regulatory requirements.
Key market drivers include the COVID-19 pandemic's lasting impact on infection control protocols, increasing surgical volumes in ambulatory settings, growing prevalence of chronic diseases requiring surgical interventions, and stricter regulatory frameworks governing sterilization practices globally. The FDA's recent emphasis on validated sterilization processes has accelerated adoption of advanced monitoring systems and documentation protocols.
Market challenges include high initial investment costs for advanced sterilization equipment, operational complexities requiring specialized training, and environmental concerns associated with certain sterilization methods. Additionally, healthcare facilities in developing regions face infrastructure limitations that hinder adoption of sophisticated sterilization technologies.
Healthcare facilities remain the dominant end-users, accounting for nearly 65% of market share, with pharmaceutical and biotechnology companies following at 20%. Hospitals specifically are investing heavily in advanced sterilization technologies to combat healthcare-associated infections (HAIs), which affect millions of patients annually and result in substantial financial burdens on healthcare systems worldwide.
Regionally, North America leads the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 22%. The Asia-Pacific region is expected to witness the fastest growth rate of 10.2% through 2028, primarily due to expanding healthcare infrastructure, increasing medical tourism, and growing awareness about sterilization standards in developing economies like China and India.
By technology segment, steam sterilization (autoclave) continues to dominate with 40% market share due to its reliability, cost-effectiveness, and broad application range. However, low-temperature sterilization methods including hydrogen peroxide gas plasma, ethylene oxide, and vaporized hydrogen peroxide systems are gaining significant traction, collectively growing at 9.7% annually.
Consumer demand is increasingly focused on sterilization solutions that offer shorter cycle times, improved energy efficiency, enhanced monitoring capabilities, and reduced environmental impact. Healthcare facilities are particularly interested in systems that provide comprehensive data logging and validation features to meet stringent regulatory requirements.
Key market drivers include the COVID-19 pandemic's lasting impact on infection control protocols, increasing surgical volumes in ambulatory settings, growing prevalence of chronic diseases requiring surgical interventions, and stricter regulatory frameworks governing sterilization practices globally. The FDA's recent emphasis on validated sterilization processes has accelerated adoption of advanced monitoring systems and documentation protocols.
Market challenges include high initial investment costs for advanced sterilization equipment, operational complexities requiring specialized training, and environmental concerns associated with certain sterilization methods. Additionally, healthcare facilities in developing regions face infrastructure limitations that hinder adoption of sophisticated sterilization technologies.
Current Autoclave Technology Limitations
Despite significant advancements in autoclave technology over recent decades, several critical limitations persist that impede optimal sterilization performance and protocol efficiency. Current autoclave systems face challenges in achieving uniform temperature distribution throughout the sterilization chamber, particularly when processing dense loads or items with complex geometries. This non-uniformity can create "cold spots" where sterilization parameters may not be fully achieved, potentially compromising sterility assurance levels.
Energy efficiency remains a significant concern with conventional autoclave systems. Most units require substantial power consumption to generate and maintain the high-pressure steam environment necessary for effective sterilization. This not only increases operational costs but also contributes to larger carbon footprints for healthcare facilities and industrial operations that rely heavily on these devices.
Cycle time optimization presents another notable limitation. Standard autoclave protocols often employ unnecessarily extended exposure times to compensate for potential variability in steam penetration. These conservative approaches, while ensuring sterility, result in reduced throughput, increased energy consumption, and accelerated wear on sterilized materials and equipment components.
Monitoring and validation capabilities in many current autoclave systems lack real-time precision. Traditional biological indicators provide retrospective confirmation of sterilization efficacy but cannot offer immediate feedback during the sterilization process. This delay between processing and verification creates operational inefficiencies and potential risks when sterilization failures occur.
Water consumption represents a substantial environmental and resource concern. Conventional autoclaves typically require significant quantities of purified water to generate steam and for cooling processes. This dependency not only increases operational costs but also places pressure on water resources, particularly in regions facing water scarcity.
Documentation and traceability systems in many existing autoclave installations remain manual or semi-automated, increasing the potential for human error in record-keeping and complicating regulatory compliance efforts. The lack of comprehensive digital integration limits data analysis capabilities that could otherwise drive continuous improvement in sterilization protocols.
Material compatibility constraints further limit autoclave versatility. High temperatures and moisture exposure can damage heat-sensitive instruments, electronic components, and certain polymers, necessitating alternative sterilization methods for these items and complicating workflow management in facilities that process diverse material types.
Energy efficiency remains a significant concern with conventional autoclave systems. Most units require substantial power consumption to generate and maintain the high-pressure steam environment necessary for effective sterilization. This not only increases operational costs but also contributes to larger carbon footprints for healthcare facilities and industrial operations that rely heavily on these devices.
Cycle time optimization presents another notable limitation. Standard autoclave protocols often employ unnecessarily extended exposure times to compensate for potential variability in steam penetration. These conservative approaches, while ensuring sterility, result in reduced throughput, increased energy consumption, and accelerated wear on sterilized materials and equipment components.
Monitoring and validation capabilities in many current autoclave systems lack real-time precision. Traditional biological indicators provide retrospective confirmation of sterilization efficacy but cannot offer immediate feedback during the sterilization process. This delay between processing and verification creates operational inefficiencies and potential risks when sterilization failures occur.
Water consumption represents a substantial environmental and resource concern. Conventional autoclaves typically require significant quantities of purified water to generate steam and for cooling processes. This dependency not only increases operational costs but also places pressure on water resources, particularly in regions facing water scarcity.
Documentation and traceability systems in many existing autoclave installations remain manual or semi-automated, increasing the potential for human error in record-keeping and complicating regulatory compliance efforts. The lack of comprehensive digital integration limits data analysis capabilities that could otherwise drive continuous improvement in sterilization protocols.
Material compatibility constraints further limit autoclave versatility. High temperatures and moisture exposure can damage heat-sensitive instruments, electronic components, and certain polymers, necessitating alternative sterilization methods for these items and complicating workflow management in facilities that process diverse material types.
Standard Autoclave Performance Optimization Methods
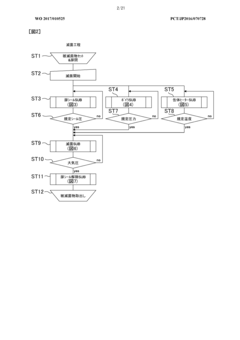
01 Monitoring and evaluation of autoclave performance parameters
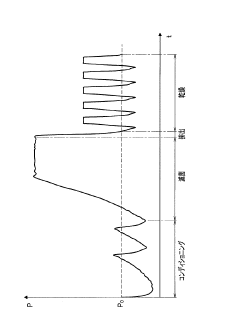
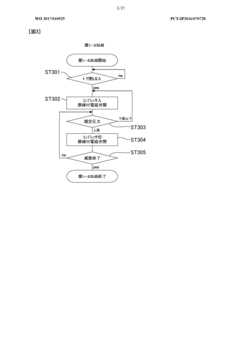
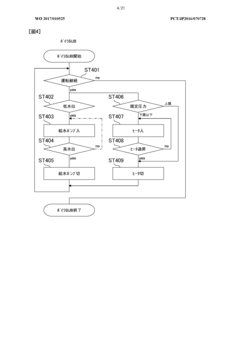
Autoclave performance can be monitored and evaluated through various parameters such as temperature, pressure, time, and sterilization efficacy. These metrics help in ensuring that the autoclave is functioning optimally and meeting the required sterilization standards. Monitoring systems can collect real-time data on these parameters and generate reports for analysis and compliance purposes.- Monitoring and evaluation of autoclave performance: Systems for monitoring and evaluating autoclave performance metrics involve real-time data collection and analysis. These systems track various parameters such as temperature, pressure, and cycle time to ensure proper sterilization. Performance monitoring tools can detect deviations from optimal operation, predict potential failures, and generate alerts when metrics fall outside acceptable ranges. This continuous monitoring helps maintain sterilization efficacy and equipment reliability.
- Predictive maintenance for autoclaves: Predictive maintenance approaches for autoclaves utilize performance metrics to anticipate equipment failures before they occur. By analyzing operational data patterns and historical performance, these systems can identify early warning signs of component degradation. This allows for scheduled maintenance interventions that minimize downtime and extend equipment lifespan. Predictive algorithms can estimate remaining useful life of critical components based on current performance metrics.
- Efficiency optimization in autoclave operations: Performance metrics are used to optimize autoclave efficiency by analyzing resource utilization, cycle times, and energy consumption. These metrics help identify operational inefficiencies and opportunities for improvement. By tracking key performance indicators such as throughput, processing time, and utility usage, operators can implement adjustments to maximize productivity while maintaining sterilization effectiveness. Optimization strategies may include load configuration changes, cycle parameter adjustments, or workflow improvements.
- Validation and compliance of sterilization processes: Performance metrics play a crucial role in validating autoclave sterilization processes and ensuring compliance with regulatory standards. These metrics include biological indicators, chemical indicators, and physical parameters that verify sterilization effectiveness. Automated systems can document and store performance data for audit purposes, creating traceable records of each sterilization cycle. This documentation helps demonstrate adherence to established protocols and regulatory requirements for medical device sterilization and laboratory safety.
- Integration with broader operational systems: Autoclave performance metrics can be integrated with broader operational systems such as laboratory information management systems (LIMS), enterprise resource planning (ERP), or facility management platforms. This integration enables comprehensive analysis of autoclave performance within the context of overall operations. Connected systems can correlate sterilization metrics with production schedules, quality control outcomes, and resource allocation. This holistic approach supports data-driven decision making and continuous improvement initiatives across the organization.
02 Performance metrics visualization and reporting systems
Visualization and reporting systems are essential for presenting autoclave performance metrics in an understandable format. These systems can display data through dashboards, graphs, and charts, allowing operators to quickly identify trends, anomalies, or potential issues. Automated reporting features can generate periodic reports on autoclave performance, facilitating compliance documentation and performance analysis.Expand Specific Solutions03 Predictive maintenance and failure detection
Predictive maintenance systems use performance metrics to anticipate potential autoclave failures before they occur. By analyzing patterns in performance data, these systems can identify early warning signs of equipment degradation or malfunction. This approach enables proactive maintenance scheduling, reduces downtime, and extends the operational life of autoclaves while ensuring consistent sterilization quality.Expand Specific Solutions04 Performance optimization and efficiency metrics
Efficiency metrics for autoclaves include energy consumption, cycle time, resource utilization, and throughput. These metrics help in optimizing autoclave operations to reduce costs while maintaining sterilization effectiveness. Performance optimization may involve adjusting cycle parameters, improving loading practices, or implementing energy-saving technologies to enhance overall autoclave efficiency.Expand Specific Solutions05 Integration with broader management systems
Autoclave performance metrics can be integrated with broader management systems such as quality management systems, enterprise resource planning, or facility management platforms. This integration enables comprehensive analysis of autoclave performance within the context of overall operations, facilitates resource allocation decisions, and supports compliance with regulatory requirements across the organization.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The autoclave sterilization market is currently in a mature growth phase with increasing technological refinement, characterized by a global market size exceeding $2 billion and steady annual growth of 5-7%. The competitive landscape features established medical equipment manufacturers like Olympus Corp. and GE Precision Healthcare dominating with comprehensive sterilization solutions, while specialized players such as Melag Medizintechnik and W&H Sterilization focus on niche innovations. Technology maturity varies across segments, with Oneighty C Technologies and Millisecond Technologies pioneering advanced DNA-breaking sterilization methods, while traditional players like 3M Innovative Properties enhance conventional autoclave efficiency. Research institutions including China Agricultural University and University of South Carolina are contributing to protocol optimization, creating a dynamic ecosystem balancing established technologies with emerging sterilization approaches.
Olympus Corp.
Technical Solution: Olympus has developed the EndoALPHA Autoclave System specifically designed for endoscopic instruments, featuring their proprietary ScopeTrack technology that monitors individual endoscope channels for proper sterilant penetration. Their system incorporates ultrasonic sensors that detect microscopic air pockets within lumens that could compromise sterilization efficacy. The company's EndoSure validation protocol combines traditional biological indicators with channel-specific process challenge devices to verify sterilization of the most challenging instrument areas. Their autoclaves feature adaptive pressure control systems that automatically adjust pressure parameters based on real-time feedback from multiple sensors, ensuring optimal sterilant distribution even in complex instrument geometries. Olympus has also pioneered the integration of RFID technology for automatic instrument identification and cycle parameter selection, reducing human error in protocol selection while creating comprehensive digital records of each instrument's sterilization history.
Strengths: Specialized design for complex endoscopic instruments provides superior assurance of sterilization in challenging lumens and channels. Their comprehensive tracking system simplifies regulatory compliance and instrument management. Weaknesses: The highly specialized nature of their systems limits versatility for general sterilization needs, and the proprietary consumables and validation materials increase operational costs compared to standard autoclaves.
Melag Medizintechnik GmbH Co. KG
Technical Solution: Melag has developed the Premium-Plus Class B autoclave series featuring their patented DRYtelligence technology, which uses adaptive algorithms to optimize drying phases based on load characteristics, reducing cycle times by up to 80% for small loads. Their MELAcontrol Pro system incorporates process challenge devices (PCDs) that simulate worst-case scenarios for sterilant penetration, providing enhanced validation beyond standard biological indicators. The company's autoclaves utilize a double-jacket chamber design that maintains uniform temperature distribution throughout the sterilization cycle, eliminating cold spots that could compromise sterilization efficacy. Their MELAconnect platform enables remote monitoring of multiple autoclaves via secure cloud connectivity, allowing for real-time performance analysis and predictive maintenance scheduling. Additionally, their water separation system prevents contaminated condensate from re-entering the chamber during vacuum phases, significantly improving sterilization consistency.
Strengths: Exceptional energy efficiency through adaptive cycle technology and superior drying capabilities reduce operational costs. Their comprehensive validation systems exceed regulatory requirements, providing enhanced safety assurance. Weaknesses: The sophisticated electronic systems require regular software updates and technical support, and the premium positioning results in higher acquisition costs compared to standard autoclaves.
Critical Patents in Sterilization Efficiency
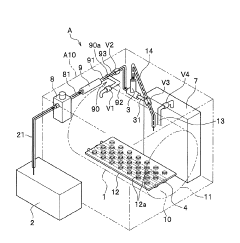
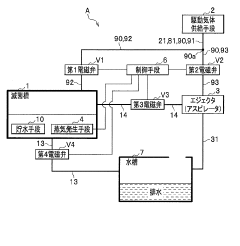
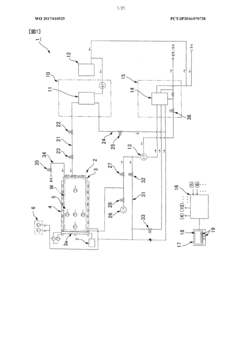
High pressure steam sterilization device
PatentActiveJP2020195539A
Innovation
- A compact high-pressure steam sterilizer incorporating a sterilization tank, driving gas supply means, ejector, steam generating means, and control means to sequentially perform sterilization, pressure reduction, and drying processes using compressed air and an ejector to enhance drying performance.
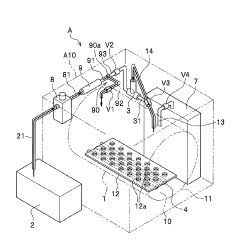
Method of flow-type high-pressure steam sterilization by soft water heat process, and flow-type sterilization device
PatentWO2017010525A1
Innovation
- A flow-through high-pressure steam sterilization method utilizing a soft hydrothermal process, which involves an air removal process, heating and pressurizing, high-pressure steam sterilization with highly saturated steam, and a controlled drying step to minimize condensed water generation and shorten drying time.
Regulatory Standards for Medical Sterilization
Medical sterilization processes are governed by stringent regulatory frameworks that vary across different regions but share common fundamental principles. In the United States, the Food and Drug Administration (FDA) establishes comprehensive guidelines for autoclave sterilization through its Quality System Regulation (21 CFR Part 820), which mandates validation of sterilization processes for medical devices. These regulations require manufacturers to demonstrate that their sterilization protocols consistently achieve a Sterility Assurance Level (SAL) of 10^-6, meaning a probability of less than one in a million that a viable microorganism remains after sterilization.
The European Union implements the Medical Device Regulation (MDR 2017/745) and the harmonized standard EN ISO 17665, which specifically addresses moist heat sterilization. These standards outline requirements for development, validation, and routine control of sterilization processes for medical devices, emphasizing the importance of documented evidence of process effectiveness.
International standards such as ISO 11134 and ISO 17665 provide globally recognized frameworks for steam sterilization validation. These standards detail specifications for temperature, pressure, and exposure time parameters that must be achieved and documented during autoclave cycles. They also establish protocols for biological indicators and chemical integrators used to verify sterilization efficacy.
Healthcare facilities must adhere to additional guidelines from organizations like the Association for the Advancement of Medical Instrumentation (AAMI) and the Centers for Disease Control and Prevention (CDC). The AAMI's ST79 comprehensive guide provides detailed recommendations for steam sterilization in healthcare facilities, covering equipment qualification, routine monitoring, and quality assurance programs.
Regulatory compliance requires rigorous documentation of all sterilization processes. This includes calibration records for autoclave equipment, validation studies demonstrating cycle effectiveness, routine monitoring results, and maintenance logs. Healthcare facilities and manufacturers must maintain these records for inspection by regulatory authorities and accreditation organizations.
Recent regulatory trends emphasize risk-based approaches to sterilization validation, requiring facilities to identify potential failure modes in their sterilization processes and implement appropriate mitigation strategies. This shift acknowledges the complexity of modern medical devices and the need for customized sterilization protocols based on specific device characteristics and bioburden levels.
Compliance with these regulatory standards necessitates ongoing staff training programs and regular audits of sterilization practices. Personnel involved in autoclave operation must demonstrate competency in cycle selection, loading techniques, and interpretation of sterilization indicators to ensure consistent adherence to established protocols.
The European Union implements the Medical Device Regulation (MDR 2017/745) and the harmonized standard EN ISO 17665, which specifically addresses moist heat sterilization. These standards outline requirements for development, validation, and routine control of sterilization processes for medical devices, emphasizing the importance of documented evidence of process effectiveness.
International standards such as ISO 11134 and ISO 17665 provide globally recognized frameworks for steam sterilization validation. These standards detail specifications for temperature, pressure, and exposure time parameters that must be achieved and documented during autoclave cycles. They also establish protocols for biological indicators and chemical integrators used to verify sterilization efficacy.
Healthcare facilities must adhere to additional guidelines from organizations like the Association for the Advancement of Medical Instrumentation (AAMI) and the Centers for Disease Control and Prevention (CDC). The AAMI's ST79 comprehensive guide provides detailed recommendations for steam sterilization in healthcare facilities, covering equipment qualification, routine monitoring, and quality assurance programs.
Regulatory compliance requires rigorous documentation of all sterilization processes. This includes calibration records for autoclave equipment, validation studies demonstrating cycle effectiveness, routine monitoring results, and maintenance logs. Healthcare facilities and manufacturers must maintain these records for inspection by regulatory authorities and accreditation organizations.
Recent regulatory trends emphasize risk-based approaches to sterilization validation, requiring facilities to identify potential failure modes in their sterilization processes and implement appropriate mitigation strategies. This shift acknowledges the complexity of modern medical devices and the need for customized sterilization protocols based on specific device characteristics and bioburden levels.
Compliance with these regulatory standards necessitates ongoing staff training programs and regular audits of sterilization practices. Personnel involved in autoclave operation must demonstrate competency in cycle selection, loading techniques, and interpretation of sterilization indicators to ensure consistent adherence to established protocols.
Sustainability in Autoclave Operations
Sustainability in autoclave operations has become a critical focus area as healthcare facilities and laboratories seek to balance effective sterilization with environmental responsibility. Modern autoclave systems consume significant resources, particularly water and energy, creating substantial environmental footprints. A typical medical-grade autoclave can use between 50-150 gallons of water per cycle and consume 3-7 kWh of electricity, contributing to both utility costs and environmental impact.
Recent advancements in autoclave technology have introduced water recirculation systems that can reduce water consumption by up to 90% compared to conventional models. These systems capture, filter, and reuse water that would otherwise be discharged as waste, significantly reducing the environmental burden while maintaining sterilization efficacy. Energy-efficient autoclaves incorporating improved insulation materials and smart heating algorithms have demonstrated 25-40% reductions in electricity usage without compromising performance metrics.
The implementation of heat recovery systems represents another sustainable innovation, capturing waste heat from exhaust steam to preheat incoming water or support facility heating needs. This approach has shown potential to recover up to 60% of thermal energy that would otherwise be lost, creating a more circular energy profile for sterilization operations.
Operational protocols also play a crucial role in sustainability efforts. Load optimization strategies that maximize chamber capacity while ensuring proper steam penetration can reduce the number of required cycles by 15-30%, directly translating to proportional resource savings. Preventive maintenance schedules designed to maintain optimal performance not only extend equipment lifespan but also prevent efficiency degradation that leads to increased resource consumption.
Chemical usage optimization presents another sustainability opportunity. Modern autoclave detergents and indicators have been reformulated to reduce environmental toxicity while maintaining effectiveness. Biodegradable alternatives have emerged that achieve comparable results with significantly reduced ecological impact during disposal.
Digital monitoring systems now enable precise tracking of resource consumption patterns, allowing facilities to identify inefficiencies and implement targeted improvements. These systems can detect anomalies in water or energy usage that may indicate maintenance needs before they impact sterilization effectiveness or further increase resource waste.
The transition toward sustainable autoclave operations requires initial investment but typically demonstrates positive return on investment within 2-4 years through reduced utility costs. Furthermore, these improvements align with broader organizational sustainability goals and increasingly stringent regulatory requirements regarding resource conservation in healthcare and laboratory settings.
Recent advancements in autoclave technology have introduced water recirculation systems that can reduce water consumption by up to 90% compared to conventional models. These systems capture, filter, and reuse water that would otherwise be discharged as waste, significantly reducing the environmental burden while maintaining sterilization efficacy. Energy-efficient autoclaves incorporating improved insulation materials and smart heating algorithms have demonstrated 25-40% reductions in electricity usage without compromising performance metrics.
The implementation of heat recovery systems represents another sustainable innovation, capturing waste heat from exhaust steam to preheat incoming water or support facility heating needs. This approach has shown potential to recover up to 60% of thermal energy that would otherwise be lost, creating a more circular energy profile for sterilization operations.
Operational protocols also play a crucial role in sustainability efforts. Load optimization strategies that maximize chamber capacity while ensuring proper steam penetration can reduce the number of required cycles by 15-30%, directly translating to proportional resource savings. Preventive maintenance schedules designed to maintain optimal performance not only extend equipment lifespan but also prevent efficiency degradation that leads to increased resource consumption.
Chemical usage optimization presents another sustainability opportunity. Modern autoclave detergents and indicators have been reformulated to reduce environmental toxicity while maintaining effectiveness. Biodegradable alternatives have emerged that achieve comparable results with significantly reduced ecological impact during disposal.
Digital monitoring systems now enable precise tracking of resource consumption patterns, allowing facilities to identify inefficiencies and implement targeted improvements. These systems can detect anomalies in water or energy usage that may indicate maintenance needs before they impact sterilization effectiveness or further increase resource waste.
The transition toward sustainable autoclave operations requires initial investment but typically demonstrates positive return on investment within 2-4 years through reduced utility costs. Furthermore, these improvements align with broader organizational sustainability goals and increasingly stringent regulatory requirements regarding resource conservation in healthcare and laboratory settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!