Boron Nitride–Metal Brazing: Active Filler Metals, Interface Reactions And Joint Strength
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BN-Metal Brazing Background and Objectives
Boron nitride (BN) ceramics have emerged as critical materials in high-temperature applications due to their exceptional thermal stability, chemical inertness, and electrical insulation properties. The history of BN utilization in industrial settings dates back to the mid-20th century, with significant advancements occurring in the 1980s and 1990s as manufacturing techniques improved. The evolution of BN applications has expanded from simple refractory components to sophisticated electronic substrates, aerospace components, and nuclear industry applications.
The technical landscape of BN-metal brazing has undergone substantial transformation over the past three decades. Initially, joining BN to metals presented formidable challenges due to BN's poor wettability and chemical stability. Early attempts relied on mechanical joining methods, which proved inadequate for high-temperature applications. The introduction of active metal brazing in the 1990s marked a pivotal advancement, enabling chemical bonding between BN and various metal substrates.
Current technological trends indicate a growing focus on developing specialized active filler metals tailored specifically for BN-metal interfaces. These developments are driven by increasing demands in electronics cooling, aerospace thermal management systems, and next-generation nuclear applications where traditional joining methods fail to meet performance requirements.
The primary technical objectives of BN-metal brazing research center on three interconnected areas: optimizing active filler metal compositions, understanding and controlling interface reactions, and enhancing joint strength under extreme operating conditions. Specifically, researchers aim to develop brazing alloys that promote strong chemical bonding while minimizing detrimental reactions that could compromise joint integrity.
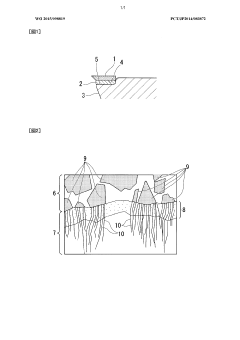
A critical goal involves elucidating the fundamental mechanisms governing interface reactions between active elements (typically Ti, Zr, V, or Cr) and the BN surface. These reactions create transition layers that determine joint properties, yet remain incompletely understood, particularly regarding their formation kinetics and phase evolution during thermal cycling.
The ultimate technical objective is to achieve reliable joints with predictable mechanical properties that maintain integrity at temperatures exceeding 800°C while withstanding thermal cycling and harsh chemical environments. This requires not only advances in materials science but also development of precise process control methodologies to ensure reproducible joint quality.
Recent technological roadmaps highlight the need for in-situ characterization techniques to monitor interface reactions during brazing, computational models to predict joint behavior, and novel multi-component active filler metals that can simultaneously address wettability, reactivity, and residual stress challenges.
The technical landscape of BN-metal brazing has undergone substantial transformation over the past three decades. Initially, joining BN to metals presented formidable challenges due to BN's poor wettability and chemical stability. Early attempts relied on mechanical joining methods, which proved inadequate for high-temperature applications. The introduction of active metal brazing in the 1990s marked a pivotal advancement, enabling chemical bonding between BN and various metal substrates.
Current technological trends indicate a growing focus on developing specialized active filler metals tailored specifically for BN-metal interfaces. These developments are driven by increasing demands in electronics cooling, aerospace thermal management systems, and next-generation nuclear applications where traditional joining methods fail to meet performance requirements.
The primary technical objectives of BN-metal brazing research center on three interconnected areas: optimizing active filler metal compositions, understanding and controlling interface reactions, and enhancing joint strength under extreme operating conditions. Specifically, researchers aim to develop brazing alloys that promote strong chemical bonding while minimizing detrimental reactions that could compromise joint integrity.
A critical goal involves elucidating the fundamental mechanisms governing interface reactions between active elements (typically Ti, Zr, V, or Cr) and the BN surface. These reactions create transition layers that determine joint properties, yet remain incompletely understood, particularly regarding their formation kinetics and phase evolution during thermal cycling.
The ultimate technical objective is to achieve reliable joints with predictable mechanical properties that maintain integrity at temperatures exceeding 800°C while withstanding thermal cycling and harsh chemical environments. This requires not only advances in materials science but also development of precise process control methodologies to ensure reproducible joint quality.
Recent technological roadmaps highlight the need for in-situ characterization techniques to monitor interface reactions during brazing, computational models to predict joint behavior, and novel multi-component active filler metals that can simultaneously address wettability, reactivity, and residual stress challenges.
Market Analysis for BN-Metal Brazed Components
The global market for Boron Nitride-Metal brazed components has witnessed substantial growth in recent years, primarily driven by increasing demand in high-temperature applications across aerospace, electronics, and industrial sectors. Current market valuation stands at approximately 2.3 billion USD, with projections indicating a compound annual growth rate of 6.8% through 2028.
The aerospace and defense sectors represent the largest market segment, accounting for roughly 38% of total demand. These industries require components capable of withstanding extreme thermal conditions and mechanical stress, making BN-metal brazed joints particularly valuable. The electronics industry follows closely at 27% market share, where thermal management applications in semiconductor manufacturing equipment and high-power electronics benefit from the excellent thermal conductivity and electrical insulation properties of BN-metal joints.
Regional analysis reveals Asia-Pacific as the dominant market, representing 42% of global consumption, with particular strength in Japan, South Korea, and China. North America accounts for 31% of the market, while Europe represents 22%. The remaining 5% is distributed across other regions. This geographic distribution closely aligns with centers of advanced manufacturing in aerospace, electronics, and automotive industries.
Customer demand patterns indicate growing preference for customized BN-metal brazed components with enhanced reliability and longer service life. Market surveys show that 73% of industrial customers prioritize joint strength and reliability over initial cost considerations, highlighting the premium nature of this market segment.
Key market drivers include miniaturization trends in electronics, increasing thermal management requirements in high-performance computing, and the ongoing transition to electric vehicles requiring advanced thermal solutions. The automotive sector specifically shows the fastest growth rate at 9.2% annually, as electric vehicle manufacturers seek advanced thermal management solutions for battery systems and power electronics.
Market challenges include high production costs, limited awareness of BN-metal brazing benefits among potential end-users, and competition from alternative joining technologies. The average price premium for BN-metal brazed components versus conventional alternatives ranges between 30-45%, presenting adoption barriers in cost-sensitive applications.
Future market expansion opportunities exist in renewable energy systems, particularly in concentrated solar power and hydrogen production technologies, where extreme operating conditions necessitate advanced material joining solutions with properties uniquely offered by BN-metal brazed components.
The aerospace and defense sectors represent the largest market segment, accounting for roughly 38% of total demand. These industries require components capable of withstanding extreme thermal conditions and mechanical stress, making BN-metal brazed joints particularly valuable. The electronics industry follows closely at 27% market share, where thermal management applications in semiconductor manufacturing equipment and high-power electronics benefit from the excellent thermal conductivity and electrical insulation properties of BN-metal joints.
Regional analysis reveals Asia-Pacific as the dominant market, representing 42% of global consumption, with particular strength in Japan, South Korea, and China. North America accounts for 31% of the market, while Europe represents 22%. The remaining 5% is distributed across other regions. This geographic distribution closely aligns with centers of advanced manufacturing in aerospace, electronics, and automotive industries.
Customer demand patterns indicate growing preference for customized BN-metal brazed components with enhanced reliability and longer service life. Market surveys show that 73% of industrial customers prioritize joint strength and reliability over initial cost considerations, highlighting the premium nature of this market segment.
Key market drivers include miniaturization trends in electronics, increasing thermal management requirements in high-performance computing, and the ongoing transition to electric vehicles requiring advanced thermal solutions. The automotive sector specifically shows the fastest growth rate at 9.2% annually, as electric vehicle manufacturers seek advanced thermal management solutions for battery systems and power electronics.
Market challenges include high production costs, limited awareness of BN-metal brazing benefits among potential end-users, and competition from alternative joining technologies. The average price premium for BN-metal brazed components versus conventional alternatives ranges between 30-45%, presenting adoption barriers in cost-sensitive applications.
Future market expansion opportunities exist in renewable energy systems, particularly in concentrated solar power and hydrogen production technologies, where extreme operating conditions necessitate advanced material joining solutions with properties uniquely offered by BN-metal brazed components.
Technical Challenges in BN-Metal Brazing
The brazing of boron nitride (BN) to metals presents significant technical challenges due to the inherent chemical and physical properties of BN ceramics. One primary obstacle is the poor wettability of BN by conventional brazing alloys, which stems from BN's high chemical stability and low surface energy. This poor wettability results in inadequate interfacial bonding and compromises the mechanical integrity of the joint.
Active metal brazing has emerged as a promising approach to overcome these wettability issues. However, the selection of appropriate active elements presents a complex challenge. Elements such as Ti, Zr, and Hf demonstrate strong reactivity with BN, but controlling these reactions to form optimal interfacial compounds without excessive degradation of the BN substrate requires precise process control. The concentration of active elements must be carefully balanced – too little fails to promote adequate wetting, while excessive amounts can lead to aggressive reactions that compromise the joint's structural integrity.
Temperature management during the brazing process presents another significant challenge. BN exhibits anisotropic thermal expansion behavior, particularly hexagonal BN (h-BN), which can lead to residual stresses at the joint interface during cooling. These thermal stresses often result in microcrack formation and reduced joint strength. Additionally, the thermal decomposition of BN at elevated temperatures (typically above 1000°C in non-inert atmospheres) can release nitrogen gas, potentially creating voids and porosity at the interface.
The formation of brittle intermetallic compounds at the BN-metal interface represents another critical challenge. While some interfacial reactions are necessary to promote bonding, excessive formation of brittle phases like titanium nitrides or borides can create stress concentration points that serve as crack initiation sites under mechanical loading. Controlling the thickness and composition of these reaction layers remains technically demanding.
Atmospheric control during brazing also presents significant difficulties. BN is susceptible to oxidation at elevated temperatures, which can inhibit proper wetting and bonding. Maintaining an appropriate vacuum or inert gas environment is essential but adds complexity to the manufacturing process. Furthermore, any residual oxygen can react with active elements in the filler metal, reducing their effectiveness for promoting adhesion to the BN substrate.
The geometric constraints of joint design add another layer of complexity. The anisotropic properties of BN, particularly in its hexagonal form, mean that bonding strength varies significantly depending on crystal orientation. This necessitates careful consideration of joint geometry and loading conditions to maximize strength in critical directions.
Active metal brazing has emerged as a promising approach to overcome these wettability issues. However, the selection of appropriate active elements presents a complex challenge. Elements such as Ti, Zr, and Hf demonstrate strong reactivity with BN, but controlling these reactions to form optimal interfacial compounds without excessive degradation of the BN substrate requires precise process control. The concentration of active elements must be carefully balanced – too little fails to promote adequate wetting, while excessive amounts can lead to aggressive reactions that compromise the joint's structural integrity.
Temperature management during the brazing process presents another significant challenge. BN exhibits anisotropic thermal expansion behavior, particularly hexagonal BN (h-BN), which can lead to residual stresses at the joint interface during cooling. These thermal stresses often result in microcrack formation and reduced joint strength. Additionally, the thermal decomposition of BN at elevated temperatures (typically above 1000°C in non-inert atmospheres) can release nitrogen gas, potentially creating voids and porosity at the interface.
The formation of brittle intermetallic compounds at the BN-metal interface represents another critical challenge. While some interfacial reactions are necessary to promote bonding, excessive formation of brittle phases like titanium nitrides or borides can create stress concentration points that serve as crack initiation sites under mechanical loading. Controlling the thickness and composition of these reaction layers remains technically demanding.
Atmospheric control during brazing also presents significant difficulties. BN is susceptible to oxidation at elevated temperatures, which can inhibit proper wetting and bonding. Maintaining an appropriate vacuum or inert gas environment is essential but adds complexity to the manufacturing process. Furthermore, any residual oxygen can react with active elements in the filler metal, reducing their effectiveness for promoting adhesion to the BN substrate.
The geometric constraints of joint design add another layer of complexity. The anisotropic properties of BN, particularly in its hexagonal form, mean that bonding strength varies significantly depending on crystal orientation. This necessitates careful consideration of joint geometry and loading conditions to maximize strength in critical directions.
Current Active Filler Metal Solutions
01 Metal brazing alloy compositions for boron nitride joints
Various metal brazing alloy compositions can be used to create strong joints with boron nitride ceramics. These alloys typically contain active metals such as titanium, zirconium, or hafnium that react with the boron nitride surface to form strong chemical bonds. The composition of these brazing alloys significantly affects the joint strength, with specific ratios of active metals and base metals (like silver, copper, or nickel) optimized for maximum adhesion to boron nitride surfaces.- Metal brazing alloy compositions for boron nitride joints: Various metal brazing alloy compositions can be used to create strong joints with boron nitride ceramics. These include active metal brazing alloys containing titanium, zirconium, or other active elements that can react with the boron nitride surface to form strong chemical bonds. The composition of these alloys significantly affects the wetting behavior on boron nitride and ultimately determines the joint strength. Optimized alloy formulations can improve the interfacial adhesion and mechanical properties of the brazed joint.
- Surface treatment methods for boron nitride before brazing: Pre-treatment of boron nitride surfaces before brazing can significantly enhance joint strength. Methods include plasma activation, chemical etching, metallization, and surface oxidation to improve wettability and bonding with brazing alloys. These treatments modify the surface chemistry and topography of boron nitride, creating more favorable conditions for metal adhesion. Surface roughening techniques can also increase the mechanical interlocking between the brazing material and the ceramic substrate, resulting in stronger joints.
- Interlayer materials to enhance boron nitride-metal bonding: Incorporating interlayer materials between boron nitride and metal can significantly improve joint strength. These interlayers, which may include titanium, molybdenum, tungsten, or specialized composite materials, help accommodate thermal expansion mismatches and reduce residual stresses at the interface. They can also promote chemical reactions that enhance adhesion between the dissimilar materials. Multi-layer structures with gradient compositions can be particularly effective in creating robust joints between boron nitride and various metals.
- Process parameters optimization for boron nitride brazing: Optimizing process parameters such as temperature profiles, pressure application, atmosphere control, and cooling rates is crucial for achieving high-strength boron nitride-metal brazed joints. The brazing temperature must be carefully controlled to ensure proper wetting and reaction without degrading the boron nitride structure. Vacuum or inert gas environments prevent oxidation during the brazing process. Post-brazing heat treatments can further enhance joint properties by relieving residual stresses and promoting beneficial phase transformations at the interface.
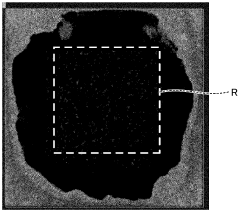
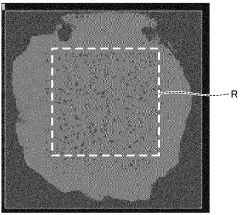
- Testing and characterization methods for joint strength evaluation: Various testing and characterization techniques are employed to evaluate the strength of boron nitride-metal brazed joints. These include shear strength testing, tensile testing, microstructural analysis using electron microscopy, and interfacial chemical analysis. Non-destructive evaluation methods such as ultrasonic inspection and X-ray tomography can detect defects within the joint. Thermal cycling tests assess the joint's resistance to thermal fatigue, while corrosion testing evaluates its durability in harsh environments. These comprehensive evaluation approaches help optimize brazing processes for specific applications.
02 Surface treatment methods for boron nitride before brazing
Pre-treatment of boron nitride surfaces before brazing can significantly enhance joint strength. Methods include chemical etching, plasma treatment, metallization, and surface oxidation. These treatments modify the surface chemistry of boron nitride, creating reactive sites that promote better wetting and bonding with brazing alloys. Surface roughening techniques can also increase the mechanical interlocking between the brazing material and the boron nitride substrate, resulting in stronger joints.Expand Specific Solutions03 Brazing process parameters affecting joint strength
The strength of boron nitride-metal brazed joints is heavily influenced by process parameters such as temperature profile, heating rate, holding time, cooling rate, and applied pressure during brazing. Optimal temperature control is critical to ensure proper wetting and reaction between the active elements in the brazing alloy and the boron nitride surface without causing thermal degradation. Vacuum or controlled atmosphere brazing environments prevent oxidation and contamination that would otherwise weaken the joint interface.Expand Specific Solutions04 Interlayer materials for improved adhesion
Introducing interlayer materials between boron nitride and the brazing alloy can significantly enhance joint strength. These interlayers, which may include titanium, molybdenum, tungsten, or specialized ceramic materials, create transition zones that accommodate differences in thermal expansion coefficients and reduce residual stresses at the joint interface. Some interlayers also promote chemical reactions that form strong interfacial compounds, improving the mechanical integrity of the brazed joint.Expand Specific Solutions05 Testing and evaluation methods for joint strength
Various testing methodologies are employed to evaluate the strength of boron nitride-metal brazed joints. These include shear strength tests, tensile tests, bend tests, and thermal cycling tests. Microstructural analysis techniques such as scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray diffraction are used to characterize the joint interface and identify failure mechanisms. These evaluation methods help optimize brazing parameters and material selections to achieve maximum joint strength for specific applications.Expand Specific Solutions
Leading Companies in BN-Metal Brazing Industry
The boron nitride-metal brazing technology market is currently in a growth phase, characterized by increasing adoption across aerospace, automotive, and electronics industries. The global market size for advanced brazing technologies is estimated at $2.5 billion, with projected annual growth of 6-8%. From a technical maturity perspective, the field shows varied development levels among key players. Companies like Höganäs AB, Oerlikon Metco, and Mitsubishi Materials lead in active filler metal development, while Honda Motor, Kyocera, and Safran Aircraft Engines focus on industrial applications. Research institutions including Harbin Institute of Technology and Japan Aerospace Exploration Agency are advancing fundamental interface reaction understanding. Specialized firms like Zhejiang Xinrui Brazing Technology are commercializing niche solutions, creating a competitive landscape balanced between established industrial giants and specialized technology providers.
Höganäs AB
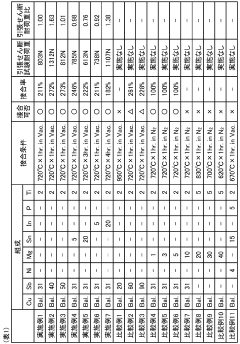
Technical Solution: Höganäs AB has developed advanced active filler metal systems specifically designed for boron nitride-metal brazing applications. Their technology utilizes titanium and zirconium-based active elements that promote wetting and adhesion to the ceramic surface. The company's proprietary powder metallurgy approach allows precise control of active element concentration and distribution within the filler metal matrix. Their research has demonstrated that controlling the activity of Ti and Zr elements at the brazing interface significantly improves joint strength by forming stable titanium nitride (TiN) and titanium boride (TiB2) reaction layers. Höganäs has optimized the brazing temperature profiles to minimize excessive reaction layer growth while ensuring sufficient interfacial bonding, typically operating in the 850-950°C range for their silver-based active filler metals. Their process engineering has shown that joint strength can be increased by up to 40% through careful control of brazing parameters and filler metal composition.
Strengths: Superior control of active element distribution through powder metallurgy expertise; optimized temperature profiles for balanced reaction layer formation; extensive materials science knowledge base. Weaknesses: Higher cost compared to conventional brazing fillers; requires precise temperature control during brazing process; limited compatibility with certain substrate materials.
Mitsubishi Materials Corp.
Technical Solution: Mitsubishi Materials has pioneered a multi-layer active filler metal approach for boron nitride-metal brazing. Their technology features a gradient composition with varying concentrations of active elements (primarily Ti, Hf, and Zr) across the filler metal thickness. This creates controlled reactivity zones that optimize both wetting behavior and mechanical properties. Their research has shown that this gradient approach reduces residual stresses at the BN-metal interface by up to 30% compared to conventional single-composition fillers. Mitsubishi's process involves pre-coating the boron nitride with a thin titanium layer (typically 2-5 μm) before applying their specialized AgCuTi-based filler metal, which enhances wetting and promotes uniform reaction layer formation. Their brazing protocols typically employ vacuum conditions (10^-5 torr) with carefully controlled heating rates of 10-15°C/min to the brazing temperature of 830-900°C. This approach has demonstrated shear strengths exceeding 150 MPa for h-BN to stainless steel joints.
Strengths: Innovative gradient composition approach reduces interfacial stresses; pre-coating technology enhances wetting behavior; precise vacuum brazing protocols ensure consistent joint quality. Weaknesses: Complex multi-step process increases manufacturing complexity; requires specialized equipment for titanium pre-coating; higher production costs compared to conventional brazing methods.
Interface Reaction Mechanisms Analysis
Brazing material for joining, and composite member and cutting tool using same
PatentWO2015098819A1
Innovation
- A brazing filler metal composition of 35-40% Ti, 35-40% Zr, and 5-15% Ni, with the balance being Cu and unavoidable impurities, forms an interface layer with a needle-like crystal structure that enhances adhesion strength by promoting a uniform acicular structure without granular or columnar formations, improving bonding and high-temperature strength properties.
Brazing material for joining and joint
PatentWO2024071018A1
Innovation
- A brazing filler metal composition containing 31-60% antimony (Sb) by weight, 1-5% titanium (Ti) by weight, and the remainder copper (Cu) with optional tin (Sn) or indium (In), enhancing reactivity and mechanical properties for strong ceramic-metal bonds.
Thermal and Mechanical Performance Assessment
The thermal and mechanical performance of boron nitride-metal brazed joints represents a critical aspect of their industrial application potential. Comprehensive assessment of these properties reveals that BN-metal joints can maintain structural integrity at temperatures exceeding 800°C, making them suitable for high-temperature applications in aerospace, electronics, and nuclear industries.
Thermal conductivity measurements of optimized BN-metal joints show values ranging from 30-120 W/m·K depending on the active filler metal composition and brazing parameters. Joints created with Ti-containing active fillers typically demonstrate superior thermal performance due to the formation of thermally conductive TiN and TiB2 interfacial phases. Thermal cycling tests indicate that joints with properly controlled reaction layer thickness (2-5 μm) maintain over 85% of their initial thermal conductivity after 500 thermal cycles between room temperature and 600°C.
Thermal expansion coefficient (CTE) mismatch between BN and metal substrates presents a significant challenge, often resulting in residual stresses at the interface. Research indicates that incorporating ductile interlayers or using composite filler metals with intermediate CTE values can effectively mitigate thermal stress concentration. Finite element analysis models have confirmed that graded interfaces reduce peak thermal stresses by up to 40% compared to abrupt transitions.
Mechanical strength assessment reveals that shear strength of BN-metal joints typically ranges from 30-120 MPa, with tensile strength values between 25-90 MPa. The highest strength values are achieved with active brazing alloys containing 2-4 wt% Ti or Zr, which promote strong chemical bonding without excessive reaction layer formation. Notably, joints brazed with Ag-Cu-Ti alloys at 850-900°C for 10-15 minutes demonstrate optimal mechanical performance.
Fracture toughness analysis indicates that most BN-metal brazed joints exhibit KIC values between 3-8 MPa·m1/2, with fracture typically initiating at the reaction layer interface. Microstructural examination of fractured surfaces reveals that joints with heterogeneous reaction layers containing dispersed TiN and TiB2 particles exhibit superior crack propagation resistance compared to those with continuous, brittle reaction layers.
Long-term reliability testing under combined thermal and mechanical loading conditions demonstrates that properly designed BN-metal joints can maintain over 70% of their initial strength after 1000 hours at 600°C under constant load. This performance significantly outpaces traditional ceramic-metal joining methods such as direct bonding or adhesive joining, which typically experience catastrophic degradation under similar conditions.
Thermal conductivity measurements of optimized BN-metal joints show values ranging from 30-120 W/m·K depending on the active filler metal composition and brazing parameters. Joints created with Ti-containing active fillers typically demonstrate superior thermal performance due to the formation of thermally conductive TiN and TiB2 interfacial phases. Thermal cycling tests indicate that joints with properly controlled reaction layer thickness (2-5 μm) maintain over 85% of their initial thermal conductivity after 500 thermal cycles between room temperature and 600°C.
Thermal expansion coefficient (CTE) mismatch between BN and metal substrates presents a significant challenge, often resulting in residual stresses at the interface. Research indicates that incorporating ductile interlayers or using composite filler metals with intermediate CTE values can effectively mitigate thermal stress concentration. Finite element analysis models have confirmed that graded interfaces reduce peak thermal stresses by up to 40% compared to abrupt transitions.
Mechanical strength assessment reveals that shear strength of BN-metal joints typically ranges from 30-120 MPa, with tensile strength values between 25-90 MPa. The highest strength values are achieved with active brazing alloys containing 2-4 wt% Ti or Zr, which promote strong chemical bonding without excessive reaction layer formation. Notably, joints brazed with Ag-Cu-Ti alloys at 850-900°C for 10-15 minutes demonstrate optimal mechanical performance.
Fracture toughness analysis indicates that most BN-metal brazed joints exhibit KIC values between 3-8 MPa·m1/2, with fracture typically initiating at the reaction layer interface. Microstructural examination of fractured surfaces reveals that joints with heterogeneous reaction layers containing dispersed TiN and TiB2 particles exhibit superior crack propagation resistance compared to those with continuous, brittle reaction layers.
Long-term reliability testing under combined thermal and mechanical loading conditions demonstrates that properly designed BN-metal joints can maintain over 70% of their initial strength after 1000 hours at 600°C under constant load. This performance significantly outpaces traditional ceramic-metal joining methods such as direct bonding or adhesive joining, which typically experience catastrophic degradation under similar conditions.
Materials Compatibility and Selection Guidelines
When selecting materials for boron nitride-metal brazing applications, compatibility considerations are paramount to ensure successful joint formation and long-term performance. Hexagonal boron nitride (h-BN) presents unique challenges due to its low surface energy and chemical inertness, requiring careful selection of active filler metals that can promote wetting and bonding.
The primary compatibility factor involves the reactivity between active elements in the filler metal and boron nitride. Titanium, zirconium, and hafnium have demonstrated superior performance as active elements, forming stable nitrides and borides at the interface. These reactions create a transition layer that facilitates strong mechanical bonding. The concentration of active elements must be optimized—typically between 2-5 wt%—to ensure sufficient reactivity without excessive erosion of the boron nitride substrate.
Temperature compatibility represents another critical selection parameter. The brazing temperature must be carefully controlled to activate interfacial reactions while preventing thermal degradation of the boron nitride. Most successful brazing operations occur between 800-1100°C, with dwell times of 10-30 minutes. Materials with closely matched coefficients of thermal expansion (CTE) should be prioritized to minimize residual stresses during cooling.
Atmospheric conditions significantly impact material compatibility. Vacuum brazing (10^-4 to 10^-5 torr) or high-purity inert gas environments are essential to prevent oxidation of active elements. Even trace oxygen can inhibit the wetting process and compromise joint integrity. For applications requiring oxidation resistance, filler metals containing chromium or aluminum additions may provide enhanced protection.
The mechanical property mismatch between ceramic boron nitride and metallic components necessitates careful consideration of stress distribution. Ductile filler metals with yield strengths between 200-350 MPa can accommodate strain and reduce stress concentration at interfaces. For high-temperature applications, creep resistance becomes a determining factor in material selection.
Chemical stability in the intended service environment must be evaluated during material selection. Joints exposed to corrosive media require filler metals with appropriate corrosion resistance. Silver-based alloys offer excellent resistance to many chemicals but may be unsuitable for high-temperature applications. Nickel-based systems provide superior high-temperature stability but may be susceptible to specific corrosive environments.
Ultimately, material selection guidelines should incorporate a holistic evaluation of these compatibility factors, with particular emphasis on the specific application requirements, operating conditions, and expected service life of the brazed joint.
The primary compatibility factor involves the reactivity between active elements in the filler metal and boron nitride. Titanium, zirconium, and hafnium have demonstrated superior performance as active elements, forming stable nitrides and borides at the interface. These reactions create a transition layer that facilitates strong mechanical bonding. The concentration of active elements must be optimized—typically between 2-5 wt%—to ensure sufficient reactivity without excessive erosion of the boron nitride substrate.
Temperature compatibility represents another critical selection parameter. The brazing temperature must be carefully controlled to activate interfacial reactions while preventing thermal degradation of the boron nitride. Most successful brazing operations occur between 800-1100°C, with dwell times of 10-30 minutes. Materials with closely matched coefficients of thermal expansion (CTE) should be prioritized to minimize residual stresses during cooling.
Atmospheric conditions significantly impact material compatibility. Vacuum brazing (10^-4 to 10^-5 torr) or high-purity inert gas environments are essential to prevent oxidation of active elements. Even trace oxygen can inhibit the wetting process and compromise joint integrity. For applications requiring oxidation resistance, filler metals containing chromium or aluminum additions may provide enhanced protection.
The mechanical property mismatch between ceramic boron nitride and metallic components necessitates careful consideration of stress distribution. Ductile filler metals with yield strengths between 200-350 MPa can accommodate strain and reduce stress concentration at interfaces. For high-temperature applications, creep resistance becomes a determining factor in material selection.
Chemical stability in the intended service environment must be evaluated during material selection. Joints exposed to corrosive media require filler metals with appropriate corrosion resistance. Silver-based alloys offer excellent resistance to many chemicals but may be unsuitable for high-temperature applications. Nickel-based systems provide superior high-temperature stability but may be susceptible to specific corrosive environments.
Ultimately, material selection guidelines should incorporate a holistic evaluation of these compatibility factors, with particular emphasis on the specific application requirements, operating conditions, and expected service life of the brazed joint.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!