Boron Nitride Qualification For Medical Devices: Biostability, Sterilization And ISO Compliance
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Boron Nitride in Medical Devices: Background and Objectives
Boron nitride (BN) has emerged as a promising material in the medical device industry due to its exceptional properties, including high thermal conductivity, excellent chemical stability, and biocompatibility. The evolution of BN applications in healthcare has accelerated significantly over the past decade, transitioning from theoretical research to practical implementations in various medical devices and components.
The historical development of BN in medical applications began in the early 2000s when researchers first identified its potential biocompatibility advantages. By 2010, preliminary studies demonstrated BN's resistance to biological degradation and its stability under various sterilization methods, sparking increased interest from medical device manufacturers. The technological trajectory has since focused on refining BN formulations specifically tailored for medical applications, with particular emphasis on hexagonal boron nitride (h-BN) and cubic boron nitride (c-BN) structures.
Current technological trends indicate a growing integration of BN in implantable devices, surgical instruments, and diagnostic equipment. The material's thermal management capabilities make it particularly valuable in electronic medical devices where heat dissipation is critical. Additionally, BN's chemical inertness presents significant advantages for applications requiring resistance to bodily fluids and sterilization processes.
The primary technical objectives for BN qualification in medical devices encompass several dimensions. First, establishing comprehensive biostability profiles under various physiological conditions to ensure long-term performance in vivo. Second, developing standardized protocols for validating BN materials' resistance to common sterilization methods including autoclave, ethylene oxide, and gamma radiation. Third, creating a robust framework for ISO compliance, particularly with standards such as ISO 10993 for biocompatibility and ISO 13485 for quality management systems.
Research goals also include optimizing BN composite formulations to enhance mechanical properties while maintaining biocompatibility, investigating surface modification techniques to improve integration with biological tissues, and developing cost-effective manufacturing processes to facilitate broader adoption in medical applications.
The qualification pathway for BN in medical devices represents a critical intersection of materials science, biomedical engineering, and regulatory compliance. Success in this domain could potentially revolutionize certain medical device categories by introducing materials with superior performance characteristics while maintaining the stringent safety standards required for healthcare applications.
The historical development of BN in medical applications began in the early 2000s when researchers first identified its potential biocompatibility advantages. By 2010, preliminary studies demonstrated BN's resistance to biological degradation and its stability under various sterilization methods, sparking increased interest from medical device manufacturers. The technological trajectory has since focused on refining BN formulations specifically tailored for medical applications, with particular emphasis on hexagonal boron nitride (h-BN) and cubic boron nitride (c-BN) structures.
Current technological trends indicate a growing integration of BN in implantable devices, surgical instruments, and diagnostic equipment. The material's thermal management capabilities make it particularly valuable in electronic medical devices where heat dissipation is critical. Additionally, BN's chemical inertness presents significant advantages for applications requiring resistance to bodily fluids and sterilization processes.
The primary technical objectives for BN qualification in medical devices encompass several dimensions. First, establishing comprehensive biostability profiles under various physiological conditions to ensure long-term performance in vivo. Second, developing standardized protocols for validating BN materials' resistance to common sterilization methods including autoclave, ethylene oxide, and gamma radiation. Third, creating a robust framework for ISO compliance, particularly with standards such as ISO 10993 for biocompatibility and ISO 13485 for quality management systems.
Research goals also include optimizing BN composite formulations to enhance mechanical properties while maintaining biocompatibility, investigating surface modification techniques to improve integration with biological tissues, and developing cost-effective manufacturing processes to facilitate broader adoption in medical applications.
The qualification pathway for BN in medical devices represents a critical intersection of materials science, biomedical engineering, and regulatory compliance. Success in this domain could potentially revolutionize certain medical device categories by introducing materials with superior performance characteristics while maintaining the stringent safety standards required for healthcare applications.
Market Analysis for Boron Nitride-Based Medical Applications
The global market for boron nitride in medical applications is experiencing significant growth, driven by the material's exceptional properties that address critical needs in the healthcare sector. Current market valuations indicate that the medical-grade boron nitride segment reached approximately $45 million in 2022, with projections suggesting a compound annual growth rate of 6.8% through 2030. This growth trajectory is primarily fueled by increasing demand for biocompatible materials in implantable devices and surgical instruments.
Regional analysis reveals that North America currently dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. The remaining regions account for about 9% of the global market. The United States and Germany lead in research and commercialization efforts, while Japan and China are rapidly expanding their market presence through increased investment in medical materials research.
Demand segmentation shows that orthopedic applications represent the largest market share at 32%, followed by cardiovascular devices at 27%, dental applications at 18%, and surgical instruments at 15%. The remaining 8% encompasses various niche applications including drug delivery systems and diagnostic equipment. This distribution reflects the versatility of boron nitride in addressing diverse medical needs.
Key market drivers include the aging global population, increasing prevalence of chronic diseases requiring implantable devices, and growing demand for minimally invasive surgical procedures. Additionally, stringent regulatory requirements for biocompatible materials have positioned boron nitride favorably against traditional materials that may present biocompatibility challenges.
Market barriers primarily revolve around high production costs, limited awareness among medical device manufacturers, and the need for extensive clinical validation. The cost factor is particularly significant, as medical-grade boron nitride can be 3-5 times more expensive than conventional materials, creating adoption hesitancy among price-sensitive segments.
Customer analysis indicates that tier-1 medical device manufacturers are the primary adopters, accounting for approximately 65% of market consumption. These companies prioritize material performance and regulatory compliance over cost considerations. Mid-sized manufacturers represent about 25% of the market, while smaller specialized device makers account for the remaining 10%.
Future market expansion is expected in emerging applications such as bioelectronics, where boron nitride's electrical insulation properties offer significant advantages, and in combination with other advanced materials to create composite structures with enhanced functionality for next-generation medical devices.
Regional analysis reveals that North America currently dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. The remaining regions account for about 9% of the global market. The United States and Germany lead in research and commercialization efforts, while Japan and China are rapidly expanding their market presence through increased investment in medical materials research.
Demand segmentation shows that orthopedic applications represent the largest market share at 32%, followed by cardiovascular devices at 27%, dental applications at 18%, and surgical instruments at 15%. The remaining 8% encompasses various niche applications including drug delivery systems and diagnostic equipment. This distribution reflects the versatility of boron nitride in addressing diverse medical needs.
Key market drivers include the aging global population, increasing prevalence of chronic diseases requiring implantable devices, and growing demand for minimally invasive surgical procedures. Additionally, stringent regulatory requirements for biocompatible materials have positioned boron nitride favorably against traditional materials that may present biocompatibility challenges.
Market barriers primarily revolve around high production costs, limited awareness among medical device manufacturers, and the need for extensive clinical validation. The cost factor is particularly significant, as medical-grade boron nitride can be 3-5 times more expensive than conventional materials, creating adoption hesitancy among price-sensitive segments.
Customer analysis indicates that tier-1 medical device manufacturers are the primary adopters, accounting for approximately 65% of market consumption. These companies prioritize material performance and regulatory compliance over cost considerations. Mid-sized manufacturers represent about 25% of the market, while smaller specialized device makers account for the remaining 10%.
Future market expansion is expected in emerging applications such as bioelectronics, where boron nitride's electrical insulation properties offer significant advantages, and in combination with other advanced materials to create composite structures with enhanced functionality for next-generation medical devices.
Current Challenges in Boron Nitride Medical Qualification
The integration of boron nitride (BN) into medical devices presents significant technical challenges that must be addressed to ensure compliance with stringent regulatory standards. Currently, one of the primary obstacles is the limited standardized testing protocols specifically designed for evaluating BN materials in medical applications. While ISO 10993 series provides general biocompatibility assessment frameworks, there are no BN-specific testing parameters established, creating uncertainty in qualification processes.
Biostability assessment of BN in physiological environments remains problematic due to the varied forms of BN (hexagonal, cubic, amorphous) exhibiting different degradation behaviors. Research indicates that while BN is generally chemically stable, its long-term performance under biological stress conditions is inadequately characterized, particularly regarding potential nanoparticle release from BN-composite materials during extended implantation periods.
Sterilization compatibility presents another significant challenge. Traditional sterilization methods including gamma irradiation, ethylene oxide, and autoclave processing can potentially alter BN's surface properties and structural integrity. Recent studies have documented changes in BN's thermal conductivity and mechanical properties following repeated sterilization cycles, but comprehensive data across different BN formulations remains scarce.
The qualification of BN-containing devices is further complicated by manufacturing inconsistencies. Current production methods yield BN materials with varying degrees of purity, crystallinity, and surface functionalization, leading to inconsistent performance in biological environments. This variability makes it difficult to establish reliable qualification protocols that can be universally applied across different BN sources and formulations.
Regulatory pathways for BN medical devices lack clarity, with different international bodies applying varying standards for novel material qualification. The FDA's regulatory approach to nanomaterials differs from the European Medical Device Regulation (MDR) framework, creating compliance challenges for global manufacturers incorporating BN into their medical products.
Toxicological profiling of BN remains incomplete, particularly regarding the potential for nano-sized BN particles to translocate within biological systems. While bulk BN shows promising biocompatibility, the safety profile of BN nanotubes and nanosheets requires further investigation, especially concerning their potential inflammatory responses and genotoxicity in long-term applications.
These challenges collectively create significant barriers to the widespread adoption of BN in medical devices despite its promising thermal, electrical, and mechanical properties. Addressing these qualification hurdles requires coordinated efforts between material scientists, regulatory experts, and medical device manufacturers to develop standardized testing protocols and clear regulatory pathways.
Biostability assessment of BN in physiological environments remains problematic due to the varied forms of BN (hexagonal, cubic, amorphous) exhibiting different degradation behaviors. Research indicates that while BN is generally chemically stable, its long-term performance under biological stress conditions is inadequately characterized, particularly regarding potential nanoparticle release from BN-composite materials during extended implantation periods.
Sterilization compatibility presents another significant challenge. Traditional sterilization methods including gamma irradiation, ethylene oxide, and autoclave processing can potentially alter BN's surface properties and structural integrity. Recent studies have documented changes in BN's thermal conductivity and mechanical properties following repeated sterilization cycles, but comprehensive data across different BN formulations remains scarce.
The qualification of BN-containing devices is further complicated by manufacturing inconsistencies. Current production methods yield BN materials with varying degrees of purity, crystallinity, and surface functionalization, leading to inconsistent performance in biological environments. This variability makes it difficult to establish reliable qualification protocols that can be universally applied across different BN sources and formulations.
Regulatory pathways for BN medical devices lack clarity, with different international bodies applying varying standards for novel material qualification. The FDA's regulatory approach to nanomaterials differs from the European Medical Device Regulation (MDR) framework, creating compliance challenges for global manufacturers incorporating BN into their medical products.
Toxicological profiling of BN remains incomplete, particularly regarding the potential for nano-sized BN particles to translocate within biological systems. While bulk BN shows promising biocompatibility, the safety profile of BN nanotubes and nanosheets requires further investigation, especially concerning their potential inflammatory responses and genotoxicity in long-term applications.
These challenges collectively create significant barriers to the widespread adoption of BN in medical devices despite its promising thermal, electrical, and mechanical properties. Addressing these qualification hurdles requires coordinated efforts between material scientists, regulatory experts, and medical device manufacturers to develop standardized testing protocols and clear regulatory pathways.
Established Qualification Protocols for Medical-Grade Boron Nitride
01 Biocompatibility and biostability of boron nitride materials
Boron nitride materials demonstrate excellent biocompatibility and biostability properties, making them suitable for medical implants and devices. These materials show minimal adverse reactions when in contact with biological tissues and maintain their structural integrity in biological environments. The biostability of boron nitride is attributed to its chemical inertness and resistance to degradation in physiological conditions, allowing for long-term applications in the body.- Biocompatibility and biostability of boron nitride materials: Boron nitride materials demonstrate excellent biocompatibility and biostability properties, making them suitable for medical and biological applications. These materials show minimal cytotoxicity and good compatibility with living tissues, allowing for their use in implantable devices and biomedical applications. The inherent chemical stability of boron nitride contributes to its long-term performance in biological environments without significant degradation.
- Sterilization methods for boron nitride-based materials: Various sterilization techniques can be applied to boron nitride-based materials without compromising their structural integrity or functional properties. These methods include autoclave sterilization, gamma radiation, ethylene oxide treatment, and dry heat sterilization. Boron nitride materials maintain their physical and chemical characteristics after sterilization processes, which is crucial for applications in medical devices, implants, and other biomedical products requiring sterile conditions.
- Boron nitride nanostructures for medical applications: Boron nitride nanostructures, including nanotubes, nanosheets, and nanoparticles, offer unique properties beneficial for medical applications. These nanostructures can be functionalized for drug delivery, tissue engineering, and bioimaging purposes. Their high surface area, thermal stability, and chemical inertness make them excellent candidates for biomedical applications. The controlled synthesis and surface modification of these nanostructures enhance their performance in biological environments.
- Boron nitride composites with enhanced biostability: Composite materials incorporating boron nitride exhibit enhanced biostability and mechanical properties suitable for biomedical applications. These composites combine the beneficial properties of boron nitride with other materials such as polymers, ceramics, or metals. The resulting materials show improved wear resistance, thermal conductivity, and biological performance. Surface treatments and coating techniques can further enhance the biostability and functionality of these composite materials in medical environments.
- Thermal and chemical stability of boron nitride for sterilizable medical devices: Boron nitride materials possess exceptional thermal and chemical stability, making them ideal for medical devices that require repeated sterilization. These materials can withstand high temperatures, pressure, and exposure to sterilizing chemicals without degradation. The thermal conductivity of boron nitride helps in uniform heat distribution during thermal sterilization processes. Additionally, its chemical inertness prevents reactions with sterilizing agents, ensuring the longevity and reliability of medical devices containing boron nitride components.
02 Sterilization methods for boron nitride-based materials
Various sterilization techniques can be applied to boron nitride-based materials without compromising their structural integrity or properties. These methods include autoclave sterilization, gamma radiation, ethylene oxide treatment, and dry heat sterilization. Boron nitride materials exhibit high thermal stability and chemical resistance, allowing them to withstand harsh sterilization conditions while maintaining their functional characteristics, which is crucial for medical and biological applications.Expand Specific Solutions03 Boron nitride nanostructures for biomedical applications
Boron nitride nanostructures, including nanotubes, nanosheets, and nanoparticles, offer unique advantages for biomedical applications due to their exceptional biostability. These nanostructures can be functionalized to enhance their biocompatibility and can serve as drug delivery vehicles, imaging agents, or components in tissue engineering scaffolds. Their high surface area, chemical stability, and ability to be modified with various functional groups make them versatile materials for advanced biomedical technologies.Expand Specific Solutions04 Boron nitride composites with enhanced biostability
Composite materials incorporating boron nitride exhibit improved biostability and mechanical properties compared to conventional materials. These composites typically combine boron nitride with polymers, ceramics, or metals to create materials with tailored characteristics suitable for specific biomedical applications. The addition of boron nitride enhances the wear resistance, thermal conductivity, and chemical stability of the composite while maintaining biocompatibility, making these materials ideal for orthopedic implants and dental applications.Expand Specific Solutions05 Surface modification of boron nitride for improved biological interaction
Surface modification techniques can enhance the biostability and functionality of boron nitride materials in biological environments. These modifications include coating with bioactive molecules, surface functionalization with specific chemical groups, or creating hierarchical surface structures. Modified boron nitride surfaces can promote cell adhesion, reduce bacterial colonization, and improve integration with surrounding tissues, extending the lifespan and effectiveness of boron nitride-based medical devices and implants.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The boron nitride medical device qualification market is currently in a growth phase, with increasing adoption driven by the material's exceptional biostability and thermal properties. The global market is expanding as medical device manufacturers seek advanced materials that meet stringent ISO compliance requirements. Companies like Ethicon (Johnson & Johnson subsidiary) and O&M Halyard are leveraging boron nitride in sterile medical applications, while specialized materials firms such as Denka Corp., Sumitomo Electric Industries, and NOF Corp. are developing medical-grade boron nitride formulations. Research institutions including Georgia Tech Research Corp. and McGill University are advancing the fundamental understanding of boron nitride biocompatibility. The technology is maturing rapidly with DiFusion and Janssen Research & Development focusing on innovative applications that combine biostability with sterilization resistance.
Ethicon, Inc.
Technical Solution: Ethicon, a Johnson & Johnson subsidiary, has pioneered the integration of boron nitride nanotubes (BNNTs) into surgical instruments and implantable medical devices. Their proprietary qualification process for boron nitride materials includes comprehensive biostability testing in simulated biological fluids for extended periods (up to 5 years) to ensure long-term stability. Ethicon's technology incorporates boron nitride into polymer matrices to create composite materials with enhanced mechanical properties while maintaining biocompatibility. Their sterilization validation protocols are particularly robust, testing BN-containing materials through multiple sterilization cycles using gamma radiation, ethylene oxide, and steam sterilization methods. The company has developed specialized surface treatments for boron nitride that improve its integration with biological tissues while minimizing foreign body responses. Ethicon's qualification process strictly adheres to ISO 10993 standards, with particular emphasis on parts 1, 5, 10, and 11 for biological evaluation of medical devices.
Strengths: Extensive experience in medical device regulatory compliance and access to Johnson & Johnson's vast resources for comprehensive testing. Their BN composites demonstrate excellent mechanical properties and tissue compatibility. Weaknesses: Their proprietary BN formulations may be limited to specific applications within surgical instruments and implants, potentially limiting broader application across diverse medical device categories.
DiFusion, Inc.
Technical Solution: DiFusion has developed ZFUZE, a proprietary technology platform incorporating boron nitride into spinal implants and orthopedic devices. Their qualification process focuses on the antimicrobial properties of boron nitride composites, demonstrating significant reduction in bacterial adhesion and biofilm formation compared to traditional materials. DiFusion's technology leverages the unique properties of hexagonal boron nitride (h-BN) to create implantable devices with enhanced osseointegration capabilities while maintaining excellent biostability. Their qualification protocol includes extensive in vitro and in vivo testing to assess long-term stability in simulated physiological environments. The company has developed specialized manufacturing processes that ensure uniform distribution of boron nitride particles within polymer matrices, creating consistent material properties across production batches. DiFusion's sterilization validation demonstrates that their BN-enhanced materials maintain structural integrity and antimicrobial properties after multiple sterilization cycles using standard hospital protocols. Their compliance testing adheres strictly to ISO 10993-1 guidelines, with particular focus on parts 5, 10, 11, and 12 for cytotoxicity, irritation, sensitization, and material-mediated pyrogenicity.
Strengths: Specialized expertise in orthopedic and spinal applications with demonstrated clinical benefits including reduced infection rates and improved osseointegration. Their materials show excellent resistance to degradation in physiological environments. Weaknesses: Their technology platform may be optimized specifically for orthopedic applications, potentially limiting transferability to other medical device categories requiring different material properties.
Critical Patents and Research on Boron Nitride Biostability
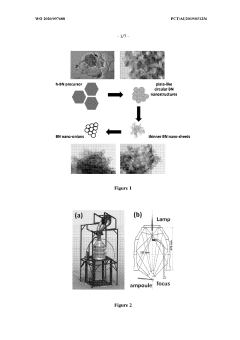
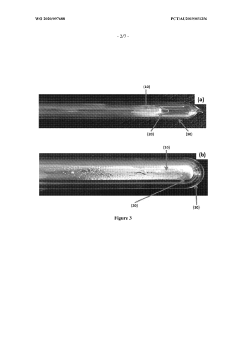
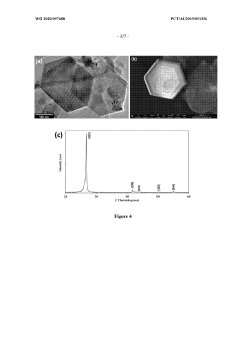
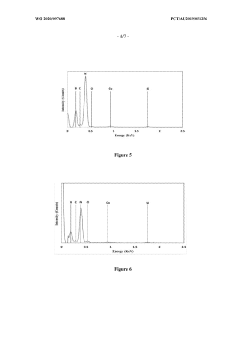
Boron nitride nanostructures
PatentWO2020097688A1
Innovation
- Subjecting boron nitride precursor material to lamp ablation within an adiabatic radiative shielding environment, such as a hermetically sealed fused quartz vessel, to produce a variety of nanostructures, particularly nano-onions, using high flux bright light that promotes reactions not attainable in conventional methods.
ISO 10993 Compliance Framework for Boron Nitride Materials
ISO 10993 compliance represents a critical framework for evaluating the biocompatibility of materials used in medical devices, including innovative materials like boron nitride (BN). This international standard series establishes systematic approaches to biological evaluation, ensuring patient safety while enabling technological advancement in healthcare applications.
The ISO 10993 framework consists of multiple parts addressing different aspects of biocompatibility assessment. For boron nitride materials, compliance begins with ISO 10993-1, which outlines the general principles and categorization based on the nature and duration of body contact. Medical devices incorporating BN must be properly categorized to determine the appropriate testing regimen.
Risk assessment forms the foundation of the compliance process, requiring manufacturers to identify potential biological hazards associated with BN materials. This includes evaluation of chemical composition, manufacturing processes, sterilization methods, and potential leachables or degradation products that might affect biological systems.
Material characterization represents another crucial element in the framework, governed by ISO 10993-18. For boron nitride, this involves comprehensive chemical analysis to identify and quantify constituent components, impurities, and manufacturing residuals. Different forms of BN (hexagonal, cubic, amorphous) require specific characterization approaches due to their distinct properties.
Cytotoxicity testing under ISO 10993-5 serves as a primary screening method for BN materials, evaluating potential cellular damage through direct contact, extract, or indirect testing methodologies. This provides initial insights into cellular compatibility before progressing to more complex biological evaluations.
Systemic toxicity assessment follows ISO 10993-11 guidelines, examining potential adverse effects of BN materials on organ systems. This may include acute, subchronic, and chronic toxicity studies depending on the intended duration of patient contact and risk classification of the device.
Genotoxicity evaluation under ISO 10993-3 investigates potential DNA damage or mutations that might be caused by BN materials or their leachables. This typically involves a battery of tests including bacterial reverse mutation assays, chromosomal aberration tests, and in vivo micronucleus studies.
Implantation studies, guided by ISO 10993-6, assess local tissue responses to implanted BN materials over time. These studies evaluate inflammation, fibrous capsule formation, and tissue integration, providing critical data for long-term implantable devices utilizing boron nitride components.
The ISO 10993 framework consists of multiple parts addressing different aspects of biocompatibility assessment. For boron nitride materials, compliance begins with ISO 10993-1, which outlines the general principles and categorization based on the nature and duration of body contact. Medical devices incorporating BN must be properly categorized to determine the appropriate testing regimen.
Risk assessment forms the foundation of the compliance process, requiring manufacturers to identify potential biological hazards associated with BN materials. This includes evaluation of chemical composition, manufacturing processes, sterilization methods, and potential leachables or degradation products that might affect biological systems.
Material characterization represents another crucial element in the framework, governed by ISO 10993-18. For boron nitride, this involves comprehensive chemical analysis to identify and quantify constituent components, impurities, and manufacturing residuals. Different forms of BN (hexagonal, cubic, amorphous) require specific characterization approaches due to their distinct properties.
Cytotoxicity testing under ISO 10993-5 serves as a primary screening method for BN materials, evaluating potential cellular damage through direct contact, extract, or indirect testing methodologies. This provides initial insights into cellular compatibility before progressing to more complex biological evaluations.
Systemic toxicity assessment follows ISO 10993-11 guidelines, examining potential adverse effects of BN materials on organ systems. This may include acute, subchronic, and chronic toxicity studies depending on the intended duration of patient contact and risk classification of the device.
Genotoxicity evaluation under ISO 10993-3 investigates potential DNA damage or mutations that might be caused by BN materials or their leachables. This typically involves a battery of tests including bacterial reverse mutation assays, chromosomal aberration tests, and in vivo micronucleus studies.
Implantation studies, guided by ISO 10993-6, assess local tissue responses to implanted BN materials over time. These studies evaluate inflammation, fibrous capsule formation, and tissue integration, providing critical data for long-term implantable devices utilizing boron nitride components.
Biocompatibility Testing Requirements and Methodologies
Biocompatibility testing for boron nitride (BN) in medical devices follows a structured approach guided by international standards, primarily ISO 10993 series. These standards establish a framework for evaluating the biological safety of materials intended for human contact during medical applications.
The testing requirements vary based on the nature and duration of contact between BN-containing devices and human tissues. For short-term contact devices, cytotoxicity, sensitization, and irritation tests form the baseline assessment. Devices with prolonged or permanent contact require additional evaluations including genotoxicity, implantation studies, and systemic toxicity assessments.
Cytotoxicity testing (ISO 10993-5) represents the initial screening method, typically utilizing in vitro techniques with mammalian cell cultures to detect potential cellular damage from BN materials. The test evaluates cell viability, growth inhibition, and morphological alterations following exposure to BN extracts or direct contact with the material.
Sensitization testing (ISO 10993-10) assesses the potential of BN to cause delayed hypersensitivity reactions. The Guinea Pig Maximization Test or Local Lymph Node Assay are commonly employed methodologies, examining immune responses following repeated exposure to the material.
Irritation testing protocols (ISO 10993-23) evaluate potential inflammatory responses upon contact with skin, eyes, or mucosal membranes. These tests typically involve application of BN extracts to appropriate test systems with subsequent evaluation of tissue reactions.
For implantable BN-containing devices, implantation studies (ISO 10993-6) become essential, involving surgical placement of test samples in appropriate animal models. These studies evaluate local tissue responses over time periods relevant to the intended clinical application, with histopathological examination of surrounding tissues.
Hemocompatibility testing (ISO 10993-4) is critical for BN materials intended for blood contact, assessing potential adverse interactions with blood components including hemolysis, thrombogenicity, and complement activation.
Advanced biocompatibility assessments may include subchronic and chronic toxicity studies (ISO 10993-11), examining potential systemic effects following long-term exposure. Genotoxicity testing (ISO 10993-3) evaluates mutagenic potential through bacterial reverse mutation tests, chromosomal aberration studies, and mammalian cell gene mutation assays.
The testing strategy must be tailored to the specific application of BN in medical devices, considering factors such as processing methods, surface treatments, and potential leachable compounds. A risk-based approach, as outlined in ISO 10993-1, guides the selection of appropriate test methods based on the device's intended use and potential biological risks.
The testing requirements vary based on the nature and duration of contact between BN-containing devices and human tissues. For short-term contact devices, cytotoxicity, sensitization, and irritation tests form the baseline assessment. Devices with prolonged or permanent contact require additional evaluations including genotoxicity, implantation studies, and systemic toxicity assessments.
Cytotoxicity testing (ISO 10993-5) represents the initial screening method, typically utilizing in vitro techniques with mammalian cell cultures to detect potential cellular damage from BN materials. The test evaluates cell viability, growth inhibition, and morphological alterations following exposure to BN extracts or direct contact with the material.
Sensitization testing (ISO 10993-10) assesses the potential of BN to cause delayed hypersensitivity reactions. The Guinea Pig Maximization Test or Local Lymph Node Assay are commonly employed methodologies, examining immune responses following repeated exposure to the material.
Irritation testing protocols (ISO 10993-23) evaluate potential inflammatory responses upon contact with skin, eyes, or mucosal membranes. These tests typically involve application of BN extracts to appropriate test systems with subsequent evaluation of tissue reactions.
For implantable BN-containing devices, implantation studies (ISO 10993-6) become essential, involving surgical placement of test samples in appropriate animal models. These studies evaluate local tissue responses over time periods relevant to the intended clinical application, with histopathological examination of surrounding tissues.
Hemocompatibility testing (ISO 10993-4) is critical for BN materials intended for blood contact, assessing potential adverse interactions with blood components including hemolysis, thrombogenicity, and complement activation.
Advanced biocompatibility assessments may include subchronic and chronic toxicity studies (ISO 10993-11), examining potential systemic effects following long-term exposure. Genotoxicity testing (ISO 10993-3) evaluates mutagenic potential through bacterial reverse mutation tests, chromosomal aberration studies, and mammalian cell gene mutation assays.
The testing strategy must be tailored to the specific application of BN in medical devices, considering factors such as processing methods, surface treatments, and potential leachable compounds. A risk-based approach, as outlined in ISO 10993-1, guides the selection of appropriate test methods based on the device's intended use and potential biological risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!