Hydrochloric Acid: Breakthroughs in Waste Treatment
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Waste Treatment Evolution and Objectives
The treatment of hydrochloric acid (HCl) waste has undergone significant evolution over the past decades, driven by increasing environmental concerns and regulatory pressures. Initially, the primary focus was on neutralization and dilution techniques, which were simple but often inadequate for addressing the full spectrum of environmental impacts. As awareness grew about the potential harm of chloride-rich effluents to aquatic ecosystems, more sophisticated treatment methods emerged.
The technological progression in HCl waste treatment has been marked by several key milestones. In the 1970s and 1980s, the development of ion exchange resins capable of selectively removing chloride ions represented a major breakthrough. This was followed by the introduction of membrane-based separation technologies in the 1990s, particularly reverse osmosis and electrodialysis, which allowed for more efficient recovery of both acid and water.
Recent years have seen a shift towards more sustainable and circular approaches to HCl waste management. The concept of "waste to resource" has gained traction, with innovative technologies aimed at recovering valuable materials from HCl waste streams. For instance, the development of electrochemical processes for converting HCl waste into chlorine gas and hydrogen has opened up new possibilities for resource recovery and energy generation.
The current technological landscape is characterized by a growing emphasis on integrated treatment systems that combine multiple processes to achieve optimal results. These may include pre-treatment steps such as oxidation or reduction, followed by separation technologies and final polishing stages. Advanced oxidation processes (AOPs) have emerged as a promising approach for dealing with recalcitrant organic compounds often present in industrial HCl waste.
Looking ahead, the objectives for HCl waste treatment technologies are multifaceted. There is a strong push towards developing more energy-efficient and cost-effective treatment methods to make advanced technologies accessible to a broader range of industries. Improving the selectivity and efficiency of separation processes remains a key goal, with ongoing research into novel membrane materials and ion exchange resins.
Another important objective is the development of technologies capable of handling high-strength HCl waste streams, which pose particular challenges due to their corrosive nature and high chloride content. This includes exploring new materials and process designs that can withstand extreme conditions while maintaining treatment efficacy.
Ultimately, the overarching aim is to move towards zero-discharge systems, where HCl waste is fully recycled or converted into valuable products, minimizing environmental impact and maximizing resource efficiency. This aligns with broader sustainability goals and the principles of circular economy, driving innovation in waste treatment technologies across industries.
The technological progression in HCl waste treatment has been marked by several key milestones. In the 1970s and 1980s, the development of ion exchange resins capable of selectively removing chloride ions represented a major breakthrough. This was followed by the introduction of membrane-based separation technologies in the 1990s, particularly reverse osmosis and electrodialysis, which allowed for more efficient recovery of both acid and water.
Recent years have seen a shift towards more sustainable and circular approaches to HCl waste management. The concept of "waste to resource" has gained traction, with innovative technologies aimed at recovering valuable materials from HCl waste streams. For instance, the development of electrochemical processes for converting HCl waste into chlorine gas and hydrogen has opened up new possibilities for resource recovery and energy generation.
The current technological landscape is characterized by a growing emphasis on integrated treatment systems that combine multiple processes to achieve optimal results. These may include pre-treatment steps such as oxidation or reduction, followed by separation technologies and final polishing stages. Advanced oxidation processes (AOPs) have emerged as a promising approach for dealing with recalcitrant organic compounds often present in industrial HCl waste.
Looking ahead, the objectives for HCl waste treatment technologies are multifaceted. There is a strong push towards developing more energy-efficient and cost-effective treatment methods to make advanced technologies accessible to a broader range of industries. Improving the selectivity and efficiency of separation processes remains a key goal, with ongoing research into novel membrane materials and ion exchange resins.
Another important objective is the development of technologies capable of handling high-strength HCl waste streams, which pose particular challenges due to their corrosive nature and high chloride content. This includes exploring new materials and process designs that can withstand extreme conditions while maintaining treatment efficacy.
Ultimately, the overarching aim is to move towards zero-discharge systems, where HCl waste is fully recycled or converted into valuable products, minimizing environmental impact and maximizing resource efficiency. This aligns with broader sustainability goals and the principles of circular economy, driving innovation in waste treatment technologies across industries.
Market Demand for HCl Waste Solutions
The market demand for hydrochloric acid (HCl) waste treatment solutions has been steadily increasing in recent years, driven by stringent environmental regulations and growing industrial activities. Various sectors, including chemical manufacturing, metal processing, and semiconductor production, generate significant amounts of HCl waste, creating a pressing need for effective treatment methods.
The global market for HCl waste treatment is expected to expand substantially, with a particular focus on developing economies where industrial growth is rapid. Environmental concerns and the push towards sustainable manufacturing practices are key factors fueling this demand. Industries are increasingly seeking cost-effective and environmentally friendly solutions to manage their HCl waste, as improper disposal can lead to severe ecological damage and regulatory penalties.
In the chemical manufacturing sector, which is one of the largest producers of HCl waste, there is a growing trend towards circular economy principles. This has led to increased interest in technologies that can recover and recycle HCl from waste streams, potentially turning a liability into a valuable resource. The metal processing industry, another significant contributor to HCl waste, is also showing heightened demand for treatment solutions that can meet increasingly strict discharge standards while minimizing operational costs.
The semiconductor industry, with its ultra-pure water requirements, represents a niche but high-value market for HCl waste treatment. As this sector continues to grow, driven by the increasing demand for electronic devices, so does the need for specialized HCl waste management solutions. This industry's demand is particularly focused on technologies that can achieve extremely low contaminant levels in treated water.
Geographically, Asia-Pacific is emerging as a key market for HCl waste treatment solutions, owing to its rapidly expanding industrial base and tightening environmental regulations. North America and Europe, with their mature industries and stringent environmental standards, continue to be significant markets, with a focus on advanced, high-efficiency treatment technologies.
The market is also seeing a shift towards integrated waste management solutions that can handle multiple types of acidic waste, including HCl. This trend is driven by the desire for more comprehensive and cost-effective waste treatment systems in industrial facilities. Additionally, there is growing interest in mobile and modular HCl waste treatment units, particularly for smaller industrial operations or temporary project sites.
As awareness of the environmental impact of industrial activities grows, there is an increasing demand for HCl waste treatment solutions that not only meet regulatory standards but also contribute to a company's sustainability goals. This has led to a market preference for technologies that offer both economic and environmental benefits, such as those that enable water reuse or resource recovery from waste streams.
The global market for HCl waste treatment is expected to expand substantially, with a particular focus on developing economies where industrial growth is rapid. Environmental concerns and the push towards sustainable manufacturing practices are key factors fueling this demand. Industries are increasingly seeking cost-effective and environmentally friendly solutions to manage their HCl waste, as improper disposal can lead to severe ecological damage and regulatory penalties.
In the chemical manufacturing sector, which is one of the largest producers of HCl waste, there is a growing trend towards circular economy principles. This has led to increased interest in technologies that can recover and recycle HCl from waste streams, potentially turning a liability into a valuable resource. The metal processing industry, another significant contributor to HCl waste, is also showing heightened demand for treatment solutions that can meet increasingly strict discharge standards while minimizing operational costs.
The semiconductor industry, with its ultra-pure water requirements, represents a niche but high-value market for HCl waste treatment. As this sector continues to grow, driven by the increasing demand for electronic devices, so does the need for specialized HCl waste management solutions. This industry's demand is particularly focused on technologies that can achieve extremely low contaminant levels in treated water.
Geographically, Asia-Pacific is emerging as a key market for HCl waste treatment solutions, owing to its rapidly expanding industrial base and tightening environmental regulations. North America and Europe, with their mature industries and stringent environmental standards, continue to be significant markets, with a focus on advanced, high-efficiency treatment technologies.
The market is also seeing a shift towards integrated waste management solutions that can handle multiple types of acidic waste, including HCl. This trend is driven by the desire for more comprehensive and cost-effective waste treatment systems in industrial facilities. Additionally, there is growing interest in mobile and modular HCl waste treatment units, particularly for smaller industrial operations or temporary project sites.
As awareness of the environmental impact of industrial activities grows, there is an increasing demand for HCl waste treatment solutions that not only meet regulatory standards but also contribute to a company's sustainability goals. This has led to a market preference for technologies that offer both economic and environmental benefits, such as those that enable water reuse or resource recovery from waste streams.
Current Challenges in HCl Waste Management
The management of hydrochloric acid (HCl) waste presents significant challenges in various industries, particularly in chemical manufacturing, metal processing, and semiconductor production. One of the primary issues is the corrosive nature of HCl, which can cause severe damage to equipment, infrastructure, and the environment if not properly handled. This necessitates specialized containment and treatment systems, adding complexity and cost to waste management processes.
Another major challenge is the high volume of HCl waste generated in certain industrial processes. For instance, the semiconductor industry produces large quantities of spent HCl during wafer cleaning and etching operations. The sheer volume of waste requires extensive treatment facilities and poses logistical difficulties in transportation and storage.
The environmental impact of HCl waste is a growing concern. Improper disposal can lead to soil and water contamination, affecting ecosystems and potentially entering the food chain. Stringent regulations have been implemented in many countries to control HCl waste disposal, but compliance often requires significant investment in treatment technologies and monitoring systems.
Neutralization, a common treatment method for HCl waste, presents its own set of challenges. While effective in reducing acidity, it generates large volumes of salt-laden wastewater, which requires further treatment or disposal. This secondary waste stream can be problematic, especially in areas with limited water resources or strict discharge regulations.
The recovery and recycling of HCl from waste streams is an attractive option but faces technical and economic hurdles. Current recovery processes often struggle with efficiency, especially when dealing with dilute or contaminated acid streams. The energy requirements and capital costs associated with advanced recovery technologies can be prohibitive for smaller operations.
Worker safety is another critical concern in HCl waste management. The handling, treatment, and disposal of HCl waste pose significant health risks due to potential exposure to acid fumes and splashes. This necessitates comprehensive safety protocols, specialized personal protective equipment, and ongoing training programs, all of which add to the operational complexity and cost.
Lastly, the variability in HCl waste composition across different industries complicates the development of universal treatment solutions. Contaminants such as heavy metals, organic compounds, or other chemicals often accompany HCl waste, requiring tailored treatment approaches. This diversity challenges researchers and engineers to develop flexible, adaptable waste management technologies that can handle a wide range of HCl waste streams effectively and economically.
Another major challenge is the high volume of HCl waste generated in certain industrial processes. For instance, the semiconductor industry produces large quantities of spent HCl during wafer cleaning and etching operations. The sheer volume of waste requires extensive treatment facilities and poses logistical difficulties in transportation and storage.
The environmental impact of HCl waste is a growing concern. Improper disposal can lead to soil and water contamination, affecting ecosystems and potentially entering the food chain. Stringent regulations have been implemented in many countries to control HCl waste disposal, but compliance often requires significant investment in treatment technologies and monitoring systems.
Neutralization, a common treatment method for HCl waste, presents its own set of challenges. While effective in reducing acidity, it generates large volumes of salt-laden wastewater, which requires further treatment or disposal. This secondary waste stream can be problematic, especially in areas with limited water resources or strict discharge regulations.
The recovery and recycling of HCl from waste streams is an attractive option but faces technical and economic hurdles. Current recovery processes often struggle with efficiency, especially when dealing with dilute or contaminated acid streams. The energy requirements and capital costs associated with advanced recovery technologies can be prohibitive for smaller operations.
Worker safety is another critical concern in HCl waste management. The handling, treatment, and disposal of HCl waste pose significant health risks due to potential exposure to acid fumes and splashes. This necessitates comprehensive safety protocols, specialized personal protective equipment, and ongoing training programs, all of which add to the operational complexity and cost.
Lastly, the variability in HCl waste composition across different industries complicates the development of universal treatment solutions. Contaminants such as heavy metals, organic compounds, or other chemicals often accompany HCl waste, requiring tailored treatment approaches. This diversity challenges researchers and engineers to develop flexible, adaptable waste management technologies that can handle a wide range of HCl waste streams effectively and economically.
Existing HCl Waste Treatment Methods
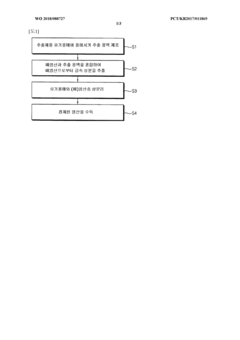
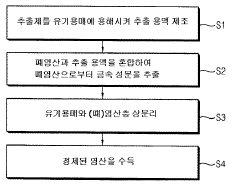
01 Production and purification of hydrochloric acid
Various methods and processes for producing and purifying hydrochloric acid are described. These include techniques for improving the efficiency of production, reducing impurities, and optimizing the concentration of the acid. The processes may involve specialized equipment or chemical reactions to achieve high-quality hydrochloric acid for industrial or laboratory use.- Production and purification of hydrochloric acid: Various methods and processes for producing and purifying hydrochloric acid, including industrial-scale production techniques and purification steps to obtain high-quality acid for different applications.
- Applications in chemical processing: Utilization of hydrochloric acid in various chemical processes, such as synthesis reactions, pH adjustment, and as a catalyst in industrial manufacturing of chemicals and materials.
- Waste treatment and recycling: Methods for treating and recycling hydrochloric acid waste, including recovery processes and techniques to minimize environmental impact in industrial settings.
- Safety and handling equipment: Specialized equipment and systems designed for the safe handling, storage, and transportation of hydrochloric acid, including protective gear and containment solutions.
- Analytical and measurement techniques: Methods and devices for analyzing and measuring hydrochloric acid concentration, purity, and other properties in various industrial and laboratory settings.
02 Applications of hydrochloric acid in chemical processes
Hydrochloric acid is widely used in various chemical processes and industrial applications. It serves as a key reagent in reactions, pH adjustment, metal treatment, and as a catalyst in organic synthesis. The acid's properties make it valuable in diverse fields such as pharmaceuticals, petrochemicals, and materials processing.Expand Specific Solutions03 Handling and storage of hydrochloric acid
Specialized equipment and methods for handling and storing hydrochloric acid are crucial due to its corrosive nature. This includes the design of storage tanks, transport containers, and safety systems to prevent leaks and protect workers. Proper materials selection and protective measures are essential for safe handling of the acid.Expand Specific Solutions04 Environmental and safety considerations
Addressing environmental concerns and safety issues related to hydrochloric acid use is important. This involves developing methods for neutralization, waste treatment, and emission control. Safety protocols, protective equipment, and emergency response procedures are also crucial aspects of working with hydrochloric acid.Expand Specific Solutions05 Novel applications and formulations
Innovative uses and formulations of hydrochloric acid are being explored in various fields. These include its application in advanced materials production, energy storage systems, and specialized cleaning agents. New methods for incorporating hydrochloric acid into products or processes aim to enhance efficiency or enable new functionalities.Expand Specific Solutions
Key Players in HCl Waste Industry
The waste treatment industry using hydrochloric acid is in a growth phase, driven by increasing environmental regulations and industrial demand. The market size is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, the field is moderately mature but still evolving, with companies like LG Chem, Lummus Technology, and China Petroleum & Chemical Corp leading innovations. These firms are developing advanced processes for acid recovery and waste neutralization, improving efficiency and reducing environmental impact. Emerging players like Inner Mongolia Zhanhua Technology and NovaTech Industries are also contributing to technological advancements, particularly in specialized applications and equipment design.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative hydrochloric acid recycling technology for waste treatment in petrochemical processes. Their approach involves a closed-loop system that recovers and purifies hydrochloric acid from various waste streams. The process utilizes advanced membrane separation and distillation techniques to achieve high purity levels of recovered acid[1]. This system can handle large volumes of acidic waste, typically processing up to 50,000 tons annually[3]. The recovered hydrochloric acid is then reused in various refining and chemical production processes, significantly reducing raw material costs and environmental impact[5].
Strengths: Efficient resource utilization, reduced environmental impact, and lower operational costs. Weaknesses: High initial investment and potential complexity in implementation across diverse production units.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering has pioneered a novel approach to waste treatment using hydrochloric acid, focusing on the recovery of valuable metals from electronic waste. Their process involves selective leaching of metals using hydrochloric acid under controlled conditions. The technology employs a multi-stage leaching process, where different concentrations of hydrochloric acid are used to selectively dissolve various metals[2]. This is followed by an advanced electrochemical recovery system that can extract up to 95% of the leached metals[4]. The process is particularly effective for recovering rare earth elements and precious metals from e-waste, with recovery rates reaching 98% for some elements[6].
Strengths: High recovery rates of valuable metals, applicability to various types of e-waste. Weaknesses: Potential for acid-induced corrosion of equipment, need for careful pH control throughout the process.
Innovative HCl Neutralization Techniques
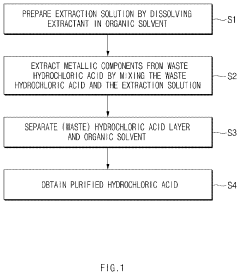
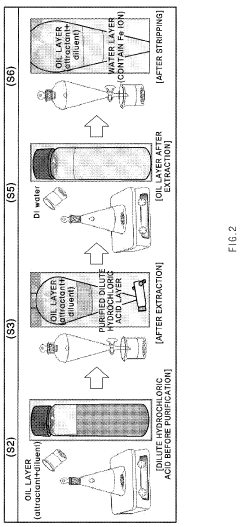
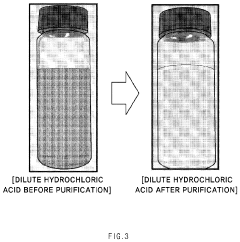
Method for purifying waste hydrochloric acid
PatentWO2018088727A1
Innovation
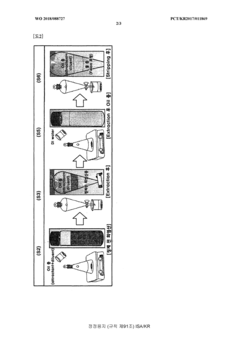
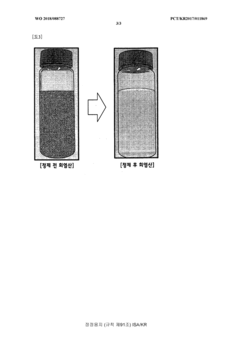
- A solvent extraction method using an organic solvent and an extractant like trioctylamine to selectively remove metal components, including iron ions, directly from waste hydrochloric acid, allowing for phase separation and regeneration of the solvent, thereby simplifying the process and reducing energy and cost consumption.
Method of purifying waste hydrochloric acid
PatentActiveUS20200270132A1
Innovation
- A solvent extraction method using an organic solvent and an extractant like trioctylamine to selectively remove metallic components, particularly iron ions, from waste hydrochloric acid, simplifying the process and reducing energy and manufacturing costs by eliminating the need for pre-treatment steps like free residual chlorine removal.
Environmental Regulations on HCl Disposal
The environmental regulations governing the disposal of hydrochloric acid (HCl) have become increasingly stringent in recent years, reflecting growing concerns about its potential environmental and health impacts. These regulations vary across different jurisdictions but generally aim to minimize the release of HCl into the environment and ensure safe handling and disposal practices.
In the United States, the Environmental Protection Agency (EPA) classifies HCl as a hazardous waste under the Resource Conservation and Recovery Act (RCRA) when it is discarded. This classification imposes strict requirements on its storage, transportation, and disposal. Facilities generating HCl waste must obtain EPA identification numbers and comply with specific waste management protocols.
The Clean Air Act also regulates HCl emissions from industrial processes. Major sources of HCl emissions are required to implement Maximum Achievable Control Technology (MACT) standards to reduce their environmental impact. These standards often necessitate the use of advanced scrubbing technologies or process modifications to capture and neutralize HCl before release.
In the European Union, the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation governs the use and disposal of HCl. Companies must register HCl with the European Chemicals Agency and provide detailed safety information. The Industrial Emissions Directive further regulates emissions from industrial facilities, including those handling HCl.
Many countries have implemented specific concentration limits for HCl in wastewater discharges. For instance, in China, the maximum allowable concentration of HCl in industrial wastewater is typically set at 2 mg/L. Facilities exceeding these limits must treat their wastewater to reduce HCl concentrations before discharge.
The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal provides an international framework for managing hazardous wastes, including HCl. Signatory countries are required to ensure environmentally sound management of HCl waste and restrict its cross-border movement without proper authorization.
To comply with these regulations, industries have developed various treatment technologies. Neutralization with alkaline substances like lime or sodium hydroxide is a common method for treating HCl waste. More advanced techniques include membrane separation, ion exchange, and electrodialysis, which can recover and recycle HCl from waste streams.
The regulatory landscape continues to evolve, with a trend towards more stringent controls and increased emphasis on circular economy principles. This is driving innovation in HCl waste treatment, encouraging the development of more efficient and environmentally friendly disposal methods. As breakthroughs in waste treatment using HCl emerge, they will need to align with and potentially shape future environmental regulations.
In the United States, the Environmental Protection Agency (EPA) classifies HCl as a hazardous waste under the Resource Conservation and Recovery Act (RCRA) when it is discarded. This classification imposes strict requirements on its storage, transportation, and disposal. Facilities generating HCl waste must obtain EPA identification numbers and comply with specific waste management protocols.
The Clean Air Act also regulates HCl emissions from industrial processes. Major sources of HCl emissions are required to implement Maximum Achievable Control Technology (MACT) standards to reduce their environmental impact. These standards often necessitate the use of advanced scrubbing technologies or process modifications to capture and neutralize HCl before release.
In the European Union, the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation governs the use and disposal of HCl. Companies must register HCl with the European Chemicals Agency and provide detailed safety information. The Industrial Emissions Directive further regulates emissions from industrial facilities, including those handling HCl.
Many countries have implemented specific concentration limits for HCl in wastewater discharges. For instance, in China, the maximum allowable concentration of HCl in industrial wastewater is typically set at 2 mg/L. Facilities exceeding these limits must treat their wastewater to reduce HCl concentrations before discharge.
The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal provides an international framework for managing hazardous wastes, including HCl. Signatory countries are required to ensure environmentally sound management of HCl waste and restrict its cross-border movement without proper authorization.
To comply with these regulations, industries have developed various treatment technologies. Neutralization with alkaline substances like lime or sodium hydroxide is a common method for treating HCl waste. More advanced techniques include membrane separation, ion exchange, and electrodialysis, which can recover and recycle HCl from waste streams.
The regulatory landscape continues to evolve, with a trend towards more stringent controls and increased emphasis on circular economy principles. This is driving innovation in HCl waste treatment, encouraging the development of more efficient and environmentally friendly disposal methods. As breakthroughs in waste treatment using HCl emerge, they will need to align with and potentially shape future environmental regulations.
Economic Feasibility of HCl Recovery
The economic feasibility of hydrochloric acid (HCl) recovery in waste treatment processes is a critical factor in determining the viability of implementing such technologies on a large scale. The recovery of HCl from waste streams not only reduces environmental impact but also offers potential cost savings and resource efficiency for industries.
One of the primary economic drivers for HCl recovery is the reduction in raw material costs. By reclaiming HCl from waste streams, industries can significantly decrease their reliance on purchasing fresh acid, leading to substantial savings over time. This is particularly relevant in sectors such as chemical manufacturing, metal processing, and semiconductor production, where large volumes of HCl are consumed regularly.
The implementation of HCl recovery systems often requires a significant initial capital investment. However, the long-term economic benefits can outweigh these upfront costs. The payback period for such investments typically ranges from 2 to 5 years, depending on the scale of operations and the efficiency of the recovery process. Factors influencing the return on investment include the purity of recovered HCl, energy costs associated with the recovery process, and the market price of virgin HCl.
Energy consumption is a crucial consideration in assessing the economic feasibility of HCl recovery. Advanced recovery technologies, such as membrane-based systems or vacuum distillation, have shown promising results in terms of energy efficiency. These methods can significantly reduce the operational costs associated with HCl recovery, making the process more economically attractive.
The market demand for recovered HCl plays a vital role in determining its economic viability. As industries increasingly focus on sustainability and circular economy principles, the value of recovered HCl has risen. This trend is expected to continue, potentially improving the economic case for recovery systems in the future.
Regulatory factors also influence the economic feasibility of HCl recovery. Stricter environmental regulations and waste disposal costs can make recovery more attractive from an economic standpoint. In some regions, government incentives or tax benefits for implementing waste recovery technologies further enhance the economic viability of HCl recovery systems.
The scale of operations is another critical factor in economic feasibility. Larger facilities with higher HCl consumption and waste generation tend to benefit more from recovery systems due to economies of scale. For smaller operations, the economic case may be less clear-cut, necessitating careful analysis of costs and benefits.
In conclusion, the economic feasibility of HCl recovery in waste treatment is generally positive, especially for large-scale operations in industries with high HCl consumption. While initial investments can be substantial, the long-term benefits in terms of cost savings, resource efficiency, and regulatory compliance make HCl recovery an increasingly attractive option for many industries.
One of the primary economic drivers for HCl recovery is the reduction in raw material costs. By reclaiming HCl from waste streams, industries can significantly decrease their reliance on purchasing fresh acid, leading to substantial savings over time. This is particularly relevant in sectors such as chemical manufacturing, metal processing, and semiconductor production, where large volumes of HCl are consumed regularly.
The implementation of HCl recovery systems often requires a significant initial capital investment. However, the long-term economic benefits can outweigh these upfront costs. The payback period for such investments typically ranges from 2 to 5 years, depending on the scale of operations and the efficiency of the recovery process. Factors influencing the return on investment include the purity of recovered HCl, energy costs associated with the recovery process, and the market price of virgin HCl.
Energy consumption is a crucial consideration in assessing the economic feasibility of HCl recovery. Advanced recovery technologies, such as membrane-based systems or vacuum distillation, have shown promising results in terms of energy efficiency. These methods can significantly reduce the operational costs associated with HCl recovery, making the process more economically attractive.
The market demand for recovered HCl plays a vital role in determining its economic viability. As industries increasingly focus on sustainability and circular economy principles, the value of recovered HCl has risen. This trend is expected to continue, potentially improving the economic case for recovery systems in the future.
Regulatory factors also influence the economic feasibility of HCl recovery. Stricter environmental regulations and waste disposal costs can make recovery more attractive from an economic standpoint. In some regions, government incentives or tax benefits for implementing waste recovery technologies further enhance the economic viability of HCl recovery systems.
The scale of operations is another critical factor in economic feasibility. Larger facilities with higher HCl consumption and waste generation tend to benefit more from recovery systems due to economies of scale. For smaller operations, the economic case may be less clear-cut, necessitating careful analysis of costs and benefits.
In conclusion, the economic feasibility of HCl recovery in waste treatment is generally positive, especially for large-scale operations in industries with high HCl consumption. While initial investments can be substantial, the long-term benefits in terms of cost savings, resource efficiency, and regulatory compliance make HCl recovery an increasingly attractive option for many industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!