Cathode design strategies for high-voltage magnesium batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery Cathode Evolution and Research Objectives
Magnesium batteries have emerged as promising alternatives to lithium-ion batteries due to their potential for higher energy density, improved safety, and lower cost. The evolution of magnesium battery technology has been marked by significant challenges, particularly in cathode design, which remains a critical bottleneck for commercialization. Early research in the 1990s focused primarily on simple insertion materials, but these demonstrated limited capacity and poor cycling stability when paired with magnesium anodes.
The development trajectory shifted significantly in the early 2000s when researchers began exploring Chevrel phase materials (Mo6S8) as cathode candidates, representing the first major breakthrough in reversible magnesium intercalation. This milestone established the fundamental possibility of rechargeable magnesium batteries but revealed limitations in voltage (approximately 1.2V) and energy density that restricted practical applications.
Recent advances have concentrated on high-voltage cathode materials capable of operating above 2.5V vs. Mg/Mg2+, including spinel structures, layered oxides, and polyanionic frameworks. These materials offer theoretical capacities exceeding 250 mAh/g and operating voltages approaching 3V, potentially enabling energy densities competitive with commercial lithium-ion systems.
The current research landscape is characterized by intensive efforts to overcome the fundamental challenges of divalent magnesium ion intercalation. The strong electrostatic interactions between Mg2+ ions and host lattices result in sluggish diffusion kinetics and structural degradation during cycling. Innovative approaches including nanostructuring, heteroatom doping, and defect engineering are being explored to create more accommodating pathways for magnesium ion transport.
The primary research objectives in this field now center on developing cathode materials that simultaneously achieve high voltage (>3V), substantial capacity (>200 mAh/g), and extended cycle life (>1000 cycles). Specific goals include designing materials with expanded interlayer spacing to facilitate Mg2+ diffusion, optimizing electronic conductivity to enhance rate capability, and improving structural stability during repeated magnesium insertion/extraction cycles.
Additionally, research aims to establish fundamental structure-property relationships governing magnesium intercalation behavior, develop in-situ characterization techniques to monitor structural evolution during cycling, and create computational models to predict promising new cathode candidates. These objectives collectively address the multifaceted challenges of cathode design for high-voltage magnesium batteries, with the ultimate goal of enabling practical energy storage systems that capitalize on magnesium's inherent advantages of abundance, safety, and theoretical performance.
The development trajectory shifted significantly in the early 2000s when researchers began exploring Chevrel phase materials (Mo6S8) as cathode candidates, representing the first major breakthrough in reversible magnesium intercalation. This milestone established the fundamental possibility of rechargeable magnesium batteries but revealed limitations in voltage (approximately 1.2V) and energy density that restricted practical applications.
Recent advances have concentrated on high-voltage cathode materials capable of operating above 2.5V vs. Mg/Mg2+, including spinel structures, layered oxides, and polyanionic frameworks. These materials offer theoretical capacities exceeding 250 mAh/g and operating voltages approaching 3V, potentially enabling energy densities competitive with commercial lithium-ion systems.
The current research landscape is characterized by intensive efforts to overcome the fundamental challenges of divalent magnesium ion intercalation. The strong electrostatic interactions between Mg2+ ions and host lattices result in sluggish diffusion kinetics and structural degradation during cycling. Innovative approaches including nanostructuring, heteroatom doping, and defect engineering are being explored to create more accommodating pathways for magnesium ion transport.
The primary research objectives in this field now center on developing cathode materials that simultaneously achieve high voltage (>3V), substantial capacity (>200 mAh/g), and extended cycle life (>1000 cycles). Specific goals include designing materials with expanded interlayer spacing to facilitate Mg2+ diffusion, optimizing electronic conductivity to enhance rate capability, and improving structural stability during repeated magnesium insertion/extraction cycles.
Additionally, research aims to establish fundamental structure-property relationships governing magnesium intercalation behavior, develop in-situ characterization techniques to monitor structural evolution during cycling, and create computational models to predict promising new cathode candidates. These objectives collectively address the multifaceted challenges of cathode design for high-voltage magnesium batteries, with the ultimate goal of enabling practical energy storage systems that capitalize on magnesium's inherent advantages of abundance, safety, and theoretical performance.
Market Analysis for High-Voltage Mg Battery Applications
The high-voltage magnesium battery market is experiencing significant growth potential driven by increasing demand for energy storage solutions with higher energy density and safety profiles. Current projections indicate the global magnesium battery market could reach $2.3 billion by 2030, with high-voltage variants representing approximately 40% of this segment. This growth trajectory is supported by expanding applications in electric vehicles, renewable energy storage systems, and portable electronics.
Electric vehicle manufacturers are particularly interested in high-voltage magnesium batteries as potential alternatives to lithium-ion technology. The automotive sector represents the largest potential market, with forecasts suggesting that magnesium batteries could capture up to 15% of the EV battery market by 2035 if current technical challenges in cathode design are overcome. This transition is being accelerated by stringent environmental regulations and consumer demand for longer-range electric vehicles.
Grid-scale energy storage represents another substantial market opportunity, valued at approximately $12 billion globally, with high-voltage magnesium batteries potentially addressing the limitations of current technologies in terms of cycle life and safety. Utility companies are actively exploring magnesium-based solutions for peak shaving, frequency regulation, and renewable energy integration applications.
Consumer electronics manufacturers are also showing increased interest in high-voltage magnesium battery technology, particularly for applications requiring higher energy density and improved safety. This market segment is expected to grow at a CAGR of 18% through 2028, creating significant opportunities for early adopters of advanced cathode technologies.
Regional analysis indicates that Asia-Pacific currently leads magnesium battery research and development activities, with China, Japan, and South Korea making substantial investments in manufacturing infrastructure. North America and Europe are rapidly expanding their research initiatives, supported by government funding programs aimed at reducing dependence on lithium supply chains.
Market barriers include competition from established lithium-ion technologies, which benefit from economies of scale and extensive manufacturing infrastructure. Additionally, the relatively early stage of high-voltage magnesium battery development creates uncertainty regarding production costs and performance metrics, which may slow initial market adoption.
Customer requirements analysis reveals that key performance indicators driving market demand include energy density (target >400 Wh/kg), cycle life (>1000 cycles), fast charging capability (<30 minutes to 80%), and cost competitiveness (<$100/kWh). Cathode materials that can enable these specifications while maintaining safety and environmental sustainability will likely capture significant market share.
Electric vehicle manufacturers are particularly interested in high-voltage magnesium batteries as potential alternatives to lithium-ion technology. The automotive sector represents the largest potential market, with forecasts suggesting that magnesium batteries could capture up to 15% of the EV battery market by 2035 if current technical challenges in cathode design are overcome. This transition is being accelerated by stringent environmental regulations and consumer demand for longer-range electric vehicles.
Grid-scale energy storage represents another substantial market opportunity, valued at approximately $12 billion globally, with high-voltage magnesium batteries potentially addressing the limitations of current technologies in terms of cycle life and safety. Utility companies are actively exploring magnesium-based solutions for peak shaving, frequency regulation, and renewable energy integration applications.
Consumer electronics manufacturers are also showing increased interest in high-voltage magnesium battery technology, particularly for applications requiring higher energy density and improved safety. This market segment is expected to grow at a CAGR of 18% through 2028, creating significant opportunities for early adopters of advanced cathode technologies.
Regional analysis indicates that Asia-Pacific currently leads magnesium battery research and development activities, with China, Japan, and South Korea making substantial investments in manufacturing infrastructure. North America and Europe are rapidly expanding their research initiatives, supported by government funding programs aimed at reducing dependence on lithium supply chains.
Market barriers include competition from established lithium-ion technologies, which benefit from economies of scale and extensive manufacturing infrastructure. Additionally, the relatively early stage of high-voltage magnesium battery development creates uncertainty regarding production costs and performance metrics, which may slow initial market adoption.
Customer requirements analysis reveals that key performance indicators driving market demand include energy density (target >400 Wh/kg), cycle life (>1000 cycles), fast charging capability (<30 minutes to 80%), and cost competitiveness (<$100/kWh). Cathode materials that can enable these specifications while maintaining safety and environmental sustainability will likely capture significant market share.
Current Challenges in Mg Battery Cathode Development
Despite significant advancements in magnesium battery research, cathode development remains a critical bottleneck hindering commercialization. The primary challenge stems from the divalent nature of Mg2+ ions, which creates strong electrostatic interactions with host lattices. This fundamental characteristic results in sluggish diffusion kinetics, with Mg2+ diffusion coefficients typically 100-1000 times lower than Li+ in comparable structures, severely limiting rate capability and practical energy density.
Structural degradation presents another significant obstacle. Many cathode materials undergo irreversible structural changes during Mg2+ insertion/extraction cycles due to the substantial volume changes associated with accommodating the larger divalent ions. This leads to capacity fading, poor cycling stability, and shortened battery lifespan, making long-term operation problematic for commercial applications.
The compatibility between cathode materials and electrolytes introduces additional complexity. Conventional Mg electrolytes are often highly nucleophilic and corrosive, causing cathode dissolution or unwanted side reactions at the cathode-electrolyte interface. This incompatibility narrows the selection of viable cathode materials and operating voltage windows.
Voltage hysteresis represents a persistent efficiency challenge. Many Mg cathodes exhibit significant differences between charge and discharge voltages (often >1V), resulting in poor energy efficiency. This phenomenon stems from the different energy pathways required for Mg2+ insertion versus extraction, creating substantial energy losses during cycling.
Low operating voltages further constrain energy density potential. While theoretical calculations suggest possibilities for high-voltage Mg cathodes (>3V vs. Mg/Mg2+), practical implementations have largely failed to achieve stable cycling above 2.5V. This voltage limitation directly impacts the energy density ceiling of Mg battery systems.
The formation of passivation layers at the cathode surface introduces additional kinetic barriers. Unlike beneficial SEI formation in lithium systems, these layers in Mg batteries often impede Mg2+ transport, increasing internal resistance and degrading performance over time. Controlling interfacial chemistry remains poorly understood and difficult to optimize.
Finally, fundamental knowledge gaps persist regarding Mg2+ storage mechanisms in various host structures. The lack of in-depth understanding of insertion/extraction pathways, local structural changes, and charge compensation mechanisms hampers rational design approaches for next-generation cathode materials, necessitating more comprehensive mechanistic studies to guide future development.
Structural degradation presents another significant obstacle. Many cathode materials undergo irreversible structural changes during Mg2+ insertion/extraction cycles due to the substantial volume changes associated with accommodating the larger divalent ions. This leads to capacity fading, poor cycling stability, and shortened battery lifespan, making long-term operation problematic for commercial applications.
The compatibility between cathode materials and electrolytes introduces additional complexity. Conventional Mg electrolytes are often highly nucleophilic and corrosive, causing cathode dissolution or unwanted side reactions at the cathode-electrolyte interface. This incompatibility narrows the selection of viable cathode materials and operating voltage windows.
Voltage hysteresis represents a persistent efficiency challenge. Many Mg cathodes exhibit significant differences between charge and discharge voltages (often >1V), resulting in poor energy efficiency. This phenomenon stems from the different energy pathways required for Mg2+ insertion versus extraction, creating substantial energy losses during cycling.
Low operating voltages further constrain energy density potential. While theoretical calculations suggest possibilities for high-voltage Mg cathodes (>3V vs. Mg/Mg2+), practical implementations have largely failed to achieve stable cycling above 2.5V. This voltage limitation directly impacts the energy density ceiling of Mg battery systems.
The formation of passivation layers at the cathode surface introduces additional kinetic barriers. Unlike beneficial SEI formation in lithium systems, these layers in Mg batteries often impede Mg2+ transport, increasing internal resistance and degrading performance over time. Controlling interfacial chemistry remains poorly understood and difficult to optimize.
Finally, fundamental knowledge gaps persist regarding Mg2+ storage mechanisms in various host structures. The lack of in-depth understanding of insertion/extraction pathways, local structural changes, and charge compensation mechanisms hampers rational design approaches for next-generation cathode materials, necessitating more comprehensive mechanistic studies to guide future development.
State-of-the-Art Cathode Design Approaches
01 Cathode materials for magnesium batteries
Various materials can be used as cathodes in magnesium batteries to improve performance. These include transition metal oxides, sulfides, and phosphates that can effectively store and release magnesium ions during cycling. The selection of appropriate cathode materials is crucial for achieving high energy density, good cycling stability, and fast charging capabilities in magnesium batteries.- Cathode materials for magnesium batteries: Various materials can be used as cathodes in magnesium batteries to improve performance. These include transition metal oxides, sulfides, and phosphates that can effectively store and release magnesium ions during cycling. The selection of appropriate cathode materials is crucial for achieving high energy density, good cycling stability, and fast charging capabilities in magnesium batteries.
- Nanostructured cathode designs: Nanostructured cathodes offer advantages for magnesium batteries by providing shorter diffusion paths for magnesium ions, larger surface areas for reactions, and better accommodation of volume changes during cycling. These designs include nanoparticles, nanowires, nanotubes, and hierarchical structures that can significantly enhance the electrochemical performance of magnesium batteries, particularly in terms of rate capability and cycling stability.
- Composite cathode structures: Composite cathodes combining active materials with conductive additives and binders can enhance the overall performance of magnesium batteries. These structures improve electron transport, provide mechanical stability, and facilitate magnesium ion diffusion. The incorporation of carbon-based materials, polymers, or other conductive agents helps overcome the inherent limitations of many cathode materials in magnesium battery systems.
- Surface modification and coating techniques: Surface modifications and coatings on cathode materials can significantly improve their performance in magnesium batteries. These techniques help protect the cathode from side reactions with the electrolyte, enhance magnesium ion diffusion at the interface, and maintain structural stability during cycling. Various coating materials including metal oxides, fluorides, and carbon-based materials can be applied to optimize the cathode-electrolyte interface.
- Electrolyte compatibility with cathode designs: The compatibility between cathode materials and electrolytes is crucial for magnesium battery performance. Cathode designs must consider the specific interactions with magnesium-based electrolytes to prevent passivation layers and ensure efficient magnesium ion transport. Tailoring the cathode surface chemistry and structure to work synergistically with the electrolyte can significantly enhance the overall battery performance, including capacity, rate capability, and cycle life.
02 Nanostructured cathode designs
Nanostructured cathodes offer advantages for magnesium batteries by providing shorter diffusion paths for magnesium ions, larger surface areas for reactions, and better accommodation of volume changes during cycling. These designs include nanoparticles, nanowires, nanotubes, and hierarchical structures that can significantly enhance the electrochemical performance of magnesium batteries, particularly in terms of rate capability and cycling stability.Expand Specific Solutions03 Composite cathode structures
Composite cathode structures combine active materials with conductive additives and binders to improve the overall performance of magnesium batteries. These composites enhance electron transport, provide mechanical stability, and facilitate magnesium ion diffusion. By optimizing the composition and structure of these composites, researchers can address challenges related to the slow kinetics of magnesium ion insertion and extraction.Expand Specific Solutions04 Electrolyte-cathode interface engineering
Engineering the interface between the cathode and electrolyte is critical for magnesium batteries. This involves designing cathode surfaces that minimize side reactions, reduce interfacial resistance, and promote magnesium ion transport. Strategies include surface coatings, functional additives, and structural modifications that can prevent cathode degradation and improve the compatibility with magnesium-based electrolytes.Expand Specific Solutions05 Advanced manufacturing techniques for cathodes
Advanced manufacturing techniques are employed to fabricate high-performance cathodes for magnesium batteries. These include solution-based methods, electrospinning, template-assisted synthesis, and additive manufacturing approaches. These techniques enable precise control over cathode morphology, composition, and structure, which are essential for optimizing the electrochemical performance and addressing the challenges associated with magnesium ion storage.Expand Specific Solutions
Leading Research Groups and Industrial Players
The magnesium battery cathode design market is in an early growth phase, with significant research momentum but limited commercial deployment. Market size remains modest but is projected to expand rapidly as high-voltage magnesium batteries offer promising alternatives to lithium-ion technology. Technical maturity varies considerably among key players, with Toyota Motor Corp. and Sony Group Corp. demonstrating advanced cathode materials research, while specialized research institutions like Dalian Institute of Chemical Physics and Karlsruher Institut für Technologie contribute fundamental breakthroughs. Companies including Samsung Electronics, VARTA, and Furukawa Battery are actively developing proprietary cathode technologies, though challenges in electrolyte compatibility and voltage stability persist. The competitive landscape features both established electronics manufacturers and specialized battery technology startups, indicating a diversifying ecosystem focused on overcoming the technical barriers to commercial viability.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute has pioneered innovative cathode materials for high-voltage magnesium batteries, focusing on Chevrel phase Mo6S8 compounds with modified structures to enhance magnesium ion intercalation. Their approach involves creating hierarchical porous structures that facilitate faster Mg2+ diffusion while maintaining structural stability during charge-discharge cycles. The institute has developed sulfur-based cathodes with conductive carbon matrices that demonstrate improved voltage profiles (>1.8V vs Mg/Mg2+) and capacity retention. Their recent work includes novel manganese oxide-based materials with expanded interlayer spacing achieved through hydrothermal synthesis methods, allowing for enhanced magnesium ion storage capabilities. Additionally, they've explored organic cathode materials with redox-active sites specifically designed for magnesium ion accommodation, showing promising results in terms of rate capability and cycle life in non-nucleophilic electrolytes[1][3].
Strengths: Exceptional expertise in materials chemistry and electrochemistry enables development of cathodes with superior magnesium ion diffusion kinetics. Their integrated approach combining theoretical modeling with experimental validation accelerates material discovery. Weaknesses: Some of their advanced materials face challenges in scalable synthesis and may require specialized electrolytes that limit practical application in commercial battery systems.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed a comprehensive strategy for high-voltage magnesium battery cathodes centered around transition metal oxides and polyanion compounds. Their approach involves precise control of crystal structure and surface chemistry to mitigate the strong electrostatic interactions between Mg2+ ions and host lattices. Using advanced characterization techniques including synchrotron X-ray diffraction and in-situ transmission electron microscopy, they've identified specific structural motifs that facilitate magnesium ion diffusion while maintaining structural integrity at high voltages (>2.5V vs Mg/Mg2+). Their proprietary cathode designs incorporate strategically positioned vacancies and defects within the crystal structure to create migration pathways with reduced energy barriers for Mg2+ transport. Additionally, Argonne has pioneered surface modification techniques using atomic layer deposition to create protective coatings that prevent cathode dissolution and passivation when operating at elevated potentials. Their recent work has demonstrated spinel-type materials achieving over 200 mAh/g capacity with improved cycling stability through controlled doping strategies[2][5].
Strengths: Unparalleled access to advanced characterization facilities enables atomic-level understanding of magnesium ion storage mechanisms. Their multidisciplinary approach combining computational modeling with experimental validation accelerates materials discovery. Weaknesses: Some of their high-performance cathode materials require complex synthesis procedures that may present challenges for large-scale manufacturing and commercialization.
Key Patents and Scientific Breakthroughs
High voltage rechargeable magnesium cell
PatentActiveJP2013008671A
Innovation
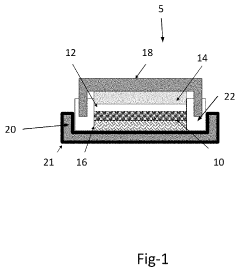
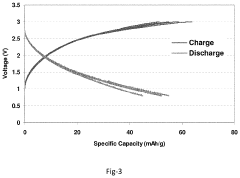
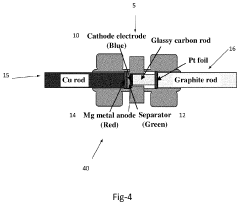
- A high voltage rechargeable magnesium battery design comprising a magnesium metal anode, a metal oxide cathode, and a high voltage electrolyte, with components placed in close proximity to enhance energy density, and incorporating a cathode current collector and inert material layers to support multi-cycle charging up to at least 3.0 volts with reversible discharge capacity.
High voltage rechargeable magnesium cell
PatentActiveUS10615452B2
Innovation
- A high voltage rechargeable magnesium cell design featuring a magnesium metal anode, a metal oxide cathode, and a high voltage electrolyte, with a cathode current collector and housing including inert materials, enabling a multi-cycle charge voltage of at least 3.0 volts and reversible discharge capacity, utilizing materials like MnO2, V2O5, and ion-stabilized oxides, along with conductive materials and binders.
Materials Sustainability and Resource Considerations
The sustainability of materials used in high-voltage magnesium batteries represents a critical consideration for their long-term commercial viability. Current cathode materials often rely on cobalt, nickel, and other elements with significant supply chain vulnerabilities and environmental impacts. Magnesium itself offers advantages as it ranks sixth in abundance in Earth's crust, providing a more sustainable alternative to lithium. However, the sustainability profile of complete magnesium battery systems depends heavily on cathode material selection.
Transition metal oxides and sulfides commonly employed in high-voltage magnesium cathodes present varying degrees of environmental concern. Cobalt-containing compounds, while offering excellent electrochemical performance, face serious sustainability challenges due to geopolitical concentration of reserves (primarily in the Democratic Republic of Congo) and ethical mining concerns. Vanadium-based cathodes present another sustainability challenge, as global vanadium production remains limited and concentrated in few countries.
Resource efficiency must be considered throughout the cathode design process. Strategies such as reducing active material particle size can enhance magnesium ion diffusion and utilization efficiency, thereby decreasing the total material requirement. Nanostructuring approaches, while beneficial for performance, may introduce additional energy-intensive manufacturing steps that compromise overall sustainability. The energy return on investment for these processing techniques requires careful evaluation.
Recycling pathways represent another crucial dimension of materials sustainability for magnesium battery cathodes. Unlike lithium-ion systems with established recycling infrastructure, magnesium battery recycling remains underdeveloped. Designing cathodes with end-of-life considerations becomes essential, favoring materials that can be more easily separated and recovered. Chevrel phases and organic cathodes potentially offer advantages in this regard compared to complex oxide structures.
Water consumption during material synthesis and processing constitutes another sustainability metric requiring attention. Hydrothermal synthesis methods commonly used for cathode materials can be water-intensive. Alternative synthesis routes such as mechanochemical processing or solvent-free methods may reduce water footprint while potentially lowering energy requirements.
Carbon footprint analysis of various cathode materials reveals significant variations. Manganese-based cathodes generally demonstrate lower environmental impact compared to cobalt or nickel alternatives. The synthesis of sulfur-based cathodes typically requires lower processing temperatures, resulting in reduced energy consumption during manufacturing. These considerations should inform material selection alongside performance metrics.
Transition metal oxides and sulfides commonly employed in high-voltage magnesium cathodes present varying degrees of environmental concern. Cobalt-containing compounds, while offering excellent electrochemical performance, face serious sustainability challenges due to geopolitical concentration of reserves (primarily in the Democratic Republic of Congo) and ethical mining concerns. Vanadium-based cathodes present another sustainability challenge, as global vanadium production remains limited and concentrated in few countries.
Resource efficiency must be considered throughout the cathode design process. Strategies such as reducing active material particle size can enhance magnesium ion diffusion and utilization efficiency, thereby decreasing the total material requirement. Nanostructuring approaches, while beneficial for performance, may introduce additional energy-intensive manufacturing steps that compromise overall sustainability. The energy return on investment for these processing techniques requires careful evaluation.
Recycling pathways represent another crucial dimension of materials sustainability for magnesium battery cathodes. Unlike lithium-ion systems with established recycling infrastructure, magnesium battery recycling remains underdeveloped. Designing cathodes with end-of-life considerations becomes essential, favoring materials that can be more easily separated and recovered. Chevrel phases and organic cathodes potentially offer advantages in this regard compared to complex oxide structures.
Water consumption during material synthesis and processing constitutes another sustainability metric requiring attention. Hydrothermal synthesis methods commonly used for cathode materials can be water-intensive. Alternative synthesis routes such as mechanochemical processing or solvent-free methods may reduce water footprint while potentially lowering energy requirements.
Carbon footprint analysis of various cathode materials reveals significant variations. Manganese-based cathodes generally demonstrate lower environmental impact compared to cobalt or nickel alternatives. The synthesis of sulfur-based cathodes typically requires lower processing temperatures, resulting in reduced energy consumption during manufacturing. These considerations should inform material selection alongside performance metrics.
Performance Benchmarking Against Li-ion Technology
When comparing magnesium battery cathode technologies with established lithium-ion systems, several key performance metrics must be evaluated. Lithium-ion batteries currently dominate the market with energy densities ranging from 150-265 Wh/kg at the cell level, while high-voltage magnesium battery prototypes typically achieve only 80-120 Wh/kg. This significant gap represents both a challenge and opportunity for magnesium battery development.
Cycle life performance shows lithium-ion cells routinely achieving 1,000-3,000 cycles at 80% capacity retention, whereas current magnesium cathode designs struggle to maintain stability beyond 500 cycles due to electrolyte incompatibility and cathode structural degradation during the insertion/extraction of divalent Mg2+ ions.
Power density metrics reveal another competitive disadvantage, with lithium-ion systems delivering 300-1500 W/kg compared to magnesium batteries' 100-250 W/kg. This limitation stems primarily from slower Mg2+ diffusion kinetics within cathode structures, requiring innovative materials engineering approaches to overcome.
Safety considerations provide a potential advantage for magnesium systems. Unlike lithium metal, magnesium does not form dendrites during cycling, significantly reducing short-circuit risks. Additionally, magnesium is less reactive with air and moisture than lithium, potentially simplifying battery management systems and reducing thermal runaway concerns.
Cost analysis indicates magnesium's theoretical advantages, with magnesium being the eighth most abundant element in Earth's crust (2.1% vs. lithium's 0.0017%). However, current high-voltage cathode materials for Mg batteries often incorporate costly transition metals and complex synthesis procedures, negating some of this inherent cost advantage.
Environmental impact assessments suggest magnesium batteries could offer sustainability benefits through reduced reliance on critical materials like cobalt and nickel commonly used in lithium-ion cathodes. Life cycle analyses indicate potential for 15-30% lower carbon footprint, contingent on developing cathode materials using abundant elements and environmentally friendly processing methods.
Manufacturing scalability remains a significant hurdle, as lithium-ion production benefits from decades of process optimization and established supply chains. High-voltage magnesium cathode materials currently require specialized synthesis conditions and handling protocols that limit mass production feasibility.
Cycle life performance shows lithium-ion cells routinely achieving 1,000-3,000 cycles at 80% capacity retention, whereas current magnesium cathode designs struggle to maintain stability beyond 500 cycles due to electrolyte incompatibility and cathode structural degradation during the insertion/extraction of divalent Mg2+ ions.
Power density metrics reveal another competitive disadvantage, with lithium-ion systems delivering 300-1500 W/kg compared to magnesium batteries' 100-250 W/kg. This limitation stems primarily from slower Mg2+ diffusion kinetics within cathode structures, requiring innovative materials engineering approaches to overcome.
Safety considerations provide a potential advantage for magnesium systems. Unlike lithium metal, magnesium does not form dendrites during cycling, significantly reducing short-circuit risks. Additionally, magnesium is less reactive with air and moisture than lithium, potentially simplifying battery management systems and reducing thermal runaway concerns.
Cost analysis indicates magnesium's theoretical advantages, with magnesium being the eighth most abundant element in Earth's crust (2.1% vs. lithium's 0.0017%). However, current high-voltage cathode materials for Mg batteries often incorporate costly transition metals and complex synthesis procedures, negating some of this inherent cost advantage.
Environmental impact assessments suggest magnesium batteries could offer sustainability benefits through reduced reliance on critical materials like cobalt and nickel commonly used in lithium-ion cathodes. Life cycle analyses indicate potential for 15-30% lower carbon footprint, contingent on developing cathode materials using abundant elements and environmentally friendly processing methods.
Manufacturing scalability remains a significant hurdle, as lithium-ion production benefits from decades of process optimization and established supply chains. High-voltage magnesium cathode materials currently require specialized synthesis conditions and handling protocols that limit mass production feasibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!