Magnesium metal anode stability in nonaqueous electrolytes
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Anode Development Background and Objectives
Magnesium batteries have emerged as a promising alternative to lithium-ion technology due to magnesium's abundance, safety, and theoretical capacity. The development of magnesium metal anodes represents a critical frontier in energy storage research, with historical roots dating back to the early 1990s when the first rechargeable magnesium batteries were demonstrated by Gregory et al. Since then, research interest has grown substantially, particularly over the past decade, as limitations of lithium-based systems become increasingly apparent.
The evolution of magnesium anode technology has been characterized by several distinct phases. Initially, research focused on understanding the fundamental electrochemical behavior of magnesium in simple electrolyte systems. This was followed by efforts to overcome the primary challenge of magnesium electrochemistry: the formation of passivation layers that, unlike in lithium systems, block rather than facilitate ion transport.
Current technological trajectories are centered on developing electrolyte formulations that enable reversible magnesium deposition and stripping while maintaining compatibility with practical cathode materials. The field has witnessed significant breakthroughs in electrolyte design, including the development of non-nucleophilic electrolytes based on magnesium aluminum chloride complexes and more recent advances in borohydride-based systems.
The primary objective of magnesium anode development is to achieve stable cycling performance in practical nonaqueous electrolytes. This entails addressing several interconnected challenges: preventing dendritic growth during deposition, minimizing side reactions with electrolyte components, ensuring high Coulombic efficiency, and maintaining low interfacial resistance over extended cycling.
Secondary objectives include improving the rate capability of magnesium anodes, as the divalent nature of magnesium ions typically results in slower kinetics compared to monovalent lithium. Additionally, there is a growing focus on developing electrolyte systems that are compatible with both magnesium anodes and high-voltage cathode materials to maximize energy density.
The field is now moving toward more sophisticated understanding of interfacial phenomena at the magnesium-electrolyte interface. Advanced characterization techniques, including in-situ electron microscopy and spectroscopic methods, are revealing the complex nature of surface films on magnesium and their role in determining electrochemical performance.
Looking forward, the trajectory of magnesium anode development is increasingly aligned with practical battery requirements, moving beyond proof-of-concept demonstrations toward metrics relevant for commercial viability, including long-term stability, rate performance, and compatibility with scalable manufacturing processes.
The evolution of magnesium anode technology has been characterized by several distinct phases. Initially, research focused on understanding the fundamental electrochemical behavior of magnesium in simple electrolyte systems. This was followed by efforts to overcome the primary challenge of magnesium electrochemistry: the formation of passivation layers that, unlike in lithium systems, block rather than facilitate ion transport.
Current technological trajectories are centered on developing electrolyte formulations that enable reversible magnesium deposition and stripping while maintaining compatibility with practical cathode materials. The field has witnessed significant breakthroughs in electrolyte design, including the development of non-nucleophilic electrolytes based on magnesium aluminum chloride complexes and more recent advances in borohydride-based systems.
The primary objective of magnesium anode development is to achieve stable cycling performance in practical nonaqueous electrolytes. This entails addressing several interconnected challenges: preventing dendritic growth during deposition, minimizing side reactions with electrolyte components, ensuring high Coulombic efficiency, and maintaining low interfacial resistance over extended cycling.
Secondary objectives include improving the rate capability of magnesium anodes, as the divalent nature of magnesium ions typically results in slower kinetics compared to monovalent lithium. Additionally, there is a growing focus on developing electrolyte systems that are compatible with both magnesium anodes and high-voltage cathode materials to maximize energy density.
The field is now moving toward more sophisticated understanding of interfacial phenomena at the magnesium-electrolyte interface. Advanced characterization techniques, including in-situ electron microscopy and spectroscopic methods, are revealing the complex nature of surface films on magnesium and their role in determining electrochemical performance.
Looking forward, the trajectory of magnesium anode development is increasingly aligned with practical battery requirements, moving beyond proof-of-concept demonstrations toward metrics relevant for commercial viability, including long-term stability, rate performance, and compatibility with scalable manufacturing processes.
Market Analysis for Mg-Based Battery Systems
The global market for magnesium-based battery systems is experiencing significant growth potential as the energy storage industry seeks alternatives to lithium-ion technology. Current market valuations indicate that magnesium battery technology represents a small but rapidly expanding segment within the broader energy storage market, which was valued at approximately $145 billion in 2022 and is projected to reach $500 billion by 2035.
Magnesium batteries offer compelling market advantages that drive interest from both investors and manufacturers. The abundance of magnesium in the Earth's crust (2.1% compared to lithium's 0.002%) translates to substantially lower raw material costs and reduced supply chain vulnerabilities. This economic advantage positions magnesium-based systems favorably in price-sensitive market segments, particularly for grid-scale storage applications where cost per kilowatt-hour is a critical factor.
Market segmentation analysis reveals several key application areas with strong growth potential. Electric vehicles represent the most promising sector, with automotive manufacturers actively seeking battery technologies that offer higher energy density and improved safety profiles compared to current lithium-ion solutions. The stationary energy storage sector also presents significant opportunities, particularly for residential and commercial applications where safety concerns are paramount.
Regional market analysis indicates that Asia-Pacific currently leads in magnesium battery research and development investments, with China, Japan, and South Korea hosting the majority of patent filings. North America follows closely, with substantial research funding allocated through programs like the U.S. Department of Energy's ARPA-E initiatives specifically targeting magnesium-based energy storage solutions.
Market adoption barriers remain significant, primarily centered around the technical challenges of electrolyte compatibility with magnesium metal anodes. The formation of passivation layers on magnesium surfaces in conventional electrolytes has limited commercial viability. However, recent breakthroughs in nonaqueous electrolyte formulations have accelerated market interest, with several startups securing venture capital funding to commercialize these advances.
Consumer demand trends increasingly favor sustainable battery technologies with reduced environmental impact and improved safety profiles. Magnesium batteries align well with these market preferences, offering potentially lower carbon footprints than lithium-ion alternatives due to less energy-intensive mining and processing requirements. This sustainability advantage represents a significant market differentiator as regulatory frameworks increasingly prioritize environmental considerations.
Industry forecasts project that magnesium battery technology could capture up to 15% of the energy storage market by 2030, contingent upon successful resolution of current technical challenges related to electrolyte stability and anode performance.
Magnesium batteries offer compelling market advantages that drive interest from both investors and manufacturers. The abundance of magnesium in the Earth's crust (2.1% compared to lithium's 0.002%) translates to substantially lower raw material costs and reduced supply chain vulnerabilities. This economic advantage positions magnesium-based systems favorably in price-sensitive market segments, particularly for grid-scale storage applications where cost per kilowatt-hour is a critical factor.
Market segmentation analysis reveals several key application areas with strong growth potential. Electric vehicles represent the most promising sector, with automotive manufacturers actively seeking battery technologies that offer higher energy density and improved safety profiles compared to current lithium-ion solutions. The stationary energy storage sector also presents significant opportunities, particularly for residential and commercial applications where safety concerns are paramount.
Regional market analysis indicates that Asia-Pacific currently leads in magnesium battery research and development investments, with China, Japan, and South Korea hosting the majority of patent filings. North America follows closely, with substantial research funding allocated through programs like the U.S. Department of Energy's ARPA-E initiatives specifically targeting magnesium-based energy storage solutions.
Market adoption barriers remain significant, primarily centered around the technical challenges of electrolyte compatibility with magnesium metal anodes. The formation of passivation layers on magnesium surfaces in conventional electrolytes has limited commercial viability. However, recent breakthroughs in nonaqueous electrolyte formulations have accelerated market interest, with several startups securing venture capital funding to commercialize these advances.
Consumer demand trends increasingly favor sustainable battery technologies with reduced environmental impact and improved safety profiles. Magnesium batteries align well with these market preferences, offering potentially lower carbon footprints than lithium-ion alternatives due to less energy-intensive mining and processing requirements. This sustainability advantage represents a significant market differentiator as regulatory frameworks increasingly prioritize environmental considerations.
Industry forecasts project that magnesium battery technology could capture up to 15% of the energy storage market by 2030, contingent upon successful resolution of current technical challenges related to electrolyte stability and anode performance.
Current Challenges in Mg Metal Anode Stability
Despite significant advancements in magnesium battery technology, the stability of magnesium metal anodes in nonaqueous electrolytes remains a critical challenge. The primary obstacle stems from the formation of a passivation layer on the magnesium surface when in contact with conventional electrolytes. Unlike lithium batteries where the solid electrolyte interphase (SEI) facilitates ion transport, the passivation layer on magnesium anodes typically blocks ion diffusion, severely limiting electrochemical performance.
Electrolyte compatibility presents another major hurdle. Most conventional electrolytes containing ethereal solvents (such as THF and glymes) suffer from narrow electrochemical windows, limiting the overall cell voltage and energy density. Additionally, these electrolytes often demonstrate poor oxidative stability at the cathode interface, leading to parasitic reactions and capacity fade during cycling.
The challenge of dendrite formation, though less pronounced than in lithium systems, still poses reliability concerns for magnesium metal anodes. Under certain conditions, especially at high current densities, uneven magnesium deposition can occur, potentially leading to internal short circuits and safety hazards. This phenomenon is exacerbated by the inherent heterogeneity of the electrode surface and local current density variations.
Interfacial kinetics represent another significant barrier. The desolvation energy for magnesium ions is substantially higher than for lithium ions, resulting in slower charge transfer processes at the electrode-electrolyte interface. This kinetic limitation manifests as high overpotentials during both plating and stripping processes, reducing energy efficiency and practical capacity utilization.
Environmental sensitivity further complicates magnesium anode development. Many magnesium-compatible electrolytes are highly sensitive to moisture and oxygen, necessitating stringent handling conditions. Even trace amounts of contaminants can trigger side reactions that compromise the reversibility of magnesium deposition/dissolution processes.
The multivalent nature of magnesium ions (Mg²⁺) introduces additional complexities. The stronger coulombic interactions between divalent magnesium ions and the host lattice or solvent molecules result in slower diffusion kinetics compared to monovalent ions. This fundamentally limits the rate capability of magnesium-based systems, particularly at high current densities relevant for practical applications.
Lastly, the lack of standardized testing protocols and comprehensive understanding of degradation mechanisms hinders systematic improvement efforts. The complex interplay between the magnesium metal surface, electrolyte components, and operating conditions creates a multidimensional problem space that requires sophisticated analytical techniques and modeling approaches to fully characterize and address.
Electrolyte compatibility presents another major hurdle. Most conventional electrolytes containing ethereal solvents (such as THF and glymes) suffer from narrow electrochemical windows, limiting the overall cell voltage and energy density. Additionally, these electrolytes often demonstrate poor oxidative stability at the cathode interface, leading to parasitic reactions and capacity fade during cycling.
The challenge of dendrite formation, though less pronounced than in lithium systems, still poses reliability concerns for magnesium metal anodes. Under certain conditions, especially at high current densities, uneven magnesium deposition can occur, potentially leading to internal short circuits and safety hazards. This phenomenon is exacerbated by the inherent heterogeneity of the electrode surface and local current density variations.
Interfacial kinetics represent another significant barrier. The desolvation energy for magnesium ions is substantially higher than for lithium ions, resulting in slower charge transfer processes at the electrode-electrolyte interface. This kinetic limitation manifests as high overpotentials during both plating and stripping processes, reducing energy efficiency and practical capacity utilization.
Environmental sensitivity further complicates magnesium anode development. Many magnesium-compatible electrolytes are highly sensitive to moisture and oxygen, necessitating stringent handling conditions. Even trace amounts of contaminants can trigger side reactions that compromise the reversibility of magnesium deposition/dissolution processes.
The multivalent nature of magnesium ions (Mg²⁺) introduces additional complexities. The stronger coulombic interactions between divalent magnesium ions and the host lattice or solvent molecules result in slower diffusion kinetics compared to monovalent ions. This fundamentally limits the rate capability of magnesium-based systems, particularly at high current densities relevant for practical applications.
Lastly, the lack of standardized testing protocols and comprehensive understanding of degradation mechanisms hinders systematic improvement efforts. The complex interplay between the magnesium metal surface, electrolyte components, and operating conditions creates a multidimensional problem space that requires sophisticated analytical techniques and modeling approaches to fully characterize and address.
State-of-the-Art Solutions for Mg Anode Passivation
01 Surface coatings and protective layers for magnesium anodes
Various protective coatings and surface treatments can be applied to magnesium metal anodes to enhance their stability and prevent rapid corrosion. These coatings create a barrier that protects the reactive magnesium surface from degradation while still allowing ion transport. Examples include polymer films, inorganic protective layers, and composite coatings that significantly improve the cycling performance and longevity of magnesium anodes in battery applications.- Surface coatings and protective layers for magnesium anodes: Various coating techniques and protective layers can be applied to magnesium metal anodes to enhance their stability and prevent rapid corrosion. These coatings create a barrier that protects the reactive magnesium surface from aggressive electrolyte components while still allowing ion transport. Examples include polymer films, inorganic protective layers, and composite coatings that significantly improve the cycling performance and longevity of magnesium anodes in battery applications.
- Electrolyte modifications for magnesium anode stability: The composition of electrolytes plays a crucial role in stabilizing magnesium metal anodes. By carefully selecting electrolyte salts, solvents, and additives, the formation of passivation layers on magnesium surfaces can be controlled. Modified electrolytes can promote the formation of stable solid electrolyte interphase (SEI) layers that protect the magnesium anode while maintaining efficient ion transport, thereby enhancing overall battery performance and cycle life.
- Alloying strategies for improved magnesium anode performance: Alloying magnesium with other metals such as aluminum, zinc, or lithium can significantly improve the stability and electrochemical performance of magnesium anodes. These alloys often exhibit reduced corrosion rates, improved mechanical properties, and enhanced electrochemical activity compared to pure magnesium. The specific composition and microstructure of these alloys can be tailored to optimize performance in different battery systems and operating conditions.
- Nanostructured magnesium anodes for enhanced stability: Nanostructuring approaches can significantly improve the stability of magnesium anodes. By creating magnesium anodes with controlled nanoscale architectures, such as nanoparticles, nanowires, or porous structures, the electrochemical performance can be enhanced. These nanostructured designs provide larger surface areas, shorter ion diffusion paths, and better accommodation of volume changes during cycling, resulting in improved stability and cycling performance of magnesium-based battery systems.
- Interface engineering for magnesium anode protection: Interface engineering focuses on modifying the interface between the magnesium anode and the electrolyte to enhance stability. This approach includes the use of artificial SEI layers, buffer layers, and functional interlayers that can prevent direct contact between the reactive magnesium surface and corrosive electrolyte components. These engineered interfaces help to suppress side reactions, prevent dendrite formation, and maintain the structural integrity of the magnesium anode during repeated charge-discharge cycles.
02 Electrolyte formulations for magnesium anode stability
Specialized electrolyte compositions can dramatically improve the stability of magnesium metal anodes. These formulations typically include specific salts, solvents, and additives that form favorable solid electrolyte interphase (SEI) layers on the magnesium surface, preventing continuous corrosion while maintaining ionic conductivity. Non-aqueous electrolytes with carefully selected components help suppress dendrite formation and parasitic reactions that would otherwise lead to capacity fade and cell failure.Expand Specific Solutions03 Alloying and doping strategies for magnesium anodes
Alloying magnesium with other metals or incorporating dopants can significantly enhance its electrochemical stability as an anode material. These modifications alter the electronic structure and surface properties of magnesium, reducing its reactivity while maintaining desirable electrochemical performance. Common alloying elements include aluminum, zinc, and lithium, which can create more stable interfaces with electrolytes and improve cycling efficiency in battery applications.Expand Specific Solutions04 Nanostructured and composite magnesium anodes
Engineering magnesium anodes at the nanoscale or creating composite structures can dramatically improve their stability. Nanostructured magnesium provides controlled reactivity, better mechanical properties, and improved ion diffusion pathways. Composite anodes that combine magnesium with carbon materials, metal oxides, or other stabilizing components can mitigate volume changes during cycling and prevent unwanted side reactions, resulting in enhanced electrochemical performance and longer service life.Expand Specific Solutions05 Manufacturing and processing techniques for stable magnesium anodes
Specialized manufacturing and processing methods can significantly enhance the stability of magnesium metal anodes. These techniques include controlled oxidation processes, mechanical treatments, and precise fabrication methods that optimize surface properties and microstructure. Advanced processing can create magnesium anodes with reduced surface defects, controlled grain boundaries, and optimized morphology, all of which contribute to improved electrochemical stability and performance in battery applications.Expand Specific Solutions
Leading Research Groups and Companies in Mg Battery Technology
The magnesium metal anode stability in nonaqueous electrolytes market is in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market size remains relatively small but is expanding rapidly due to increasing demand for high-energy density batteries. Technologically, this field is still evolving, with major players demonstrating varying levels of maturity. Toyota, Panasonic, and GS Yuasa lead commercial development with established research programs, while academic institutions like Tsinghua University and Arizona State University contribute fundamental research. Research organizations such as Argonne National Laboratory and Battelle Memorial Institute bridge the gap between academic discovery and industrial application. The technology faces challenges in electrolyte stability and dendrite formation, but recent breakthroughs by companies like Pellion Technologies suggest promising advancement toward commercialization.
Toyota Motor Corp.
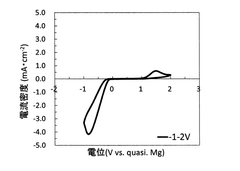
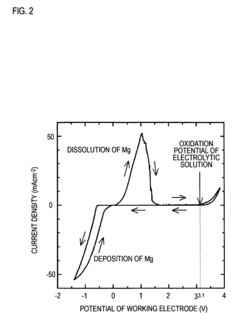
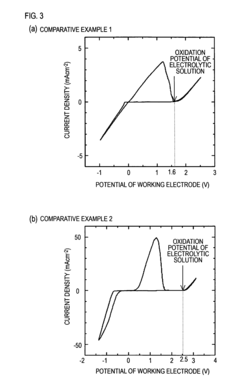
Technical Solution: Toyota has developed a proprietary non-corrosive electrolyte system for magnesium metal anodes based on magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) salt in ether-based solvents. Their approach incorporates specific additives that form a stable passivation layer on the magnesium surface, preventing continuous electrolyte decomposition while maintaining efficient magnesium ion transport. The company has demonstrated reversible magnesium plating/stripping with over 85% coulombic efficiency in their electrolyte system, significantly higher than conventional electrolytes that typically achieve less than 50% efficiency. Toyota's research also focuses on the molecular-level interactions between solvent molecules and magnesium ions to optimize coordination environments and reduce desolvation energy barriers at the electrode-electrolyte interface.
Strengths: Toyota's electrolyte formulation demonstrates superior cycling stability and reduced corrosion of current collectors compared to conventional systems. Their extensive testing infrastructure allows for rapid iteration and validation in realistic battery conditions. Weaknesses: The system still exhibits relatively high internal resistance and slower kinetics compared to lithium-based systems, limiting power density in practical applications.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed an innovative approach to magnesium metal anode stabilization using a non-nucleophilic electrolyte system based on magnesium bis(hexamethyldisilazide) [Mg(HMDS)2] combined with aluminum chloride in ethereal solvents. Their research has identified specific solvent combinations that minimize parasitic reactions while maintaining high ionic conductivity (>5 mS/cm at room temperature). Using advanced characterization techniques including in-situ X-ray absorption spectroscopy and cryogenic transmission electron microscopy, Argonne researchers have mapped the evolution of the electrode-electrolyte interphase during cycling, revealing that controlled formation of magnesium fluoride and magnesium oxide species can actually enhance long-term stability rather than causing detrimental passivation when properly engineered. Their electrolyte design incorporates fluorinated additives that promote the formation of this beneficial interphase layer, achieving reversible magnesium plating/stripping with coulombic efficiencies consistently above 98% for over 1000 cycles.
Strengths: Argonne's solution demonstrates exceptional electrochemical stability windows (>3.5V vs Mg/Mg2+), enabling compatibility with high-voltage cathode materials. Their fundamental understanding of interfacial phenomena provides clear pathways for further optimization. Weaknesses: The current formulation requires relatively high operating temperatures (>40°C) to achieve optimal performance, limiting practical applications in consumer electronics and certain automotive contexts.
Critical Patents and Literature on Mg-Electrolyte Interfaces
Nonaqueous electrolyte solution for magnesium secondary batteries and magnesium secondary battery using same
PatentWO2020115938A1
Innovation
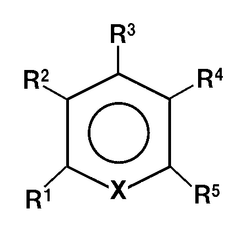
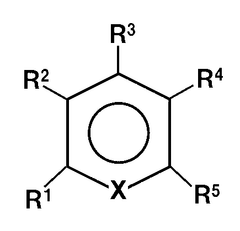
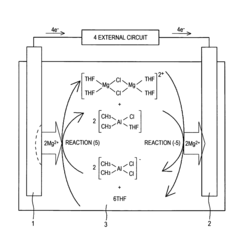
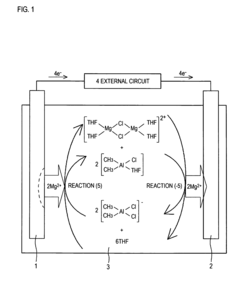
- A non-aqueous electrolyte comprising a non-aqueous solvent, a magnesium salt, and an aromatic heterocyclic compound with an aliphatic hydrocarbon group as a substituent, which forms a coordination bond with magnesium ions, promoting precipitation and dissolution of magnesium metal.
Magnesium ion-containing nonaqueous electrolytic solution and method for manufacturing the same, and electrochemical device
PatentActiveUS8993178B2
Innovation
- A magnesium ion-containing nonaqueous electrolytic solution is developed with a polynuclear complex ion structure, incorporating multiple magnesium ions and another metal ion, forming stable metal complexes with a shared ligand anion, which enhances the oxidation potential and conductivity, using chemically stable materials and a simplified manufacturing process.
Environmental Impact of Mg Battery Technologies
The environmental implications of magnesium battery technologies represent a critical consideration in their development and potential widespread adoption. Compared to lithium-ion batteries, magnesium-based systems offer several environmental advantages. The abundance of magnesium in the Earth's crust (approximately 2.3% versus lithium's 0.0017%) significantly reduces the ecological footprint associated with resource extraction. Mining operations for magnesium typically disturb less land area and generate fewer toxic byproducts than lithium mining, which often involves extensive brine evaporation processes that can deplete water resources in arid regions.
The production of magnesium metal anodes generally requires less energy than lithium extraction and refinement, potentially reducing greenhouse gas emissions associated with battery manufacturing. Additionally, the stability of magnesium metal in nonaqueous electrolytes, when properly engineered, can contribute to longer battery lifespans, thereby reducing electronic waste generation and the frequency of replacement.
Regarding end-of-life considerations, magnesium batteries present promising recycling opportunities. The metal is more easily recoverable from spent batteries than lithium, with established recycling processes already in place for magnesium in other industries. This recyclability could establish a more circular economy for energy storage technologies, reducing the need for continuous primary resource extraction.
Water pollution risks are also comparatively lower with magnesium battery technologies. While electrolyte development remains an ongoing challenge, the nonaqueous electrolytes being researched for magnesium batteries typically pose less severe contamination threats to aquatic ecosystems than the organic electrolytes and lithium salts used in conventional batteries.
Safety aspects further enhance the environmental profile of magnesium batteries. The reduced fire and explosion risks associated with magnesium systems (when properly designed with appropriate electrolytes) minimize the potential for environmental contamination through catastrophic failure events. This safety advantage becomes particularly significant in large-scale energy storage applications where thermal runaway in lithium systems could result in substantial environmental damage.
However, challenges remain in optimizing the environmental performance of magnesium battery technologies. Current research into electrolyte formulations must balance stability and performance with environmental considerations, as some promising electrolyte components may present toxicity concerns. Additionally, manufacturing processes for specialized magnesium anodes and compatible cathode materials require further refinement to minimize environmental impacts throughout the production chain.
The production of magnesium metal anodes generally requires less energy than lithium extraction and refinement, potentially reducing greenhouse gas emissions associated with battery manufacturing. Additionally, the stability of magnesium metal in nonaqueous electrolytes, when properly engineered, can contribute to longer battery lifespans, thereby reducing electronic waste generation and the frequency of replacement.
Regarding end-of-life considerations, magnesium batteries present promising recycling opportunities. The metal is more easily recoverable from spent batteries than lithium, with established recycling processes already in place for magnesium in other industries. This recyclability could establish a more circular economy for energy storage technologies, reducing the need for continuous primary resource extraction.
Water pollution risks are also comparatively lower with magnesium battery technologies. While electrolyte development remains an ongoing challenge, the nonaqueous electrolytes being researched for magnesium batteries typically pose less severe contamination threats to aquatic ecosystems than the organic electrolytes and lithium salts used in conventional batteries.
Safety aspects further enhance the environmental profile of magnesium batteries. The reduced fire and explosion risks associated with magnesium systems (when properly designed with appropriate electrolytes) minimize the potential for environmental contamination through catastrophic failure events. This safety advantage becomes particularly significant in large-scale energy storage applications where thermal runaway in lithium systems could result in substantial environmental damage.
However, challenges remain in optimizing the environmental performance of magnesium battery technologies. Current research into electrolyte formulations must balance stability and performance with environmental considerations, as some promising electrolyte components may present toxicity concerns. Additionally, manufacturing processes for specialized magnesium anodes and compatible cathode materials require further refinement to minimize environmental impacts throughout the production chain.
Safety Considerations for Mg-Based Energy Storage
Safety considerations for magnesium-based energy storage systems are paramount as this technology advances toward commercial applications. Unlike lithium-ion batteries, magnesium metal anodes in nonaqueous electrolytes present distinct safety advantages and challenges that require thorough evaluation.
Magnesium batteries inherently offer enhanced safety profiles compared to lithium systems due to magnesium's lower reactivity with air and moisture. The formation of a passivating oxide layer on magnesium surfaces provides natural protection against catastrophic reactions. Additionally, magnesium does not form dendrites during cycling, significantly reducing short-circuit risks that plague lithium metal anodes.
However, several safety concerns remain unresolved. The compatibility between magnesium anodes and electrolytes can lead to exothermic reactions under certain conditions. Particularly, chloride-containing electrolytes, while effective for magnesium deposition/dissolution, may present corrosion risks to battery components and potential hazards if cell integrity is compromised.
Thermal runaway scenarios, though less severe than in lithium systems, require investigation specific to magnesium chemistry. The behavior of magnesium-based systems under abuse conditions (overcharging, physical damage, extreme temperatures) remains inadequately characterized. Standard safety protocols developed for lithium-ion batteries may not address magnesium-specific failure modes.
Gas evolution during cycling represents another safety consideration. Electrolyte decomposition can generate hydrogen and other gases, potentially leading to pressure buildup within sealed cells. This necessitates appropriate venting mechanisms and pressure management strategies for commercial designs.
Environmental and toxicological aspects must also be evaluated. While magnesium itself has low environmental toxicity, various electrolyte components, particularly those containing chlorides or organometallic compounds, may present handling and disposal challenges. Comprehensive lifecycle safety assessments are essential for regulatory compliance.
Standardized safety testing protocols specifically designed for magnesium-based energy storage systems are currently lacking. The development of such standards is critical for industry adoption and regulatory approval. These should address the unique characteristics of magnesium electrochemistry in nonaqueous environments and provide meaningful safety metrics for comparison with existing technologies.
Magnesium batteries inherently offer enhanced safety profiles compared to lithium systems due to magnesium's lower reactivity with air and moisture. The formation of a passivating oxide layer on magnesium surfaces provides natural protection against catastrophic reactions. Additionally, magnesium does not form dendrites during cycling, significantly reducing short-circuit risks that plague lithium metal anodes.
However, several safety concerns remain unresolved. The compatibility between magnesium anodes and electrolytes can lead to exothermic reactions under certain conditions. Particularly, chloride-containing electrolytes, while effective for magnesium deposition/dissolution, may present corrosion risks to battery components and potential hazards if cell integrity is compromised.
Thermal runaway scenarios, though less severe than in lithium systems, require investigation specific to magnesium chemistry. The behavior of magnesium-based systems under abuse conditions (overcharging, physical damage, extreme temperatures) remains inadequately characterized. Standard safety protocols developed for lithium-ion batteries may not address magnesium-specific failure modes.
Gas evolution during cycling represents another safety consideration. Electrolyte decomposition can generate hydrogen and other gases, potentially leading to pressure buildup within sealed cells. This necessitates appropriate venting mechanisms and pressure management strategies for commercial designs.
Environmental and toxicological aspects must also be evaluated. While magnesium itself has low environmental toxicity, various electrolyte components, particularly those containing chlorides or organometallic compounds, may present handling and disposal challenges. Comprehensive lifecycle safety assessments are essential for regulatory compliance.
Standardized safety testing protocols specifically designed for magnesium-based energy storage systems are currently lacking. The development of such standards is critical for industry adoption and regulatory approval. These should address the unique characteristics of magnesium electrochemistry in nonaqueous environments and provide meaningful safety metrics for comparison with existing technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!