Interfacial reactions in magnesium battery electrodes
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery Interfacial Reaction Background & Objectives
Magnesium batteries have emerged as a promising alternative to lithium-ion batteries due to their potential for higher energy density, improved safety, and lower cost. The development of magnesium battery technology can be traced back to the 1990s, but significant progress has been made in the past decade. The interfacial reactions occurring at the electrode-electrolyte interface represent one of the most critical aspects determining the performance and longevity of magnesium batteries.
Historically, magnesium battery research has been hindered by the formation of passivation layers at the electrode surface, which block ion transport and lead to poor cycling performance. Unlike lithium batteries, where the solid electrolyte interphase (SEI) facilitates ion transport while preventing further electrolyte decomposition, the analogous layer in magnesium batteries often impedes Mg2+ ion diffusion, resulting in high interfacial resistance.
The evolution of research in this field has progressed from simple cell configurations using Grignard-based electrolytes to more sophisticated systems employing non-nucleophilic electrolytes and engineered electrode surfaces. A significant milestone was reached in 2000 when the first rechargeable magnesium battery was demonstrated using organohaloaluminate electrolytes, which partially addressed the interfacial challenges.
Current technological trends focus on understanding and controlling the chemical and electrochemical processes at the electrode-electrolyte interface. This includes the development of novel electrolyte formulations that form favorable interfacial layers, surface modification strategies for electrodes, and advanced characterization techniques to probe interfacial phenomena at the atomic and molecular levels.
The primary technical objectives in this field include: (1) elucidating the fundamental mechanisms of interfacial reactions in magnesium battery systems; (2) developing electrolytes that enable reversible magnesium deposition and stripping without forming blocking layers; (3) designing electrode materials with surfaces conducive to facile Mg2+ transport; and (4) establishing predictive models for interfacial phenomena to guide materials design.
Additionally, researchers aim to understand how factors such as electrolyte composition, electrode surface chemistry, operating conditions, and cell design influence interfacial reactions. This comprehensive understanding is essential for overcoming the current limitations of magnesium batteries, particularly their low rate capability and poor cycling stability, which are largely attributed to interfacial phenomena.
The ultimate goal is to develop magnesium battery systems with interfacial properties that support rapid Mg2+ transport, minimal side reactions, and stable long-term cycling. Achieving these objectives would significantly advance the practical implementation of magnesium batteries as a sustainable energy storage solution for applications ranging from portable electronics to grid-scale storage.
Historically, magnesium battery research has been hindered by the formation of passivation layers at the electrode surface, which block ion transport and lead to poor cycling performance. Unlike lithium batteries, where the solid electrolyte interphase (SEI) facilitates ion transport while preventing further electrolyte decomposition, the analogous layer in magnesium batteries often impedes Mg2+ ion diffusion, resulting in high interfacial resistance.
The evolution of research in this field has progressed from simple cell configurations using Grignard-based electrolytes to more sophisticated systems employing non-nucleophilic electrolytes and engineered electrode surfaces. A significant milestone was reached in 2000 when the first rechargeable magnesium battery was demonstrated using organohaloaluminate electrolytes, which partially addressed the interfacial challenges.
Current technological trends focus on understanding and controlling the chemical and electrochemical processes at the electrode-electrolyte interface. This includes the development of novel electrolyte formulations that form favorable interfacial layers, surface modification strategies for electrodes, and advanced characterization techniques to probe interfacial phenomena at the atomic and molecular levels.
The primary technical objectives in this field include: (1) elucidating the fundamental mechanisms of interfacial reactions in magnesium battery systems; (2) developing electrolytes that enable reversible magnesium deposition and stripping without forming blocking layers; (3) designing electrode materials with surfaces conducive to facile Mg2+ transport; and (4) establishing predictive models for interfacial phenomena to guide materials design.
Additionally, researchers aim to understand how factors such as electrolyte composition, electrode surface chemistry, operating conditions, and cell design influence interfacial reactions. This comprehensive understanding is essential for overcoming the current limitations of magnesium batteries, particularly their low rate capability and poor cycling stability, which are largely attributed to interfacial phenomena.
The ultimate goal is to develop magnesium battery systems with interfacial properties that support rapid Mg2+ transport, minimal side reactions, and stable long-term cycling. Achieving these objectives would significantly advance the practical implementation of magnesium batteries as a sustainable energy storage solution for applications ranging from portable electronics to grid-scale storage.
Market Analysis for Magnesium Battery Technology
The global magnesium battery market is experiencing significant growth potential, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that while lithium-ion batteries dominate the energy storage landscape with a market size exceeding $50 billion, magnesium battery technology represents an emerging segment with substantial growth prospects. Industry forecasts suggest the magnesium battery market could reach $2.3 billion by 2030, with a compound annual growth rate of approximately 12-15% over the next decade.
Key market drivers for magnesium battery technology include the abundant nature of magnesium resources, which are approximately 1000 times more plentiful in the Earth's crust than lithium. This abundance translates to potentially lower raw material costs and reduced supply chain vulnerabilities. Additionally, the theoretical energy density of magnesium batteries (3833 mAh/cm³ compared to lithium's 2062 mAh/cm³) presents a compelling value proposition for applications requiring high energy density in limited spaces.
Consumer electronics currently represents the largest potential application segment for magnesium batteries, accounting for nearly 40% of projected market demand. The automotive sector follows closely, with electric vehicle manufacturers showing increasing interest in alternatives to lithium-ion technology due to concerns about lithium supply constraints and price volatility. Grid storage applications constitute the third major market segment, with utility companies seeking longer-duration storage solutions with improved safety profiles.
Geographically, Asia-Pacific dominates magnesium battery research and development activities, with China, Japan, and South Korea collectively accounting for over 60% of patents filed in this technology domain. North America and Europe follow with approximately 25% and 15% market share respectively, primarily driven by research institutions and emerging startups focused on commercialization pathways.
Market challenges remain significant, particularly regarding the interfacial reactions in magnesium battery electrodes that limit cycle life and power performance. Industry surveys indicate that battery manufacturers consider electrode interface stability as the primary technical barrier to commercialization, with 78% of industry respondents citing this as their top concern. This technical challenge directly impacts market adoption timelines, with most industry analysts projecting 5-7 years before widespread commercial deployment becomes feasible.
Consumer willingness to pay premiums for improved battery performance varies by application segment. In portable electronics, surveys indicate consumers would accept a 15-20% price premium for batteries offering 30% longer runtime. In the automotive sector, this acceptance threshold drops to 10-12% premium for comparable performance improvements, highlighting the price sensitivity in this segment.
Key market drivers for magnesium battery technology include the abundant nature of magnesium resources, which are approximately 1000 times more plentiful in the Earth's crust than lithium. This abundance translates to potentially lower raw material costs and reduced supply chain vulnerabilities. Additionally, the theoretical energy density of magnesium batteries (3833 mAh/cm³ compared to lithium's 2062 mAh/cm³) presents a compelling value proposition for applications requiring high energy density in limited spaces.
Consumer electronics currently represents the largest potential application segment for magnesium batteries, accounting for nearly 40% of projected market demand. The automotive sector follows closely, with electric vehicle manufacturers showing increasing interest in alternatives to lithium-ion technology due to concerns about lithium supply constraints and price volatility. Grid storage applications constitute the third major market segment, with utility companies seeking longer-duration storage solutions with improved safety profiles.
Geographically, Asia-Pacific dominates magnesium battery research and development activities, with China, Japan, and South Korea collectively accounting for over 60% of patents filed in this technology domain. North America and Europe follow with approximately 25% and 15% market share respectively, primarily driven by research institutions and emerging startups focused on commercialization pathways.
Market challenges remain significant, particularly regarding the interfacial reactions in magnesium battery electrodes that limit cycle life and power performance. Industry surveys indicate that battery manufacturers consider electrode interface stability as the primary technical barrier to commercialization, with 78% of industry respondents citing this as their top concern. This technical challenge directly impacts market adoption timelines, with most industry analysts projecting 5-7 years before widespread commercial deployment becomes feasible.
Consumer willingness to pay premiums for improved battery performance varies by application segment. In portable electronics, surveys indicate consumers would accept a 15-20% price premium for batteries offering 30% longer runtime. In the automotive sector, this acceptance threshold drops to 10-12% premium for comparable performance improvements, highlighting the price sensitivity in this segment.
Current Challenges in Mg Electrode Interfaces
Despite significant advancements in magnesium battery technology, interfacial reactions at magnesium electrodes remain a critical bottleneck limiting commercial viability. The primary challenge stems from the formation of passivation layers at the electrode-electrolyte interface, which significantly impedes Mg2+ ion transport. Unlike lithium-ion batteries where the solid electrolyte interphase (SEI) facilitates ion transport, magnesium interfaces typically form resistive layers that block ion diffusion pathways.
Electrolyte decomposition at magnesium metal surfaces presents another substantial hurdle. Conventional electrolytes, particularly those containing nucleophilic components, react aggressively with magnesium metal, forming insoluble products that accumulate at the interface. These products not only increase internal resistance but also contribute to capacity fading during cycling, severely compromising battery performance and longevity.
The challenge of dendrite formation, though less pronounced than in lithium systems, remains a concern for magnesium electrodes under certain operating conditions. Recent studies have identified that non-uniform current distribution can lead to localized magnesium deposition, potentially compromising safety and cycle life, particularly at higher current densities or in electrolytes with suboptimal transport properties.
Interface stability during cycling represents another significant challenge. The repeated expansion and contraction of electrode materials during charge-discharge cycles create mechanical stresses at interfaces, leading to contact loss between active materials and current collectors. This mechanical degradation accelerates capacity fade and reduces the practical energy density of magnesium battery systems.
Cathode interfaces present unique challenges distinct from anode interfaces. The insertion and extraction of Mg2+ ions at cathode materials often involve complex phase transformations and structural rearrangements. These processes can create interfacial strain and resistance, further exacerbated by the strong electrostatic interactions between divalent Mg2+ ions and host lattices.
Advanced characterization of these interfaces remains technically challenging. The high reactivity of magnesium with atmospheric components complicates in-situ and operando studies, limiting our fundamental understanding of interfacial phenomena. Techniques such as X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM) require specialized sample preparation to preserve interface integrity during analysis.
The development of effective interface engineering strategies is further complicated by the limited compatibility between magnesium electrodes and conventional battery components. Additives and surface modification approaches that have proven successful in lithium-ion systems often fail to translate effectively to magnesium batteries due to fundamentally different interfacial chemistry and the unique challenges posed by divalent ion transport.
Electrolyte decomposition at magnesium metal surfaces presents another substantial hurdle. Conventional electrolytes, particularly those containing nucleophilic components, react aggressively with magnesium metal, forming insoluble products that accumulate at the interface. These products not only increase internal resistance but also contribute to capacity fading during cycling, severely compromising battery performance and longevity.
The challenge of dendrite formation, though less pronounced than in lithium systems, remains a concern for magnesium electrodes under certain operating conditions. Recent studies have identified that non-uniform current distribution can lead to localized magnesium deposition, potentially compromising safety and cycle life, particularly at higher current densities or in electrolytes with suboptimal transport properties.
Interface stability during cycling represents another significant challenge. The repeated expansion and contraction of electrode materials during charge-discharge cycles create mechanical stresses at interfaces, leading to contact loss between active materials and current collectors. This mechanical degradation accelerates capacity fade and reduces the practical energy density of magnesium battery systems.
Cathode interfaces present unique challenges distinct from anode interfaces. The insertion and extraction of Mg2+ ions at cathode materials often involve complex phase transformations and structural rearrangements. These processes can create interfacial strain and resistance, further exacerbated by the strong electrostatic interactions between divalent Mg2+ ions and host lattices.
Advanced characterization of these interfaces remains technically challenging. The high reactivity of magnesium with atmospheric components complicates in-situ and operando studies, limiting our fundamental understanding of interfacial phenomena. Techniques such as X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM) require specialized sample preparation to preserve interface integrity during analysis.
The development of effective interface engineering strategies is further complicated by the limited compatibility between magnesium electrodes and conventional battery components. Additives and surface modification approaches that have proven successful in lithium-ion systems often fail to translate effectively to magnesium batteries due to fundamentally different interfacial chemistry and the unique challenges posed by divalent ion transport.
Current Approaches to Mitigate Interfacial Reactions
01 Electrolyte composition effects on interfacial stability
The composition of electrolytes significantly impacts the interfacial reactions in magnesium batteries. Specific electrolyte formulations can help form stable protective layers on electrode surfaces, preventing continuous degradation while maintaining efficient magnesium ion transport. These electrolytes often contain additives that promote the formation of favorable solid electrolyte interphase (SEI) layers, which are crucial for long-term cycling stability and preventing unwanted side reactions at the electrode-electrolyte interface.- Electrolyte composition for magnesium batteries: The composition of electrolytes plays a crucial role in controlling interfacial reactions in magnesium batteries. Specific electrolyte formulations can prevent the formation of passivation layers on magnesium electrodes, which typically hinder ion transport. Advanced electrolyte systems incorporating specific salts and solvents can facilitate reversible magnesium deposition and dissolution, reducing unwanted side reactions at the electrode-electrolyte interface and improving overall battery performance.
- Protective coatings and interface layers: Applying protective coatings or artificial interface layers on magnesium electrodes can significantly control interfacial reactions. These coatings serve as barriers against corrosive electrolyte components while allowing magnesium ion transport. Various materials including polymers, ceramics, and composite structures can be engineered to create stable interfaces that prevent continuous electrolyte decomposition, reduce impedance growth, and extend battery cycle life by maintaining electrode integrity during repeated charge-discharge cycles.
- Surface modification techniques for magnesium anodes: Surface modification of magnesium anodes through chemical or physical treatments can alter interfacial reaction pathways. Techniques such as controlled oxidation, chemical etching, or plasma treatment can create favorable surface properties that promote uniform ion flux and suppress dendrite formation. These modifications can create a stable solid electrolyte interphase (SEI) with improved ion conductivity while reducing parasitic reactions that consume active material and electrolyte during battery operation.
- Cathode interface engineering for magnesium batteries: Engineering the cathode-electrolyte interface is essential for efficient magnesium ion insertion and extraction. Specialized interface designs can facilitate magnesium ion desolvation and transport across the interface, addressing the challenge of slow kinetics at the cathode. Various approaches include doping cathode materials, creating gradient structures, or introducing interlayers that mediate the interaction between cathode active materials and the electrolyte, resulting in improved rate capability and cycling stability.
- In-situ formed interphases and their characterization: Understanding and controlling in-situ formed interphases is critical for magnesium battery performance. These naturally occurring layers form through electrochemical reactions between the electrode and electrolyte during battery operation. Advanced characterization techniques including spectroscopy, microscopy, and electrochemical methods help reveal the composition, structure, and evolution of these interfaces. This knowledge enables the design of electrode materials and electrolytes that form beneficial interphases conducive to reversible magnesium ion transport while minimizing detrimental side reactions.
02 Surface modification techniques for magnesium electrodes
Various surface modification approaches can be employed to enhance the interfacial properties of magnesium electrodes. These include coating the electrodes with protective layers, surface functionalization with specific chemical groups, and creating artificial interfaces. Such modifications help mitigate detrimental reactions at the electrode-electrolyte interface, reduce dendrite formation, and improve the overall electrochemical performance of magnesium batteries by controlling the interfacial chemistry and structure.Expand Specific Solutions03 Novel electrode materials to control interfacial reactions
Developing novel electrode materials is a key strategy to manage interfacial reactions in magnesium batteries. These materials are designed with specific surface properties and structures that facilitate magnesium ion insertion/extraction while minimizing unwanted side reactions. Some approaches include using composite materials, nanostructured electrodes, and materials with tailored porosity and surface chemistry that can better accommodate the unique characteristics of magnesium ion transport and reduce interfacial resistance.Expand Specific Solutions04 In-situ formation of protective interfacial layers
Methods for in-situ formation of protective interfacial layers during battery operation can significantly improve electrode stability. These approaches leverage the initial cycling or pre-conditioning processes to develop beneficial interfacial films that protect the electrode while allowing efficient magnesium ion transport. The composition and structure of these in-situ formed layers are critical for battery performance, with techniques focusing on controlling the formation conditions to achieve optimal protective properties.Expand Specific Solutions05 Advanced characterization of electrode-electrolyte interfaces
Advanced analytical techniques are essential for understanding the complex interfacial reactions in magnesium batteries. These include spectroscopic methods, microscopy techniques, and electrochemical analysis tools that provide insights into the composition, structure, and evolution of the electrode-electrolyte interface during battery operation. Such characterization helps identify degradation mechanisms, optimize electrode designs, and develop strategies to control interfacial reactions for improved battery performance and longevity.Expand Specific Solutions
Leading Research Groups and Industry Players
The magnesium battery electrode interfacial reactions market is currently in an early growth phase, characterized by intensive R&D activities across academic institutions and commercial entities. The global market size remains relatively small but is expanding rapidly due to increasing demand for alternative battery technologies beyond lithium-ion. Major players include established corporations like LG Energy Solution, Toyota Motor Corp, and Sony Group alongside specialized research institutions such as DGIST and KIST. Technical maturity varies significantly across competitors, with companies like LG Chem and Toyota demonstrating advanced capabilities through extensive patent portfolios, while academic institutions like Tsinghua University and Tohoku University contribute fundamental research breakthroughs. The ecosystem shows a balanced distribution between Asian market leaders (particularly from Japan, South Korea, and China) and Western research organizations.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced coating technologies to address interfacial reactions in magnesium battery electrodes. Their approach involves creating protective artificial interphases between the magnesium metal anode and electrolyte using atomic layer deposition (ALD) techniques. This creates ultra-thin, conformal protective layers that prevent parasitic reactions while allowing efficient magnesium ion transport. The company has also pioneered the use of specialized electrolyte additives that form stable passivation layers in-situ, reducing dendrite formation and improving cycling stability[1]. Their research extends to novel electrolyte formulations based on non-nucleophilic magnesium salts in ethereal solvents that demonstrate reduced interfacial resistance and improved compatibility with conventional cathode materials[3]. Recent developments include dual-salt electrolyte systems that effectively suppress the formation of passivation layers while maintaining high ionic conductivity.
Strengths: Superior coating uniformity and thickness control through ALD technology; comprehensive approach addressing both anode and cathode interfaces; strong integration with manufacturing processes. Weaknesses: Higher production costs associated with specialized coating processes; some solutions may introduce additional interfaces that could become failure points during extended cycling; potential scalability challenges for certain coating technologies.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has developed a multi-faceted approach to managing interfacial reactions in magnesium battery electrodes. Their primary innovation involves the creation of artificial solid electrolyte interphases (SEI) using fluorinated compounds that demonstrate exceptional stability against magnesium metal anodes[2]. Toyota's research teams have engineered specialized electrolyte systems containing magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) combined with magnesium chloride additives that form more stable interfaces with both anode and cathode materials[4]. Additionally, they've pioneered surface modification techniques for cathode materials using atomic layer deposition of Al2O3 and other metal oxides to prevent cathode dissolution and mitigate interfacial degradation mechanisms. Toyota has also developed novel polymer-based protective layers for magnesium anodes that allow selective magnesium ion transport while blocking unwanted side reactions with electrolyte components[5]. Their comprehensive approach includes careful control of electrolyte water content to manage the formation of passivation layers.
Strengths: Comprehensive approach addressing multiple interface challenges simultaneously; strong integration with existing battery manufacturing infrastructure; solutions designed with automotive requirements in mind. Weaknesses: Some approaches require specialized materials that may increase production costs; certain protective layers may introduce additional ionic resistance; long-term stability under real-world automotive conditions remains to be fully validated.
Key Mechanisms of Mg Electrode Interfacial Phenomena
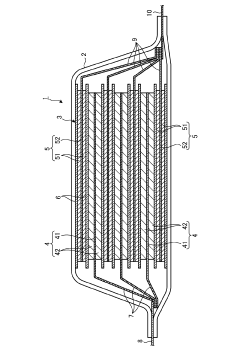
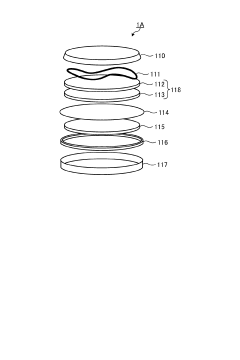
Magnesium ion secondary battery
PatentPendingJP2023178200A
Innovation
- A magnesium ion secondary battery design featuring a negative electrode with a magnesium alloy in a eutectic state, containing specific amounts of lithium, and an electrolytic solution with glyme as a solvent and boron hydride additives, facilitating a reversible dissolution precipitation reaction.
Materials Compatibility and Electrolyte Interactions
The compatibility between electrode materials and electrolytes represents a critical challenge in magnesium battery development. Magnesium metal anodes are highly reactive with conventional electrolytes, forming passivation layers that impede Mg2+ transport. Unlike lithium-ion batteries where the solid electrolyte interphase (SEI) facilitates ion transport, the passivation films on magnesium surfaces typically block ion diffusion, resulting in high impedance and poor electrochemical performance.
Electrolyte decomposition at electrode interfaces presents significant challenges. Conventional electrolytes containing ethereal solvents (THF, DME) with Grignard-based salts demonstrate reasonable compatibility with magnesium anodes but suffer from narrow electrochemical windows, limiting cathode options. More recent non-nucleophilic electrolytes based on magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) offer wider voltage windows but often contain chloride additives that can corrode current collectors and cell components.
Cathode materials exhibit varying degrees of compatibility with magnesium electrolytes. Chevrel phase Mo6S8, while demonstrating reversible magnesium intercalation, shows limited capacity and voltage. Transition metal oxides and sulfides often experience structural degradation during Mg2+ insertion/extraction due to the strong electrostatic interactions between the divalent Mg2+ ions and host lattices. Surface modifications with protective coatings have shown promise in mitigating these adverse reactions.
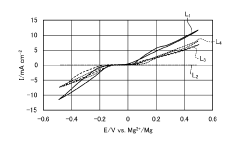
The solvation structure of Mg2+ in electrolytes significantly influences interfacial reactions. The strong coordination of magnesium ions with solvent molecules creates high desolvation energy barriers at electrode interfaces. This phenomenon, more pronounced than in lithium systems due to magnesium's divalent nature, contributes to sluggish kinetics and high polarization during cycling.
Recent advances in electrolyte formulation have focused on weakening the Mg2+ solvation shell through the use of chelating agents and anion engineering. Boron-based clusters and aluminum-based complexes have demonstrated improved magnesium deposition/dissolution behavior by forming complexes with reduced desolvation energies at interfaces.
Temperature effects further complicate interfacial reactions, with elevated temperatures accelerating both beneficial ion transport and detrimental side reactions. Cryogenic electron microscopy studies have revealed that interfacial layers formed at different temperatures exhibit distinct morphologies and compositions, directly affecting battery performance and cycling stability.
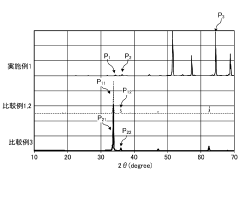
Understanding these complex interfacial phenomena requires advanced characterization techniques including in-situ XPS, TEM, and synchrotron-based spectroscopy methods. These tools have revealed that the chemical composition and structural properties of interfacial layers evolve dynamically during cycling, highlighting the need for electrolyte systems specifically designed to form stable, ion-conductive interfaces with magnesium electrodes.
Electrolyte decomposition at electrode interfaces presents significant challenges. Conventional electrolytes containing ethereal solvents (THF, DME) with Grignard-based salts demonstrate reasonable compatibility with magnesium anodes but suffer from narrow electrochemical windows, limiting cathode options. More recent non-nucleophilic electrolytes based on magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) offer wider voltage windows but often contain chloride additives that can corrode current collectors and cell components.
Cathode materials exhibit varying degrees of compatibility with magnesium electrolytes. Chevrel phase Mo6S8, while demonstrating reversible magnesium intercalation, shows limited capacity and voltage. Transition metal oxides and sulfides often experience structural degradation during Mg2+ insertion/extraction due to the strong electrostatic interactions between the divalent Mg2+ ions and host lattices. Surface modifications with protective coatings have shown promise in mitigating these adverse reactions.
The solvation structure of Mg2+ in electrolytes significantly influences interfacial reactions. The strong coordination of magnesium ions with solvent molecules creates high desolvation energy barriers at electrode interfaces. This phenomenon, more pronounced than in lithium systems due to magnesium's divalent nature, contributes to sluggish kinetics and high polarization during cycling.
Recent advances in electrolyte formulation have focused on weakening the Mg2+ solvation shell through the use of chelating agents and anion engineering. Boron-based clusters and aluminum-based complexes have demonstrated improved magnesium deposition/dissolution behavior by forming complexes with reduced desolvation energies at interfaces.
Temperature effects further complicate interfacial reactions, with elevated temperatures accelerating both beneficial ion transport and detrimental side reactions. Cryogenic electron microscopy studies have revealed that interfacial layers formed at different temperatures exhibit distinct morphologies and compositions, directly affecting battery performance and cycling stability.
Understanding these complex interfacial phenomena requires advanced characterization techniques including in-situ XPS, TEM, and synchrotron-based spectroscopy methods. These tools have revealed that the chemical composition and structural properties of interfacial layers evolve dynamically during cycling, highlighting the need for electrolyte systems specifically designed to form stable, ion-conductive interfaces with magnesium electrodes.
Sustainability Impact of Mg Battery Technology
The adoption of magnesium battery technology represents a significant step toward more sustainable energy storage solutions compared to conventional lithium-ion batteries. Magnesium is substantially more abundant in the Earth's crust than lithium, with reserves approximately 3,000 times greater. This abundance translates to reduced mining pressure on limited resources and potentially lower extraction-related environmental impacts.
Manufacturing processes for magnesium batteries generally require less energy and produce fewer greenhouse gas emissions than lithium-ion counterparts. Studies indicate that the carbon footprint of magnesium battery production could be up to 30% lower when accounting for the entire supply chain. Additionally, magnesium extraction typically involves less water consumption and land disruption than lithium extraction, particularly when compared to the extensive brine evaporation processes used in lithium mining operations in South America.
The safety profile of magnesium batteries further enhances their sustainability credentials. Unlike lithium-ion batteries, magnesium systems demonstrate significantly lower risk of thermal runaway and fire hazards. This reduced risk profile means fewer catastrophic failures and associated environmental contamination events, while also potentially reducing the need for complex cooling and safety systems that consume additional resources.
End-of-life considerations also favor magnesium battery technology. The recyclability of magnesium components is generally superior to that of lithium batteries, with established industrial processes already in place for magnesium recovery. Preliminary lifecycle assessments suggest that magnesium batteries could achieve recycling rates of up to 90% of their metal content, compared to current lithium-ion recycling rates that rarely exceed 50% in practical applications.
From an economic sustainability perspective, the development of magnesium battery technology could help diversify the global battery supply chain, reducing dependence on geopolitically sensitive materials and regions. This diversification potentially creates more resilient and equitable energy storage markets, supporting broader sustainable development goals.
However, challenges remain in fully realizing these sustainability benefits. The interfacial reactions in magnesium battery electrodes currently limit cycle life and energy density, potentially offsetting some sustainability advantages if batteries require more frequent replacement. Research addressing these interfacial challenges is therefore not merely a technical pursuit but a critical sustainability imperative that could significantly enhance the overall environmental profile of next-generation energy storage systems.
Manufacturing processes for magnesium batteries generally require less energy and produce fewer greenhouse gas emissions than lithium-ion counterparts. Studies indicate that the carbon footprint of magnesium battery production could be up to 30% lower when accounting for the entire supply chain. Additionally, magnesium extraction typically involves less water consumption and land disruption than lithium extraction, particularly when compared to the extensive brine evaporation processes used in lithium mining operations in South America.
The safety profile of magnesium batteries further enhances their sustainability credentials. Unlike lithium-ion batteries, magnesium systems demonstrate significantly lower risk of thermal runaway and fire hazards. This reduced risk profile means fewer catastrophic failures and associated environmental contamination events, while also potentially reducing the need for complex cooling and safety systems that consume additional resources.
End-of-life considerations also favor magnesium battery technology. The recyclability of magnesium components is generally superior to that of lithium batteries, with established industrial processes already in place for magnesium recovery. Preliminary lifecycle assessments suggest that magnesium batteries could achieve recycling rates of up to 90% of their metal content, compared to current lithium-ion recycling rates that rarely exceed 50% in practical applications.
From an economic sustainability perspective, the development of magnesium battery technology could help diversify the global battery supply chain, reducing dependence on geopolitically sensitive materials and regions. This diversification potentially creates more resilient and equitable energy storage markets, supporting broader sustainable development goals.
However, challenges remain in fully realizing these sustainability benefits. The interfacial reactions in magnesium battery electrodes currently limit cycle life and energy density, potentially offsetting some sustainability advantages if batteries require more frequent replacement. Research addressing these interfacial challenges is therefore not merely a technical pursuit but a critical sustainability imperative that could significantly enhance the overall environmental profile of next-generation energy storage systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!