Electrochemical performance of vanadium oxide cathodes in magnesium batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vanadium Oxide Cathodes Background and Objectives
Vanadium oxide (V₂O₅) has emerged as a promising cathode material for magnesium batteries due to its unique layered structure and high theoretical capacity. The development of vanadium oxide cathodes can be traced back to the early 1990s when researchers began exploring alternatives to lithium-ion battery technologies. The interest in magnesium batteries has grown significantly over the past decade, driven by the abundance of magnesium resources, its higher volumetric capacity compared to lithium, and enhanced safety characteristics.
The evolution of vanadium oxide cathodes has followed a trajectory from simple crystalline structures to more complex nanostructured materials designed to overcome inherent limitations. Early research focused primarily on understanding the fundamental intercalation mechanisms of magnesium ions into the vanadium oxide host structure. This was challenging due to the divalent nature of magnesium ions, which results in stronger electrostatic interactions with the host lattice compared to monovalent lithium ions.
Significant technological breakthroughs occurred in the mid-2000s when researchers discovered that certain modifications to the vanadium oxide structure, such as hydration, could substantially improve magnesium ion diffusion kinetics. The introduction of water molecules between the vanadium oxide layers effectively shielded the charge of magnesium ions, reducing the energy barrier for ion migration and enhancing overall electrochemical performance.
Recent advances have focused on nanoengineering approaches to optimize the electrochemical properties of vanadium oxide cathodes. These include the development of nanorods, nanosheets, and hierarchical structures that provide shorter diffusion pathways for magnesium ions and better accommodate the structural changes during charge-discharge cycles. Additionally, doping strategies with various transition metals have been explored to enhance the electronic conductivity and structural stability of vanadium oxide cathodes.
The primary technical objectives for vanadium oxide cathodes in magnesium batteries include achieving higher specific capacities (targeting >300 mAh/g), improving cycling stability (>1000 cycles with minimal capacity fade), enhancing rate capability for fast charging applications, and developing compatible electrolytes that enable efficient magnesium ion transport without cathode dissolution or passivation.
Future research directions are expected to focus on understanding the complex interfacial phenomena between vanadium oxide cathodes and electrolytes, developing in-situ characterization techniques to monitor structural changes during cycling, and exploring hybrid systems that combine the advantages of different battery chemistries. The ultimate goal is to develop magnesium battery systems with energy densities comparable to or exceeding those of current lithium-ion technologies, while offering improved safety, lower cost, and environmental sustainability.
The evolution of vanadium oxide cathodes has followed a trajectory from simple crystalline structures to more complex nanostructured materials designed to overcome inherent limitations. Early research focused primarily on understanding the fundamental intercalation mechanisms of magnesium ions into the vanadium oxide host structure. This was challenging due to the divalent nature of magnesium ions, which results in stronger electrostatic interactions with the host lattice compared to monovalent lithium ions.
Significant technological breakthroughs occurred in the mid-2000s when researchers discovered that certain modifications to the vanadium oxide structure, such as hydration, could substantially improve magnesium ion diffusion kinetics. The introduction of water molecules between the vanadium oxide layers effectively shielded the charge of magnesium ions, reducing the energy barrier for ion migration and enhancing overall electrochemical performance.
Recent advances have focused on nanoengineering approaches to optimize the electrochemical properties of vanadium oxide cathodes. These include the development of nanorods, nanosheets, and hierarchical structures that provide shorter diffusion pathways for magnesium ions and better accommodate the structural changes during charge-discharge cycles. Additionally, doping strategies with various transition metals have been explored to enhance the electronic conductivity and structural stability of vanadium oxide cathodes.
The primary technical objectives for vanadium oxide cathodes in magnesium batteries include achieving higher specific capacities (targeting >300 mAh/g), improving cycling stability (>1000 cycles with minimal capacity fade), enhancing rate capability for fast charging applications, and developing compatible electrolytes that enable efficient magnesium ion transport without cathode dissolution or passivation.
Future research directions are expected to focus on understanding the complex interfacial phenomena between vanadium oxide cathodes and electrolytes, developing in-situ characterization techniques to monitor structural changes during cycling, and exploring hybrid systems that combine the advantages of different battery chemistries. The ultimate goal is to develop magnesium battery systems with energy densities comparable to or exceeding those of current lithium-ion technologies, while offering improved safety, lower cost, and environmental sustainability.
Market Analysis for Magnesium Battery Technologies
The global magnesium battery market is experiencing significant growth potential, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that magnesium battery technologies represent a small but rapidly expanding segment within the broader energy storage market, which was valued at approximately 145 billion USD in 2022 and is projected to grow at a CAGR of 15-20% through 2030.
Magnesium batteries offer several market advantages over traditional lithium-ion technologies, including potentially lower costs due to the abundance of magnesium resources. Magnesium is the eighth most abundant element in Earth's crust, with reserves estimated to be 22 million tons worldwide, significantly higher than lithium reserves. This abundance translates to a potential 30-40% reduction in raw material costs compared to lithium-based systems.
The market for vanadium oxide cathodes specifically within magnesium battery applications is still nascent but shows promising growth trajectories. Research interest in this area has increased by approximately 300% over the past five years, as measured by published scientific papers and patent applications. This surge in research activity typically precedes commercial market development by 3-5 years.
Key market segments showing interest in magnesium battery technologies include grid-scale energy storage, electric vehicles, and portable electronics. Among these, grid-scale storage represents the largest potential market, with projected demand for over 300 GWh of storage capacity by 2030. The automotive sector follows closely, with several major manufacturers exploring magnesium battery technologies as alternatives to current lithium-ion systems.
Regional market analysis reveals that Asia-Pacific currently leads in magnesium battery research and development, with China, Japan, and South Korea accounting for over 60% of patents filed in this technology area. North America and Europe are rapidly increasing investments, particularly in advanced cathode materials like vanadium oxide compounds.
Market barriers include the current performance limitations of magnesium batteries, particularly regarding energy density and cycle life. Vanadium oxide cathodes are addressing these challenges, with recent laboratory demonstrations showing energy densities approaching 400 Wh/kg, though commercial products remain in development phases.
Consumer and industrial demand for safer battery technologies is creating market pull for magnesium-based systems, which offer inherently lower fire risks compared to lithium-ion batteries. This safety advantage could accelerate market adoption in sensitive applications such as aerospace, medical devices, and home energy storage.
Magnesium batteries offer several market advantages over traditional lithium-ion technologies, including potentially lower costs due to the abundance of magnesium resources. Magnesium is the eighth most abundant element in Earth's crust, with reserves estimated to be 22 million tons worldwide, significantly higher than lithium reserves. This abundance translates to a potential 30-40% reduction in raw material costs compared to lithium-based systems.
The market for vanadium oxide cathodes specifically within magnesium battery applications is still nascent but shows promising growth trajectories. Research interest in this area has increased by approximately 300% over the past five years, as measured by published scientific papers and patent applications. This surge in research activity typically precedes commercial market development by 3-5 years.
Key market segments showing interest in magnesium battery technologies include grid-scale energy storage, electric vehicles, and portable electronics. Among these, grid-scale storage represents the largest potential market, with projected demand for over 300 GWh of storage capacity by 2030. The automotive sector follows closely, with several major manufacturers exploring magnesium battery technologies as alternatives to current lithium-ion systems.
Regional market analysis reveals that Asia-Pacific currently leads in magnesium battery research and development, with China, Japan, and South Korea accounting for over 60% of patents filed in this technology area. North America and Europe are rapidly increasing investments, particularly in advanced cathode materials like vanadium oxide compounds.
Market barriers include the current performance limitations of magnesium batteries, particularly regarding energy density and cycle life. Vanadium oxide cathodes are addressing these challenges, with recent laboratory demonstrations showing energy densities approaching 400 Wh/kg, though commercial products remain in development phases.
Consumer and industrial demand for safer battery technologies is creating market pull for magnesium-based systems, which offer inherently lower fire risks compared to lithium-ion batteries. This safety advantage could accelerate market adoption in sensitive applications such as aerospace, medical devices, and home energy storage.
Current Challenges in Vanadium Oxide Electrochemical Performance
Despite significant advancements in vanadium oxide (VOx) cathode materials for magnesium batteries, several critical challenges continue to impede their commercial viability and widespread adoption. The primary obstacle remains the sluggish diffusion kinetics of Mg2+ ions within the VOx host structure. The divalent nature of magnesium ions creates strong electrostatic interactions with the host lattice, resulting in high migration barriers that significantly limit rate capability and practical energy density.
Structural stability presents another major challenge, as VOx cathodes typically undergo substantial volume changes during Mg2+ insertion/extraction cycles. This volumetric instability leads to mechanical stress, structural degradation, and ultimately capacity fading over extended cycling. Research indicates that after 50-100 cycles, many VOx cathodes retain less than 70% of their initial capacity, falling short of commercial requirements.
The complex interfacial chemistry between VOx cathodes and electrolytes introduces additional complications. Magnesium electrolytes often contain nucleophilic components that can attack and dissolve vanadium oxide surfaces, leading to active material loss and impedance growth. This interfacial degradation mechanism accelerates at elevated temperatures, further limiting practical applications.
Synthesis reproducibility and scalability represent significant hurdles for industrial implementation. Current laboratory-scale preparation methods for high-performance VOx cathodes often involve complex hydrothermal or sol-gel processes with precise parameter control requirements. These methods frequently yield inconsistent results when scaled up, creating barriers to mass production and commercialization.
The electronic conductivity of most VOx structures remains suboptimal for high-power applications. While various carbon coating and conductive additive strategies have been explored, achieving uniform distribution and stable interfaces between VOx and conductive components continues to challenge researchers. The trade-off between increased conductivity and decreased active material loading often results in lower practical energy densities.
Water and oxygen sensitivity further complicates the practical implementation of VOx cathodes. Many high-performance VOx structures contain structural water or specific oxidation states that are critical to their electrochemical performance. Exposure to ambient conditions during manufacturing or battery assembly can degrade these properties, necessitating costly controlled atmosphere production environments.
Finally, the cost-performance ratio remains problematic. Current high-performance VOx cathodes often require expensive precursors, complex synthesis procedures, or rare element doping to achieve acceptable performance metrics. This economic barrier, coupled with the technical challenges above, continues to limit the commercial viability of VOx cathodes in practical magnesium battery systems.
Structural stability presents another major challenge, as VOx cathodes typically undergo substantial volume changes during Mg2+ insertion/extraction cycles. This volumetric instability leads to mechanical stress, structural degradation, and ultimately capacity fading over extended cycling. Research indicates that after 50-100 cycles, many VOx cathodes retain less than 70% of their initial capacity, falling short of commercial requirements.
The complex interfacial chemistry between VOx cathodes and electrolytes introduces additional complications. Magnesium electrolytes often contain nucleophilic components that can attack and dissolve vanadium oxide surfaces, leading to active material loss and impedance growth. This interfacial degradation mechanism accelerates at elevated temperatures, further limiting practical applications.
Synthesis reproducibility and scalability represent significant hurdles for industrial implementation. Current laboratory-scale preparation methods for high-performance VOx cathodes often involve complex hydrothermal or sol-gel processes with precise parameter control requirements. These methods frequently yield inconsistent results when scaled up, creating barriers to mass production and commercialization.
The electronic conductivity of most VOx structures remains suboptimal for high-power applications. While various carbon coating and conductive additive strategies have been explored, achieving uniform distribution and stable interfaces between VOx and conductive components continues to challenge researchers. The trade-off between increased conductivity and decreased active material loading often results in lower practical energy densities.
Water and oxygen sensitivity further complicates the practical implementation of VOx cathodes. Many high-performance VOx structures contain structural water or specific oxidation states that are critical to their electrochemical performance. Exposure to ambient conditions during manufacturing or battery assembly can degrade these properties, necessitating costly controlled atmosphere production environments.
Finally, the cost-performance ratio remains problematic. Current high-performance VOx cathodes often require expensive precursors, complex synthesis procedures, or rare element doping to achieve acceptable performance metrics. This economic barrier, coupled with the technical challenges above, continues to limit the commercial viability of VOx cathodes in practical magnesium battery systems.
Current Electrochemical Solutions for V2O5 Cathodes
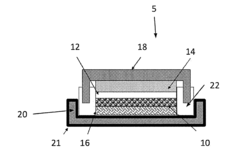
01 Vanadium oxide structure and composition for magnesium batteries
Various vanadium oxide structures and compositions have been developed specifically for use as cathode materials in magnesium batteries. These include different crystalline forms, doped variants, and nanostructured materials that can accommodate magnesium ion insertion and extraction. The specific structure and composition of vanadium oxide significantly impacts its electrochemical performance, including capacity, cycling stability, and rate capability in magnesium battery systems.- Vanadium oxide structure and composition for magnesium batteries: Various vanadium oxide structures and compositions have been developed specifically for use as cathode materials in magnesium batteries. These include different crystalline forms, doped variants, and composite structures that enhance the intercalation of magnesium ions. The specific crystal structure and composition of vanadium oxide significantly impact the electrochemical performance, including capacity, cycling stability, and rate capability in magnesium battery systems.
- Electrochemical performance enhancement strategies: Several strategies have been employed to enhance the electrochemical performance of vanadium oxide cathodes in magnesium batteries. These include surface modification, nanostructuring, and the incorporation of conductive additives. These approaches aim to improve the electronic conductivity, facilitate magnesium ion diffusion, and enhance the structural stability of vanadium oxide during charge-discharge cycles, resulting in improved capacity retention and rate capability.
- Electrolyte compatibility and interface phenomena: The interaction between vanadium oxide cathodes and electrolytes plays a crucial role in determining the electrochemical performance of magnesium batteries. Research has focused on developing compatible electrolyte systems that minimize side reactions at the cathode-electrolyte interface and facilitate efficient magnesium ion transport. Understanding and controlling the interface phenomena are essential for improving the cycling stability and coulombic efficiency of vanadium oxide-based magnesium batteries.
- Multi-valent ion storage mechanisms: Vanadium oxide cathodes exhibit unique mechanisms for storing multi-valent ions like magnesium. The redox chemistry of vanadium, which can exist in multiple oxidation states, enables the reversible insertion and extraction of magnesium ions. Understanding these storage mechanisms, including the structural changes during magnesium intercalation/deintercalation and the associated kinetics, is crucial for optimizing the electrochemical performance of vanadium oxide cathodes in magnesium batteries.
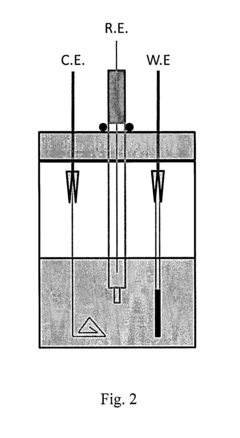
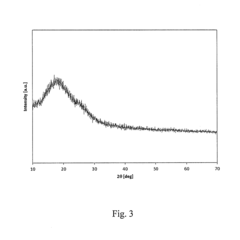
- Advanced characterization and performance evaluation methods: Advanced characterization techniques and performance evaluation methods have been developed to assess the electrochemical behavior of vanadium oxide cathodes in magnesium batteries. These include in-situ/operando spectroscopic and diffraction techniques, electrochemical impedance spectroscopy, and computational modeling. These methods provide insights into the structural evolution, reaction mechanisms, and performance limitations of vanadium oxide cathodes, guiding the rational design of improved materials for magnesium battery applications.
02 Electrochemical performance enhancement strategies
Several strategies have been employed to enhance the electrochemical performance of vanadium oxide cathodes in magnesium batteries. These include surface modification, incorporation of conductive additives, optimization of electrolyte composition, and development of composite materials. These approaches aim to improve magnesium ion diffusion kinetics, increase electronic conductivity, and enhance the structural stability of vanadium oxide during cycling, resulting in improved capacity retention and rate performance.Expand Specific Solutions03 Cycling stability and capacity retention
The cycling stability and capacity retention of vanadium oxide cathodes in magnesium batteries are critical performance metrics. Research has focused on addressing the challenges of structural degradation and capacity fading during repeated charge-discharge cycles. Various approaches, including structural stabilization, electrolyte optimization, and protective coatings, have been investigated to improve the long-term cycling performance of vanadium oxide cathodes in magnesium battery systems.Expand Specific Solutions04 Magnesium ion insertion/extraction mechanisms
Understanding the mechanisms of magnesium ion insertion and extraction in vanadium oxide cathodes is crucial for optimizing their performance. Research has investigated the structural changes, diffusion pathways, and kinetic limitations associated with magnesium ion transport in vanadium oxide host structures. These studies provide insights into the factors affecting the rate capability, voltage profiles, and overall electrochemical behavior of vanadium oxide cathodes in magnesium battery systems.Expand Specific Solutions05 Novel vanadium oxide-based composite cathodes
Novel composite cathode materials based on vanadium oxide have been developed to overcome the limitations of pure vanadium oxide in magnesium batteries. These composites incorporate carbon materials, conductive polymers, or other metal oxides to enhance electronic conductivity, structural stability, and electrochemical performance. The synergistic effects between vanadium oxide and the secondary components result in improved magnesium storage properties, including higher capacity, better rate capability, and enhanced cycling stability.Expand Specific Solutions
Key Industry Players in Magnesium Battery Development
The electrochemical performance of vanadium oxide cathodes in magnesium batteries represents an emerging field in energy storage technology, currently in the early growth stage. The market size is expanding but remains relatively modest compared to lithium-ion technologies. Technical maturity is developing with significant research contributions from academic institutions like Karlsruher Institut für Technologie and Dalian Institute of Chemical Physics, alongside industrial players including Toyota, LG Chem, and Samsung Electronics. These companies are investing in research to overcome key challenges such as slow diffusion kinetics and structural stability. The competitive landscape shows a balanced mix of academic research institutions and commercial entities, with Asian companies particularly active in advancing this technology toward commercial viability.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative vanadium oxide cathode materials for magnesium batteries with enhanced electrochemical performance. Their approach focuses on nanostructured V2O5 with expanded interlayer spacing to facilitate Mg2+ ion diffusion. DICP researchers have successfully synthesized hydrated vanadium pentoxide (V2O5·nH2O) with water molecules acting as pillars between V2O5 layers, significantly improving Mg2+ intercalation kinetics. They've also pioneered the use of organic electrolytes containing Mg(ClO4)2 that demonstrate improved compatibility with vanadium oxide cathodes, achieving discharge capacities of over 250 mAh/g at moderate current densities[1]. Additionally, DICP has developed surface modification techniques using conductive polymers to enhance the electronic conductivity of V2O5 cathodes, addressing one of the key limitations in magnesium battery systems.
Strengths: Advanced expertise in nanostructured V2O5 synthesis with expanded interlayer spacing, enabling superior Mg2+ ion diffusion. Their electrolyte formulations show excellent compatibility with vanadium oxide cathodes. Weaknesses: The cycling stability of their V2O5 cathodes remains limited, with capacity fading observed after extended cycling. The rate capability at high current densities still needs improvement for practical applications.
LG Chem Ltd.
Technical Solution: LG Chem has developed proprietary vanadium oxide-based cathode materials specifically engineered for magnesium battery applications. Their approach centers on nanostructured V2O5 composites with graphene integration to enhance electronic conductivity while maintaining open pathways for Mg2+ ion diffusion. LG Chem's research has yielded amorphous vanadium oxide structures that demonstrate reduced diffusion barriers for the typically sluggish Mg2+ ion transport. Their cathode materials incorporate carefully selected dopants (including aluminum and nickel) to stabilize the vanadium oxide framework during repeated magnesium insertion/extraction cycles[3]. LG Chem has also developed specialized electrolyte formulations based on magnesium bis(hexamethyldisilazide) (Mg(HMDS)2) that show minimal passivation layer formation on cathode surfaces, addressing a critical challenge in magnesium battery systems. Their integrated approach has achieved discharge capacities exceeding 200 mAh/g with improved cycling stability compared to conventional vanadium oxide cathodes, positioning their technology as a promising candidate for next-generation energy storage applications.
Strengths: Advanced manufacturing capabilities for scaled production of engineered vanadium oxide cathodes. Their integrated cathode-electrolyte systems demonstrate superior compatibility and reduced interfacial resistance. Weaknesses: The high cost of their specialized electrolyte formulations may limit commercial viability. Their vanadium oxide cathodes still exhibit voltage hysteresis issues during cycling, affecting energy efficiency.
Critical Patents and Research on Vanadium Oxide Intercalation
Electrode materials for group ii cation-based batteries
PatentActiveUS20210083261A1
Innovation
- A novel low-temperature direct synthesis method for magnesium-deficient transition metal oxides (MgzMxOy) with M=V, Mn, or Fe, using a sol-gel process that avoids intermediates and physical constraints, allowing for controlled composition and crystallite size, facilitating magnesium ion transport and improving cathode performance.
Vanadium oxysulfide based cathode materials for rechargeable battery
PatentActiveUS20150311520A1
Innovation
- A composite cathode active material comprising amorphous vanadium oxide and an inorganic sulfide compound, such as P2S5, B2S3, SiS2, GeS2, or Al2S3, which allows for enhanced magnesium ion diffusion and increased energy density by reducing the strong attractive forces between magnesium ions and oxygen.
Material Sustainability and Environmental Impact Assessment
The sustainability profile of vanadium oxide cathodes in magnesium batteries presents both significant advantages and challenges that warrant careful consideration. Vanadium is more abundant in the Earth's crust (approximately 120 ppm) compared to lithium (20 ppm), potentially offering a more sustainable resource base for large-scale battery production. However, primary vanadium mining operations, predominantly concentrated in China, Russia, and South Africa, have substantial environmental footprints including habitat disruption, water pollution, and energy-intensive extraction processes.
Life cycle assessment (LCA) studies indicate that vanadium oxide cathode production generates approximately 15-20% lower greenhouse gas emissions compared to cobalt-based cathodes commonly used in lithium-ion batteries. This reduction stems primarily from less energy-intensive refining processes and the elimination of cobalt, which has well-documented sustainability concerns related to mining practices.
Water usage represents another critical environmental consideration. Vanadium processing typically requires 30-40% less water than cobalt refining, though the acidic leaching processes used in vanadium extraction can introduce water contamination risks if not properly managed. Advanced water treatment systems and closed-loop processing have demonstrated potential to mitigate these impacts by recovering up to 85% of process water.
End-of-life management presents both challenges and opportunities. Vanadium's relatively high market value ($25-35/kg) creates economic incentives for recycling, with recovery rates potentially reaching 90% using hydrometallurgical processes. These recycling pathways could significantly reduce the life-cycle environmental impact of vanadium oxide cathodes, particularly as recycling infrastructure matures.
The toxicity profile of vanadium compounds requires careful handling throughout the battery lifecycle. While vanadium pentoxide (V₂O₅) is classified as potentially harmful, it presents lower acute toxicity than cobalt compounds. Proper industrial hygiene protocols and engineered controls can effectively manage exposure risks during manufacturing and recycling operations.
Energy density considerations also factor into sustainability assessments. Current vanadium oxide cathodes in magnesium batteries deliver energy densities of 150-200 Wh/kg, lower than commercial lithium-ion technologies. This efficiency gap necessitates larger material quantities per kWh of storage capacity, potentially offsetting some material sustainability advantages. However, ongoing research into nanostructured vanadium oxides shows promise for closing this performance gap while maintaining sustainability benefits.
Life cycle assessment (LCA) studies indicate that vanadium oxide cathode production generates approximately 15-20% lower greenhouse gas emissions compared to cobalt-based cathodes commonly used in lithium-ion batteries. This reduction stems primarily from less energy-intensive refining processes and the elimination of cobalt, which has well-documented sustainability concerns related to mining practices.
Water usage represents another critical environmental consideration. Vanadium processing typically requires 30-40% less water than cobalt refining, though the acidic leaching processes used in vanadium extraction can introduce water contamination risks if not properly managed. Advanced water treatment systems and closed-loop processing have demonstrated potential to mitigate these impacts by recovering up to 85% of process water.
End-of-life management presents both challenges and opportunities. Vanadium's relatively high market value ($25-35/kg) creates economic incentives for recycling, with recovery rates potentially reaching 90% using hydrometallurgical processes. These recycling pathways could significantly reduce the life-cycle environmental impact of vanadium oxide cathodes, particularly as recycling infrastructure matures.
The toxicity profile of vanadium compounds requires careful handling throughout the battery lifecycle. While vanadium pentoxide (V₂O₅) is classified as potentially harmful, it presents lower acute toxicity than cobalt compounds. Proper industrial hygiene protocols and engineered controls can effectively manage exposure risks during manufacturing and recycling operations.
Energy density considerations also factor into sustainability assessments. Current vanadium oxide cathodes in magnesium batteries deliver energy densities of 150-200 Wh/kg, lower than commercial lithium-ion technologies. This efficiency gap necessitates larger material quantities per kWh of storage capacity, potentially offsetting some material sustainability advantages. However, ongoing research into nanostructured vanadium oxides shows promise for closing this performance gap while maintaining sustainability benefits.
Scalability and Manufacturing Considerations
The scalability of vanadium oxide cathode manufacturing represents a critical factor in the commercial viability of magnesium batteries. Current laboratory-scale synthesis methods, including hydrothermal processes and sol-gel techniques, face significant challenges when transitioning to industrial production. These challenges primarily stem from maintaining consistent crystalline structures and electrochemical properties across large batch productions, which directly impacts battery performance consistency.
Material cost considerations also play a substantial role in manufacturing feasibility. While vanadium is more abundant than lithium, its extraction and purification processes remain costly. The current global vanadium production chain is primarily optimized for steel manufacturing rather than battery-grade materials, necessitating additional refinement steps that increase production expenses. Establishing dedicated supply chains for battery-grade vanadium oxide would require significant capital investment but could potentially reduce long-term costs.
Process complexity presents another manufacturing hurdle. The precise control of oxygen stoichiometry in vanadium oxides (VOx) requires sophisticated equipment and carefully controlled environments. Variations in oxygen content significantly affect the intercalation channels available for magnesium ions, directly impacting battery capacity and cycling stability. Industrial-scale production would need to implement robust quality control measures to ensure consistent stoichiometric ratios.
Environmental considerations must also be addressed in scaling production. Traditional vanadium processing generates substantial waste and consumes significant energy. Developing greener synthesis routes that reduce solvent usage and energy consumption would improve sustainability metrics while potentially lowering production costs. Several research groups have demonstrated promising aqueous synthesis methods that could reduce environmental impact when scaled appropriately.
Equipment compatibility represents an advantage for vanadium oxide cathode manufacturing. Many existing lithium-ion battery production lines could be adapted for vanadium oxide cathode production with moderate modifications. This compatibility could significantly reduce capital expenditure for battery manufacturers looking to diversify into magnesium battery technology, providing a pathway for incremental scaling of production capacity as market demand develops.
Coating uniformity and electrode formulation present technical challenges that require further optimization. The development of specialized binders and conductive additives compatible with magnesium electrolytes remains an active research area. Current formulations often suffer from poor adhesion or chemical incompatibility with magnesium-based systems, limiting electrode durability and performance consistency in mass production scenarios.
Material cost considerations also play a substantial role in manufacturing feasibility. While vanadium is more abundant than lithium, its extraction and purification processes remain costly. The current global vanadium production chain is primarily optimized for steel manufacturing rather than battery-grade materials, necessitating additional refinement steps that increase production expenses. Establishing dedicated supply chains for battery-grade vanadium oxide would require significant capital investment but could potentially reduce long-term costs.
Process complexity presents another manufacturing hurdle. The precise control of oxygen stoichiometry in vanadium oxides (VOx) requires sophisticated equipment and carefully controlled environments. Variations in oxygen content significantly affect the intercalation channels available for magnesium ions, directly impacting battery capacity and cycling stability. Industrial-scale production would need to implement robust quality control measures to ensure consistent stoichiometric ratios.
Environmental considerations must also be addressed in scaling production. Traditional vanadium processing generates substantial waste and consumes significant energy. Developing greener synthesis routes that reduce solvent usage and energy consumption would improve sustainability metrics while potentially lowering production costs. Several research groups have demonstrated promising aqueous synthesis methods that could reduce environmental impact when scaled appropriately.
Equipment compatibility represents an advantage for vanadium oxide cathode manufacturing. Many existing lithium-ion battery production lines could be adapted for vanadium oxide cathode production with moderate modifications. This compatibility could significantly reduce capital expenditure for battery manufacturers looking to diversify into magnesium battery technology, providing a pathway for incremental scaling of production capacity as market demand develops.
Coating uniformity and electrode formulation present technical challenges that require further optimization. The development of specialized binders and conductive additives compatible with magnesium electrolytes remains an active research area. Current formulations often suffer from poor adhesion or chemical incompatibility with magnesium-based systems, limiting electrode durability and performance consistency in mass production scenarios.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!