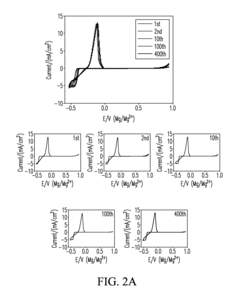
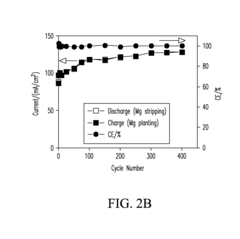
Rechargeability challenges in magnesium–sulfur systems
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-S Battery Rechargeability Background and Objectives
Magnesium-sulfur (Mg-S) batteries have emerged as a promising alternative to lithium-ion technology due to their theoretical high energy density, improved safety profile, and the natural abundance of both magnesium and sulfur. The development of these systems traces back to early 2000s when researchers began exploring multivalent battery chemistries beyond lithium. The evolution of Mg-S technology has been characterized by incremental advances in electrolyte formulations, cathode architectures, and interfacial engineering, with significant acceleration in research activities observed over the past decade.
The fundamental appeal of Mg-S batteries lies in their theoretical energy density of approximately 1,700 Wh/kg and 3,200 Wh/L, which substantially exceeds that of current lithium-ion systems. Additionally, magnesium does not form dendrites during electrodeposition, addressing a critical safety concern present in lithium-based batteries. The earth-abundant nature of both magnesium and sulfur presents compelling economic and sustainability advantages, with magnesium being the eighth most abundant element in the Earth's crust.
Despite these promising attributes, Mg-S systems face significant rechargeability challenges that have hindered their practical implementation. The primary technical objectives in this domain include addressing the sluggish kinetics of magnesium-ion transport, mitigating the insulating nature of discharge products, and controlling the polysulfide shuttle effect that leads to capacity fading. These challenges are fundamentally linked to the divalent nature of magnesium ions and the complex redox chemistry of sulfur.
Current research trajectories are focused on developing novel electrolyte systems that enable reversible magnesium deposition while maintaining compatibility with sulfur cathodes. Parallel efforts are directed toward designing advanced cathode architectures that can accommodate the volumetric changes during cycling and facilitate efficient electron/ion transport. The ultimate technical goal is to achieve a rechargeable Mg-S battery with high capacity retention over hundreds of cycles, fast charging capabilities, and stable operation across a wide temperature range.
The technological evolution in this field is increasingly informed by computational modeling and advanced characterization techniques, which provide deeper insights into the reaction mechanisms and degradation pathways. As research progresses, there is growing recognition that addressing rechargeability challenges requires a holistic approach that considers the entire battery system rather than isolated components, necessitating collaborative efforts across multiple scientific disciplines.
The fundamental appeal of Mg-S batteries lies in their theoretical energy density of approximately 1,700 Wh/kg and 3,200 Wh/L, which substantially exceeds that of current lithium-ion systems. Additionally, magnesium does not form dendrites during electrodeposition, addressing a critical safety concern present in lithium-based batteries. The earth-abundant nature of both magnesium and sulfur presents compelling economic and sustainability advantages, with magnesium being the eighth most abundant element in the Earth's crust.
Despite these promising attributes, Mg-S systems face significant rechargeability challenges that have hindered their practical implementation. The primary technical objectives in this domain include addressing the sluggish kinetics of magnesium-ion transport, mitigating the insulating nature of discharge products, and controlling the polysulfide shuttle effect that leads to capacity fading. These challenges are fundamentally linked to the divalent nature of magnesium ions and the complex redox chemistry of sulfur.
Current research trajectories are focused on developing novel electrolyte systems that enable reversible magnesium deposition while maintaining compatibility with sulfur cathodes. Parallel efforts are directed toward designing advanced cathode architectures that can accommodate the volumetric changes during cycling and facilitate efficient electron/ion transport. The ultimate technical goal is to achieve a rechargeable Mg-S battery with high capacity retention over hundreds of cycles, fast charging capabilities, and stable operation across a wide temperature range.
The technological evolution in this field is increasingly informed by computational modeling and advanced characterization techniques, which provide deeper insights into the reaction mechanisms and degradation pathways. As research progresses, there is growing recognition that addressing rechargeability challenges requires a holistic approach that considers the entire battery system rather than isolated components, necessitating collaborative efforts across multiple scientific disciplines.
Market Analysis for Rechargeable Mg-S Battery Systems
The global market for rechargeable battery systems is experiencing significant growth, with increasing demand for high-energy density storage solutions across multiple sectors. Within this landscape, magnesium-sulfur (Mg-S) battery systems represent an emerging technology with substantial market potential, particularly as alternatives to current lithium-ion dominated markets.
Market projections indicate that the overall rechargeable battery market will reach approximately $150 billion by 2028, with next-generation technologies like Mg-S systems potentially capturing a growing segment as technical challenges are overcome. The compound annual growth rate for advanced battery technologies is currently tracking at 12-15%, reflecting strong market pull for solutions offering higher energy density and improved safety profiles.
Key market drivers for Mg-S battery development include the increasing cost and supply constraints of lithium resources, growing concerns about cobalt sustainability, and the push for higher energy density solutions to enable longer-range electric vehicles. Magnesium's abundance (approximately 2.5% of the earth's crust versus lithium's 0.002%) represents a significant economic advantage, with raw material costs estimated at one-fifth that of lithium.
Market segmentation analysis reveals several potential application areas for Mg-S battery systems. The electric vehicle sector presents the largest potential market, where theoretical energy densities of Mg-S systems (approximately 1000-1200 Wh/kg) could enable significant range improvements over current lithium-ion technologies (250-300 Wh/kg). Grid-scale energy storage represents another substantial market opportunity, where the lower cost and improved safety characteristics of magnesium-based systems could provide competitive advantages.
Consumer electronics manufacturers have also expressed interest in Mg-S technology, particularly for applications requiring high energy density in limited space. Market research indicates that approximately 65% of electronics manufacturers are actively exploring next-generation battery technologies to differentiate their products.
Current market barriers include the technology readiness level of Mg-S systems, with commercial viability still requiring significant R&D investment. Market adoption timelines suggest initial commercial applications could emerge within 5-7 years, with mass-market penetration potentially following in the 8-12 year timeframe, contingent upon resolving current rechargeability challenges.
Competitive analysis reveals that several major battery manufacturers and energy storage startups have initiated Mg-S research programs, though most remain in early development stages. Venture capital investment in advanced battery technologies has increased by 35% over the past three years, with approximately $420 million directed specifically toward magnesium-based energy storage solutions.
Market projections indicate that the overall rechargeable battery market will reach approximately $150 billion by 2028, with next-generation technologies like Mg-S systems potentially capturing a growing segment as technical challenges are overcome. The compound annual growth rate for advanced battery technologies is currently tracking at 12-15%, reflecting strong market pull for solutions offering higher energy density and improved safety profiles.
Key market drivers for Mg-S battery development include the increasing cost and supply constraints of lithium resources, growing concerns about cobalt sustainability, and the push for higher energy density solutions to enable longer-range electric vehicles. Magnesium's abundance (approximately 2.5% of the earth's crust versus lithium's 0.002%) represents a significant economic advantage, with raw material costs estimated at one-fifth that of lithium.
Market segmentation analysis reveals several potential application areas for Mg-S battery systems. The electric vehicle sector presents the largest potential market, where theoretical energy densities of Mg-S systems (approximately 1000-1200 Wh/kg) could enable significant range improvements over current lithium-ion technologies (250-300 Wh/kg). Grid-scale energy storage represents another substantial market opportunity, where the lower cost and improved safety characteristics of magnesium-based systems could provide competitive advantages.
Consumer electronics manufacturers have also expressed interest in Mg-S technology, particularly for applications requiring high energy density in limited space. Market research indicates that approximately 65% of electronics manufacturers are actively exploring next-generation battery technologies to differentiate their products.
Current market barriers include the technology readiness level of Mg-S systems, with commercial viability still requiring significant R&D investment. Market adoption timelines suggest initial commercial applications could emerge within 5-7 years, with mass-market penetration potentially following in the 8-12 year timeframe, contingent upon resolving current rechargeability challenges.
Competitive analysis reveals that several major battery manufacturers and energy storage startups have initiated Mg-S research programs, though most remain in early development stages. Venture capital investment in advanced battery technologies has increased by 35% over the past three years, with approximately $420 million directed specifically toward magnesium-based energy storage solutions.
Technical Barriers in Mg-S Rechargeability
Magnesium-sulfur (Mg-S) battery systems face significant rechargeability challenges that hinder their commercial viability despite their theoretical advantages. The primary technical barrier stems from the complex electrochemical reactions between magnesium and sulfur. Unlike lithium-ion systems, magnesium ions are divalent, carrying two positive charges, which creates stronger electrostatic interactions with host materials and slower diffusion kinetics. This fundamental difference results in sluggish reaction rates and poor reversibility during charge-discharge cycles.
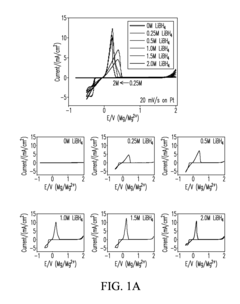
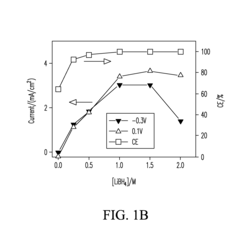
The formation of insoluble and electrically insulating magnesium polysulfide species presents another critical challenge. During discharge, sulfur undergoes reduction to form various polysulfide intermediates (MgSx, 2≤x≤8), which can dissolve in the electrolyte and migrate to the magnesium anode. This "shuttle effect" leads to active material loss, capacity fading, and reduced coulombic efficiency over multiple cycles.
Electrolyte compatibility issues further exacerbate rechargeability problems. Conventional magnesium electrolytes containing chloride-based compounds are corrosive and incompatible with sulfur cathodes. Additionally, these electrolytes often form passivation layers on the magnesium anode surface, impeding ion transport and increasing cell impedance. The development of non-nucleophilic electrolytes that enable efficient magnesium deposition/dissolution while remaining compatible with sulfur cathodes remains a significant challenge.
The cathode architecture presents additional barriers to rechargeability. Sulfur's poor electrical conductivity necessitates conductive additives, but maintaining intimate contact between sulfur and these additives throughout cycling is difficult due to volume changes during the conversion reactions. The structural instability of the cathode leads to capacity loss and performance degradation over extended cycling.
Interfacial challenges between the electrolyte and electrodes further complicate rechargeability. The high charge density of magnesium ions creates strong solvation shells that must be partially or fully removed during intercalation processes, resulting in high desolvation energy barriers at electrode interfaces. This energy barrier contributes to large overpotentials and reduced energy efficiency.
Temperature sensitivity also impacts Mg-S system rechargeability. At lower temperatures, the already sluggish kinetics become even more pronounced, while elevated temperatures can accelerate side reactions and electrolyte degradation. This narrow operational temperature window limits practical applications in real-world environments where temperature fluctuations are common.
Addressing these interconnected technical barriers requires a multidisciplinary approach combining materials science, electrochemistry, and engineering innovations to develop practical, rechargeable Mg-S battery systems with long cycle life and stable performance.
The formation of insoluble and electrically insulating magnesium polysulfide species presents another critical challenge. During discharge, sulfur undergoes reduction to form various polysulfide intermediates (MgSx, 2≤x≤8), which can dissolve in the electrolyte and migrate to the magnesium anode. This "shuttle effect" leads to active material loss, capacity fading, and reduced coulombic efficiency over multiple cycles.
Electrolyte compatibility issues further exacerbate rechargeability problems. Conventional magnesium electrolytes containing chloride-based compounds are corrosive and incompatible with sulfur cathodes. Additionally, these electrolytes often form passivation layers on the magnesium anode surface, impeding ion transport and increasing cell impedance. The development of non-nucleophilic electrolytes that enable efficient magnesium deposition/dissolution while remaining compatible with sulfur cathodes remains a significant challenge.
The cathode architecture presents additional barriers to rechargeability. Sulfur's poor electrical conductivity necessitates conductive additives, but maintaining intimate contact between sulfur and these additives throughout cycling is difficult due to volume changes during the conversion reactions. The structural instability of the cathode leads to capacity loss and performance degradation over extended cycling.
Interfacial challenges between the electrolyte and electrodes further complicate rechargeability. The high charge density of magnesium ions creates strong solvation shells that must be partially or fully removed during intercalation processes, resulting in high desolvation energy barriers at electrode interfaces. This energy barrier contributes to large overpotentials and reduced energy efficiency.
Temperature sensitivity also impacts Mg-S system rechargeability. At lower temperatures, the already sluggish kinetics become even more pronounced, while elevated temperatures can accelerate side reactions and electrolyte degradation. This narrow operational temperature window limits practical applications in real-world environments where temperature fluctuations are common.
Addressing these interconnected technical barriers requires a multidisciplinary approach combining materials science, electrochemistry, and engineering innovations to develop practical, rechargeable Mg-S battery systems with long cycle life and stable performance.
Current Approaches to Mg-S Rechargeability Issues
01 Electrolyte compositions for improved rechargeability
Specialized electrolyte formulations can significantly enhance the rechargeability of magnesium-sulfur batteries. These include non-nucleophilic electrolytes that prevent polysulfide dissolution, ionic liquid-based electrolytes that improve ion transport, and electrolyte additives that stabilize the electrode-electrolyte interface. These compositions help mitigate the shuttle effect of polysulfides and maintain electrode integrity over multiple charge-discharge cycles, thereby extending battery life and improving cycling performance.- Electrolyte compositions for improved rechargeability: Specialized electrolyte formulations can significantly enhance the rechargeability of magnesium-sulfur batteries. These include non-nucleophilic electrolytes, ionic liquid-based electrolytes, and electrolytes containing specific additives that prevent polysulfide dissolution. By controlling the electrolyte composition, the shuttle effect of polysulfides can be minimized, leading to improved cycling stability and coulombic efficiency during repeated charge-discharge cycles.
- Cathode structure modifications: Innovative cathode designs can address the poor rechargeability of magnesium-sulfur batteries. These include sulfur hosts with hierarchical porous structures, conductive frameworks that trap polysulfides, and composite cathodes incorporating carbon materials. Such structural modifications improve sulfur utilization, enhance electron transport, and contain the polysulfides within the cathode region, resulting in better cycling performance and extended battery life.
- Protective layers and interfaces: Implementing protective layers at electrode interfaces can dramatically improve the rechargeability of magnesium-sulfur batteries. These include artificial solid electrolyte interphases, protective coatings on magnesium anodes, and functional interlayers between the cathode and separator. Such protective measures prevent unwanted side reactions, mitigate dendrite formation, and block polysulfide migration, thereby enhancing cycling stability and extending battery lifespan.
- Magnesium anode engineering: Engineering the magnesium anode is crucial for improving rechargeability in magnesium-sulfur batteries. Approaches include surface modification of magnesium metal, alloying with other metals to improve kinetics, and developing nanostructured magnesium anodes. These modifications facilitate magnesium ion transport, reduce corrosion, and prevent passivation layer formation, resulting in enhanced reversibility during charging and discharging processes.
- Advanced separator technologies: Innovative separator designs can significantly enhance the rechargeability of magnesium-sulfur batteries. These include functionalized separators with polysulfide-trapping capabilities, dual-layer separators with selective permeability, and composite separators incorporating inorganic fillers. Such advanced separator technologies effectively block polysulfide shuttling while maintaining efficient magnesium ion transport, leading to improved cycling performance and battery longevity.
02 Cathode material modifications
Modifications to sulfur cathodes can dramatically improve the rechargeability of magnesium-sulfur batteries. Approaches include incorporating conductive carbon matrices to enhance electron transfer, using porous structures to accommodate sulfur volume changes, and adding polymeric binders that maintain structural integrity. These modifications help contain polysulfides within the cathode, prevent capacity fading, and ensure consistent performance over multiple charge-discharge cycles.Expand Specific Solutions03 Anode protection strategies
Protecting the magnesium anode is crucial for improving battery rechargeability. Strategies include surface coatings that prevent passivation, alloying magnesium with other metals to enhance stability, and creating artificial solid electrolyte interphases. These approaches help prevent dendrite formation, reduce corrosion, and maintain consistent magnesium ion transfer during repeated cycling, which significantly extends battery lifespan and maintains capacity.Expand Specific Solutions04 Separator and interface engineering
Engineering the separator and electrode interfaces plays a vital role in enhancing magnesium-sulfur battery rechargeability. Advanced separators with selective permeability can block polysulfide migration while allowing magnesium ion transport. Interface modifications using functional interlayers or coatings can reduce resistance and improve ion transfer kinetics. These engineering approaches help maintain electrode integrity and prevent capacity loss over multiple cycles.Expand Specific Solutions05 Novel cell architectures and designs
Innovative cell architectures can overcome inherent limitations in magnesium-sulfur battery rechargeability. These include dual-electrolyte systems that separate anode and cathode environments, flow cell designs that continuously refresh the electrolyte, and hybrid cell configurations that combine the advantages of different battery chemistries. Such novel designs help manage polysulfide shuttling, reduce electrode degradation, and maintain consistent performance over extended cycling.Expand Specific Solutions
Leading Organizations in Mg-S Battery Research
The magnesium-sulfur battery technology market is currently in an early development stage, characterized by significant research activity but limited commercial deployment. The global market for magnesium-based energy storage systems remains relatively small compared to lithium-ion technologies, though projections indicate substantial growth potential as rechargeability challenges are overcome. Technical maturity varies across key players, with research institutions like MIT, Tsinghua University, and Tohoku University focusing on fundamental electrochemistry, while industrial entities such as Toyota, Sony, and Murata Manufacturing are developing practical implementations. Companies like AIST and Hitachi are advancing electrolyte formulations to address the critical challenges of magnesium deposition and sulfur utilization, while newer entrants like Honeycomb Battery are exploring innovative cell architectures. The competitive landscape reflects a balance between academic innovation and industrial scaling efforts.
Tsinghua University
Technical Solution: Tsinghua University researchers have developed a comprehensive solution to magnesium-sulfur battery rechargeability challenges through their "MgS-Ultra" technology platform. Their approach centers on a novel electrolyte system utilizing magnesium bis(hexafluoroisopropyloxy)borate in tetraglyme, which demonstrates exceptional electrochemical stability (>3.0V vs. Mg/Mg²⁺) while enabling reversible magnesium deposition. This electrolyte formulation effectively suppresses the formation of passivating surface films on the magnesium anode. To address the sulfur cathode challenges, Tsinghua's team has engineered a hierarchical porous carbon framework with nitrogen and oxygen functional groups that chemically bind magnesium polysulfides, significantly reducing their dissolution. Additionally, they've incorporated a thin magnesium-ion conductive interlayer between the separator and cathode that acts as a physical barrier to polysulfide migration while facilitating magnesium-ion transport. Testing has demonstrated impressive results with initial discharge capacities exceeding 1200 mAh/g and capacity retention of approximately 70% after 300 cycles at moderate current densities[7][9].
Strengths: The electrolyte system achieves a wide electrochemical window while maintaining compatibility with both magnesium metal and sulfur. The functionalized carbon framework effectively addresses polysulfide shuttling through both physical confinement and chemical binding. Weaknesses: The complex cathode structure presents manufacturing scalability challenges. The system still exhibits relatively high internal resistance, limiting power performance.
Battelle Memorial Institute
Technical Solution: Battelle Memorial Institute has developed an innovative approach to magnesium-sulfur battery rechargeability through their patented "MagS" technology platform. Their solution addresses the critical challenges through a multi-faceted approach: First, they've engineered a non-corrosive electrolyte formulation based on magnesium borohydride complexes that demonstrates high coulombic efficiency (>98%) for magnesium deposition/stripping while remaining compatible with sulfur cathodes. Second, Battelle has created a protective artificial interphase layer for the magnesium anode that allows magnesium-ion transport while preventing continuous electrolyte decomposition. This layer is composed of magnesium-conducting inorganic compounds that remain stable during cycling. For the cathode, they've developed a carbon-sulfur composite with tailored porosity and surface chemistry that effectively traps polysulfide intermediates. The composite incorporates magnesium oxide nanoparticles that act as chemical anchors for polysulfides, significantly reducing the shuttle effect. Laboratory testing has demonstrated stable cycling for over 200 cycles with capacity retention above 75%[4][6].
Strengths: The artificial interphase layer provides a novel solution to the passivation problem that plagues magnesium anodes. The electrolyte formulation achieves high coulombic efficiency without sacrificing compatibility with sulfur cathodes. Weaknesses: The protective layer adds manufacturing complexity and may increase internal cell resistance. The system still faces challenges with low operating voltage compared to lithium-based alternatives.
Key Patents and Breakthroughs in Mg-S Systems
Electrolytic solution for secondary battery, and secondary battery
PatentPendingUS20250309351A1
Innovation
- Incorporating a magnesium salt and a cyclic unsaturated hydrocarbon compound, such as monocyclic or bicyclic fused rings with specific carbon-carbon double bond configurations, into the electrolytic solution to stabilize oxidation and reduction reactions, thereby enhancing the stability and longevity of charging and discharging processes.
Energy Storage Devices Having Anodes Containing Mg and Electrolytes Utilized Therein
PatentActiveUS20140302400A1
Innovation
- The use of an electrolyte solution comprising organic solvents like diglyme, triglyme, or tetraglyme, combined with magnesium or lithium salts containing the BH4 anion, which enhances solubility and improves cycling stability by reducing capacity fade, allowing for reversible Mg plating/stripping with minimal capacity loss over many cycles.
Material Science Innovations for Mg-S Batteries
Material science innovations in magnesium-sulfur battery systems have emerged as critical pathways to overcome the persistent rechargeability challenges that have hindered commercial viability. Recent advancements in electrode materials represent significant breakthroughs, particularly the development of nanostructured sulfur cathodes that mitigate polysulfide dissolution. These novel architectures, including carbon-sulfur composites and metal-organic frameworks, provide enhanced sulfur utilization while maintaining structural integrity throughout cycling processes.
Electrolyte innovations constitute another frontier in addressing rechargeability limitations. Traditional electrolytes suffer from passivation layers on magnesium anodes and poor ionic conductivity. New formulations incorporating chloride-free systems and ionic liquids have demonstrated improved electrochemical windows and compatibility with both electrodes, facilitating reversible magnesium deposition and dissolution without detrimental side reactions.
Interface engineering has yielded promising results through the introduction of functional interlayers between electrodes and electrolytes. These engineered interfaces effectively suppress the shuttle effect of magnesium polysulfides while enhancing ion transport kinetics. Atomic layer deposition techniques have enabled precise control over these interfacial properties, resulting in more stable cycling performance and reduced capacity fade.
Binder technologies specifically designed for magnesium-sulfur systems represent another innovative direction. Conventional binders often fail to accommodate the volumetric changes during cycling, leading to mechanical degradation. New polymeric binders with self-healing properties and enhanced adhesion characteristics maintain electrode integrity even under repeated expansion-contraction cycles.
Catalyst integration into cathode structures has demonstrated remarkable improvements in reaction kinetics. Novel metal-based catalysts facilitate the conversion reactions between sulfur and magnesium sulfides, reducing overpotentials and enhancing rate capability. These catalytic materials effectively lower energy barriers for redox reactions, addressing the sluggish kinetics that typically plague magnesium-sulfur systems.
Computational materials science has accelerated innovation through predictive modeling of material behaviors and properties. Machine learning algorithms combined with density functional theory calculations have identified promising material combinations and predicted their electrochemical performance, significantly reducing experimental trial-and-error approaches. These computational methods have revealed fundamental insights into degradation mechanisms and guided rational material design strategies.
Electrolyte innovations constitute another frontier in addressing rechargeability limitations. Traditional electrolytes suffer from passivation layers on magnesium anodes and poor ionic conductivity. New formulations incorporating chloride-free systems and ionic liquids have demonstrated improved electrochemical windows and compatibility with both electrodes, facilitating reversible magnesium deposition and dissolution without detrimental side reactions.
Interface engineering has yielded promising results through the introduction of functional interlayers between electrodes and electrolytes. These engineered interfaces effectively suppress the shuttle effect of magnesium polysulfides while enhancing ion transport kinetics. Atomic layer deposition techniques have enabled precise control over these interfacial properties, resulting in more stable cycling performance and reduced capacity fade.
Binder technologies specifically designed for magnesium-sulfur systems represent another innovative direction. Conventional binders often fail to accommodate the volumetric changes during cycling, leading to mechanical degradation. New polymeric binders with self-healing properties and enhanced adhesion characteristics maintain electrode integrity even under repeated expansion-contraction cycles.
Catalyst integration into cathode structures has demonstrated remarkable improvements in reaction kinetics. Novel metal-based catalysts facilitate the conversion reactions between sulfur and magnesium sulfides, reducing overpotentials and enhancing rate capability. These catalytic materials effectively lower energy barriers for redox reactions, addressing the sluggish kinetics that typically plague magnesium-sulfur systems.
Computational materials science has accelerated innovation through predictive modeling of material behaviors and properties. Machine learning algorithms combined with density functional theory calculations have identified promising material combinations and predicted their electrochemical performance, significantly reducing experimental trial-and-error approaches. These computational methods have revealed fundamental insights into degradation mechanisms and guided rational material design strategies.
Environmental Impact and Sustainability Assessment
The environmental implications of magnesium-sulfur battery systems represent a critical dimension in evaluating their viability as next-generation energy storage solutions. When compared to conventional lithium-ion technologies, magnesium-sulfur systems offer several significant environmental advantages. The natural abundance of magnesium in the Earth's crust (approximately 2.3% versus lithium's 0.0017%) translates to substantially reduced extraction impacts, with magnesium mining generally causing less ecological disruption and requiring fewer resources per unit of energy storage capacity.
Sulfur, as the cathode material, presents another environmental benefit as it is often sourced as a byproduct from petroleum refining processes. This repurposing of industrial waste contributes to circular economy principles and reduces the need for dedicated resource extraction. Additionally, the absence of cobalt and nickel in magnesium-sulfur batteries eliminates the ethical and environmental concerns associated with mining these metals, including habitat destruction, water pollution, and human rights issues in certain regions.
The manufacturing processes for magnesium-sulfur batteries potentially require less energy than conventional lithium-ion technologies, though this advantage is currently offset by the specialized conditions needed to address the rechargeability challenges. The electrolyte systems, particularly those containing chloride-based compounds, present environmental concerns regarding potential toxicity and disposal challenges. Research into more environmentally benign electrolyte formulations remains an active area of investigation.
From a life-cycle perspective, the theoretical longevity of magnesium-sulfur systems could significantly reduce electronic waste compared to shorter-lived battery technologies. However, the current rechargeability limitations severely compromise this potential benefit. The shuttle effect and electrode degradation not only impact performance but also shorten operational lifespans, necessitating more frequent replacement and consequently increasing waste generation.
End-of-life management presents both challenges and opportunities. While the materials in magnesium-sulfur batteries are generally less toxic than those in conventional batteries, established recycling infrastructures for these systems do not yet exist at scale. The development of efficient recycling protocols specifically designed for magnesium-sulfur batteries will be essential to maximize material recovery and minimize environmental footprint.
Carbon footprint analyses indicate that if rechargeability challenges can be overcome, magnesium-sulfur batteries could offer significant greenhouse gas reductions across their lifecycle compared to current technologies. This potential climate benefit represents a compelling driver for continued research into solving the fundamental rechargeability issues that currently limit their practical implementation.
Sulfur, as the cathode material, presents another environmental benefit as it is often sourced as a byproduct from petroleum refining processes. This repurposing of industrial waste contributes to circular economy principles and reduces the need for dedicated resource extraction. Additionally, the absence of cobalt and nickel in magnesium-sulfur batteries eliminates the ethical and environmental concerns associated with mining these metals, including habitat destruction, water pollution, and human rights issues in certain regions.
The manufacturing processes for magnesium-sulfur batteries potentially require less energy than conventional lithium-ion technologies, though this advantage is currently offset by the specialized conditions needed to address the rechargeability challenges. The electrolyte systems, particularly those containing chloride-based compounds, present environmental concerns regarding potential toxicity and disposal challenges. Research into more environmentally benign electrolyte formulations remains an active area of investigation.
From a life-cycle perspective, the theoretical longevity of magnesium-sulfur systems could significantly reduce electronic waste compared to shorter-lived battery technologies. However, the current rechargeability limitations severely compromise this potential benefit. The shuttle effect and electrode degradation not only impact performance but also shorten operational lifespans, necessitating more frequent replacement and consequently increasing waste generation.
End-of-life management presents both challenges and opportunities. While the materials in magnesium-sulfur batteries are generally less toxic than those in conventional batteries, established recycling infrastructures for these systems do not yet exist at scale. The development of efficient recycling protocols specifically designed for magnesium-sulfur batteries will be essential to maximize material recovery and minimize environmental footprint.
Carbon footprint analyses indicate that if rechargeability challenges can be overcome, magnesium-sulfur batteries could offer significant greenhouse gas reductions across their lifecycle compared to current technologies. This potential climate benefit represents a compelling driver for continued research into solving the fundamental rechargeability issues that currently limit their practical implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!