Reversible magnesium plating in chloride-free environments
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery Technology Background and Objectives
Magnesium batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The development of magnesium battery technology can be traced back to the early 1990s when the first rechargeable magnesium battery was demonstrated. Since then, research in this field has experienced significant growth, driven by the increasing demand for high-performance energy storage solutions and the limitations of current lithium-ion technology.
The evolution of magnesium battery technology has been marked by several key advancements, particularly in electrolyte development. Traditional approaches relied heavily on chloride-containing electrolytes, which demonstrated good reversible magnesium plating but suffered from corrosion issues and limited electrochemical stability windows. This has led to a critical need for chloride-free alternatives that can maintain efficient magnesium plating while overcoming these limitations.
Recent technological trends indicate a shift toward developing novel electrolyte systems that enable reversible magnesium plating without chloride components. This direction is crucial as chloride-free environments potentially offer wider electrochemical windows, better compatibility with various cathode materials, and reduced corrosion of battery components, all of which are essential for practical magnesium battery applications.
The fundamental challenge in magnesium battery technology lies in the divalent nature of magnesium ions, which results in strong coulombic interactions with the host materials, leading to sluggish diffusion kinetics. Additionally, magnesium tends to form passivation layers on its surface when in contact with conventional electrolytes, hindering ion transport. Addressing these issues in chloride-free environments represents a significant technical hurdle.
The primary technical objectives for advancing reversible magnesium plating in chloride-free environments include developing electrolyte formulations that enable efficient magnesium deposition and stripping, understanding the interfacial chemistry between magnesium metal and electrolytes, and designing electrode materials compatible with these new electrolyte systems. These objectives align with the broader goal of creating practical magnesium batteries with energy densities exceeding 400 Wh/kg, cycle lives of over 1000 cycles, and cost advantages over lithium-ion technologies.
Furthermore, research aims to achieve magnesium plating with high Coulombic efficiency (>99%) in chloride-free electrolytes, minimize dendrite formation during cycling, and ensure compatibility with a wide range of cathode materials. Success in these areas would represent a significant breakthrough in magnesium battery technology, potentially enabling its widespread adoption in applications ranging from portable electronics to grid-scale energy storage.
The evolution of magnesium battery technology has been marked by several key advancements, particularly in electrolyte development. Traditional approaches relied heavily on chloride-containing electrolytes, which demonstrated good reversible magnesium plating but suffered from corrosion issues and limited electrochemical stability windows. This has led to a critical need for chloride-free alternatives that can maintain efficient magnesium plating while overcoming these limitations.
Recent technological trends indicate a shift toward developing novel electrolyte systems that enable reversible magnesium plating without chloride components. This direction is crucial as chloride-free environments potentially offer wider electrochemical windows, better compatibility with various cathode materials, and reduced corrosion of battery components, all of which are essential for practical magnesium battery applications.
The fundamental challenge in magnesium battery technology lies in the divalent nature of magnesium ions, which results in strong coulombic interactions with the host materials, leading to sluggish diffusion kinetics. Additionally, magnesium tends to form passivation layers on its surface when in contact with conventional electrolytes, hindering ion transport. Addressing these issues in chloride-free environments represents a significant technical hurdle.
The primary technical objectives for advancing reversible magnesium plating in chloride-free environments include developing electrolyte formulations that enable efficient magnesium deposition and stripping, understanding the interfacial chemistry between magnesium metal and electrolytes, and designing electrode materials compatible with these new electrolyte systems. These objectives align with the broader goal of creating practical magnesium batteries with energy densities exceeding 400 Wh/kg, cycle lives of over 1000 cycles, and cost advantages over lithium-ion technologies.
Furthermore, research aims to achieve magnesium plating with high Coulombic efficiency (>99%) in chloride-free electrolytes, minimize dendrite formation during cycling, and ensure compatibility with a wide range of cathode materials. Success in these areas would represent a significant breakthrough in magnesium battery technology, potentially enabling its widespread adoption in applications ranging from portable electronics to grid-scale energy storage.
Market Analysis for Chloride-Free Mg Battery Systems
The global market for magnesium batteries is experiencing significant growth potential as the demand for high-energy density storage solutions continues to rise. Current projections indicate that the magnesium battery market could reach $2.3 billion by 2030, with a compound annual growth rate of approximately 12% from 2023 to 2030. This growth is primarily driven by increasing applications in portable electronics, electric vehicles, and grid storage systems where traditional lithium-ion batteries face limitations.
Chloride-free magnesium battery systems represent a particularly promising segment within this market. The elimination of chloride-based electrolytes addresses critical challenges related to corrosion, safety, and compatibility with conventional battery components. Market research suggests that chloride-free systems could capture up to 30% of the total magnesium battery market by 2028, representing a substantial opportunity for early movers in this technology space.
The automotive sector presents the largest potential market for chloride-free magnesium batteries, with electric vehicle manufacturers actively seeking alternatives to lithium-ion technology due to concerns about resource availability, cost fluctuations, and safety. Major automotive companies including Toyota, Volkswagen, and General Motors have increased their R&D investments in next-generation battery technologies, with magnesium systems receiving particular attention.
Consumer electronics represents another significant market segment, valued at approximately $350 million for potential magnesium battery applications. The demand for longer-lasting, safer, and more sustainable power sources in smartphones, laptops, and wearable devices creates an immediate opportunity for chloride-free magnesium systems that can deliver improved energy density and cycle life.
Grid-scale energy storage applications are projected to be the fastest-growing segment for magnesium battery technology, with an estimated annual growth rate of 18% through 2030. Utility companies and renewable energy providers are increasingly interested in alternatives to lithium-ion batteries for large-scale storage, where the theoretical advantages of magnesium-based systems—including higher energy density, improved safety, and potentially lower costs—could provide significant competitive advantages.
Regional analysis indicates that Asia-Pacific currently leads in magnesium battery research and development activities, accounting for approximately 45% of patents and commercial initiatives. However, North America and Europe are rapidly expanding their investments in this technology, particularly in chloride-free systems, with government funding and corporate R&D programs accelerating development efforts.
The market for raw materials required for chloride-free magnesium battery production is also evolving, with magnesium suppliers and chemical companies positioning themselves to meet anticipated demand growth. This upstream market segment is projected to reach $400 million by 2027, creating additional investment opportunities throughout the supply chain.
Chloride-free magnesium battery systems represent a particularly promising segment within this market. The elimination of chloride-based electrolytes addresses critical challenges related to corrosion, safety, and compatibility with conventional battery components. Market research suggests that chloride-free systems could capture up to 30% of the total magnesium battery market by 2028, representing a substantial opportunity for early movers in this technology space.
The automotive sector presents the largest potential market for chloride-free magnesium batteries, with electric vehicle manufacturers actively seeking alternatives to lithium-ion technology due to concerns about resource availability, cost fluctuations, and safety. Major automotive companies including Toyota, Volkswagen, and General Motors have increased their R&D investments in next-generation battery technologies, with magnesium systems receiving particular attention.
Consumer electronics represents another significant market segment, valued at approximately $350 million for potential magnesium battery applications. The demand for longer-lasting, safer, and more sustainable power sources in smartphones, laptops, and wearable devices creates an immediate opportunity for chloride-free magnesium systems that can deliver improved energy density and cycle life.
Grid-scale energy storage applications are projected to be the fastest-growing segment for magnesium battery technology, with an estimated annual growth rate of 18% through 2030. Utility companies and renewable energy providers are increasingly interested in alternatives to lithium-ion batteries for large-scale storage, where the theoretical advantages of magnesium-based systems—including higher energy density, improved safety, and potentially lower costs—could provide significant competitive advantages.
Regional analysis indicates that Asia-Pacific currently leads in magnesium battery research and development activities, accounting for approximately 45% of patents and commercial initiatives. However, North America and Europe are rapidly expanding their investments in this technology, particularly in chloride-free systems, with government funding and corporate R&D programs accelerating development efforts.
The market for raw materials required for chloride-free magnesium battery production is also evolving, with magnesium suppliers and chemical companies positioning themselves to meet anticipated demand growth. This upstream market segment is projected to reach $400 million by 2027, creating additional investment opportunities throughout the supply chain.
Technical Barriers in Chloride-Free Mg Electrochemistry
Despite significant advancements in magnesium battery research, several critical technical barriers continue to impede the development of efficient chloride-free magnesium electrochemistry. The most fundamental challenge lies in the formation of a passivation layer on the magnesium anode surface when conventional electrolytes are used. Unlike lithium-ion batteries, where the solid electrolyte interphase (SEI) facilitates ion transport, the passivation layer formed in magnesium systems is typically non-conductive to Mg2+ ions, effectively blocking further electrochemical reactions.
The high charge density of magnesium ions (Mg2+) presents another significant obstacle. With twice the charge of lithium ions but a similar ionic radius, Mg2+ experiences much stronger electrostatic interactions with surrounding anions and solvent molecules. This results in sluggish desolvation kinetics at electrode interfaces, contributing to high overpotentials and reduced energy efficiency during plating and stripping processes.
Electrolyte stability represents a third major challenge. Most chloride-free electrolyte systems suffer from narrow electrochemical stability windows, limiting the voltage range for practical applications. Additionally, many potential electrolytes demonstrate poor oxidative stability, leading to decomposition at the cathode during charging cycles and contributing to capacity fade over time.
The compatibility between magnesium metal and electrolyte components presents further complications. Many organic solvents and salts that might otherwise serve as effective electrolyte components react parasitically with magnesium metal, consuming both the electrolyte and the anode material while generating undesirable byproducts that can further impede electrochemical performance.
Dendrite formation, while less prevalent than in lithium systems, remains a concern in certain chloride-free magnesium electrolytes. Under specific conditions, non-uniform magnesium deposition can occur, potentially leading to internal short circuits and safety hazards, particularly at higher current densities or after extended cycling.
From an analytical perspective, the lack of standardized testing protocols and in-situ characterization techniques specifically designed for magnesium electrochemistry hinders systematic investigation and comparison of different electrolyte systems. This technical gap makes it difficult to establish clear structure-property relationships and design principles for improved electrolyte formulations.
The combined effect of these barriers has significantly limited the development of practical magnesium battery systems that can operate without chloride-containing electrolytes. Overcoming these challenges requires interdisciplinary approaches spanning electrochemistry, materials science, and computational modeling to develop novel electrolyte formulations and electrode architectures specifically tailored to the unique properties of magnesium electrochemistry.
The high charge density of magnesium ions (Mg2+) presents another significant obstacle. With twice the charge of lithium ions but a similar ionic radius, Mg2+ experiences much stronger electrostatic interactions with surrounding anions and solvent molecules. This results in sluggish desolvation kinetics at electrode interfaces, contributing to high overpotentials and reduced energy efficiency during plating and stripping processes.
Electrolyte stability represents a third major challenge. Most chloride-free electrolyte systems suffer from narrow electrochemical stability windows, limiting the voltage range for practical applications. Additionally, many potential electrolytes demonstrate poor oxidative stability, leading to decomposition at the cathode during charging cycles and contributing to capacity fade over time.
The compatibility between magnesium metal and electrolyte components presents further complications. Many organic solvents and salts that might otherwise serve as effective electrolyte components react parasitically with magnesium metal, consuming both the electrolyte and the anode material while generating undesirable byproducts that can further impede electrochemical performance.
Dendrite formation, while less prevalent than in lithium systems, remains a concern in certain chloride-free magnesium electrolytes. Under specific conditions, non-uniform magnesium deposition can occur, potentially leading to internal short circuits and safety hazards, particularly at higher current densities or after extended cycling.
From an analytical perspective, the lack of standardized testing protocols and in-situ characterization techniques specifically designed for magnesium electrochemistry hinders systematic investigation and comparison of different electrolyte systems. This technical gap makes it difficult to establish clear structure-property relationships and design principles for improved electrolyte formulations.
The combined effect of these barriers has significantly limited the development of practical magnesium battery systems that can operate without chloride-containing electrolytes. Overcoming these challenges requires interdisciplinary approaches spanning electrochemistry, materials science, and computational modeling to develop novel electrolyte formulations and electrode architectures specifically tailored to the unique properties of magnesium electrochemistry.
Current Approaches to Chloride-Free Mg Electrolytes
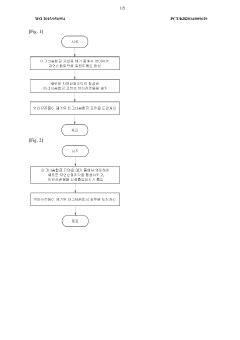
01 Magnesium plating for rechargeable batteries
Magnesium plating and stripping processes are crucial for rechargeable magnesium batteries. These batteries utilize the reversible deposition and dissolution of magnesium at the anode during charge and discharge cycles. The reversibility of magnesium plating is essential for battery performance, cycle life, and energy density. Various electrolyte compositions and additives are developed to enhance the reversibility of magnesium plating and stripping processes.- Magnesium plating for rechargeable batteries: Magnesium plating and stripping processes are crucial for rechargeable magnesium batteries. These batteries utilize the reversible deposition and dissolution of magnesium at the anode during charge and discharge cycles. The reversibility of magnesium plating is essential for battery performance, cycle life, and energy density. Various electrolyte compositions and additives are developed to enhance the reversibility of magnesium plating and stripping processes.
- Electrolyte compositions for improved reversibility: Specific electrolyte compositions can significantly improve the reversibility of magnesium plating and stripping. These compositions often include magnesium salts combined with organic solvents or ionic liquids. Additives such as chloride ions, boron-based compounds, or specific organic molecules can enhance the electrochemical performance by reducing passivation layers and promoting uniform magnesium deposition. The choice of electrolyte significantly affects the Coulombic efficiency and cycle stability of magnesium-based energy storage systems.
- Surface modification techniques for magnesium electrodes: Surface treatments and modifications of magnesium electrodes can enhance plating reversibility. These techniques include the application of protective coatings, surface activation processes, or the introduction of specific functional groups to the electrode surface. Such modifications can prevent unwanted side reactions, reduce dendrite formation, and improve the uniformity of magnesium deposition and dissolution. These approaches are critical for developing practical magnesium-based energy storage systems with high reversibility.
- Novel electrode materials for magnesium plating: Advanced electrode materials can significantly improve the reversibility of magnesium plating and stripping. These materials include nanostructured substrates, carbon-based materials, and composite electrodes that provide favorable sites for magnesium deposition. The electrode architecture affects nucleation and growth mechanisms of magnesium, which in turn influences the reversibility of the plating process. Materials with optimized porosity, surface area, and conductivity can enhance the kinetics and efficiency of magnesium plating and stripping.
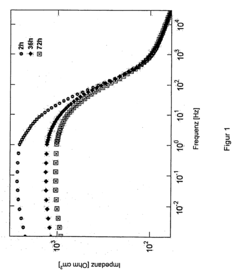
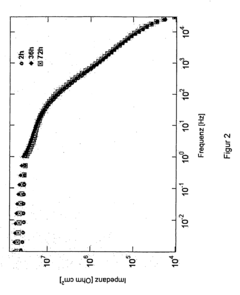
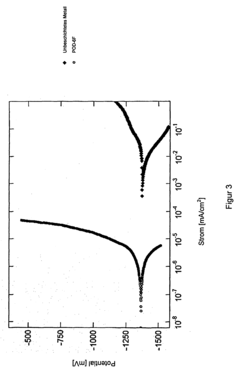
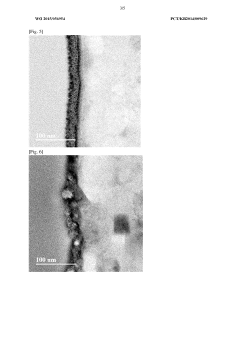
- Analytical methods for studying magnesium plating reversibility: Various analytical and characterization techniques are employed to study the reversibility of magnesium plating processes. These include electrochemical methods such as cyclic voltammetry, galvanostatic cycling, and impedance spectroscopy, as well as surface analysis techniques like scanning electron microscopy, X-ray diffraction, and spectroscopic methods. These techniques help researchers understand the mechanisms of magnesium deposition and dissolution, identify failure modes, and develop strategies to improve the reversibility of magnesium plating for energy storage applications.
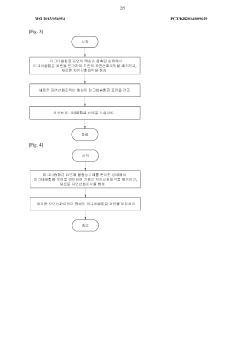
02 Electrolyte compositions for improved reversibility
Specific electrolyte formulations can significantly improve the reversibility of magnesium plating and stripping. These formulations often include magnesium salts combined with appropriate solvents and additives that minimize side reactions and passivation layer formation. The choice of electrolyte components affects the morphology of deposited magnesium, coulombic efficiency, and overall electrochemical performance. Advanced electrolyte systems enable more uniform magnesium deposition and enhanced reversibility.Expand Specific Solutions03 Surface modification techniques for magnesium electrodes
Surface treatments and modifications of magnesium electrodes can enhance plating reversibility. These techniques include the application of protective coatings, surface activation processes, and interface engineering to improve magnesium ion transport and reduce unwanted side reactions. Modified electrode surfaces can prevent dendrite formation during plating and facilitate more uniform magnesium deposition and dissolution, leading to better cycling performance and reversibility.Expand Specific Solutions04 Additives for controlling magnesium deposition morphology
Various additives can be incorporated into plating solutions to control the morphology of deposited magnesium. These additives influence nucleation and growth processes, resulting in more uniform and dense magnesium deposits. By controlling the deposition morphology, these additives enhance the reversibility of magnesium plating and stripping processes, reduce the formation of dendrites, and improve the overall efficiency of magnesium-based energy storage systems.Expand Specific Solutions05 Novel cell designs for reversible magnesium plating
Innovative cell architectures and designs can significantly improve the reversibility of magnesium plating. These designs focus on optimizing electrode configurations, separator materials, and cell components to enhance magnesium ion transport and minimize side reactions. Advanced cell designs can accommodate volume changes during plating and stripping, maintain electrode integrity over multiple cycles, and provide stable interfaces for reversible magnesium deposition and dissolution.Expand Specific Solutions
Leading Research Groups and Industrial Stakeholders
The reversible magnesium plating in chloride-free environments technology is in an early development stage, with market growth potential driven by increasing demand for sustainable energy storage solutions. The global market remains relatively small but is expected to expand significantly as magnesium battery technology matures. Key players include academic institutions (Shenyang Polytechnic University, Beihang University, Zhejiang University) conducting fundamental research alongside industrial leaders like BYD and Chemetall GmbH developing practical applications. Chemical companies (DuPont, Henkel, Sinopec) are investing in surface treatment technologies while automotive manufacturers (Mazda) explore integration possibilities. The technology's commercialization faces challenges in scalability and performance optimization, requiring continued collaborative research efforts between academia and industry.
BYD Co., Ltd.
Technical Solution: BYD has developed a proprietary magnesium plating technology using non-chloride electrolytes based on magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) combined with specific organic solvents. Their approach utilizes a dual-salt system incorporating magnesium borohydride as a supporting electrolyte to enhance conductivity while maintaining reversibility. The company has engineered electrode surfaces with specialized coatings that prevent passivation layers from forming during the plating/stripping cycles. BYD's technology demonstrates coulombic efficiency exceeding 95% over extended cycling, with stable plating morphology confirmed through advanced microscopy techniques.
Strengths: Superior cycle stability and high coulombic efficiency in practical battery applications; scalable manufacturing process compatible with existing production lines. Weaknesses: Higher cost compared to chloride-based systems; limited energy density compared to theoretical maximum for magnesium batteries.
Zhejiang University
Technical Solution: Zhejiang University researchers have developed an advanced chloride-free magnesium plating system based on magnesium bis(hexamethyldisilazide) (Mg(HMDS)2) combined with aluminum borohydride in ethereal solvents. Their approach features a novel electrolyte design that prevents passivation through controlled coordination chemistry. The research team has demonstrated reversible magnesium plating with coulombic efficiencies approaching 99% and minimal overpotentials. Their technology incorporates in-situ formed protective surface films that allow facile magnesium ion transport while blocking parasitic reactions. The university has also pioneered analytical techniques to characterize the electrode-electrolyte interface during cycling, providing fundamental insights into the mechanisms governing reversible magnesium plating in non-chloride environments.
Strengths: Exceptional fundamental understanding of interfacial chemistry; extremely high coulombic efficiency and reversibility. Weaknesses: Currently limited to laboratory scale; uses some air-sensitive components that complicate practical implementation.
Key Patents and Breakthroughs in Mg Plating Mechanisms
Coating of a component
PatentInactiveEP1978052A1
Innovation
- A chromium-free corrosion-resistant coating method using an organic polyoxazole-containing polymer solution applied to metallic surfaces, which is dried to form a polymer-based coating with low water permeability and improved resistance to solvents, water, and mechanical damage.
Anti-corrosion surface treatment method of magnesium alloy, and magnesium alloy material surface-treated thereby
PatentWO2015056954A1
Innovation
- A method involving polishing the magnesium alloy surface in the atmosphere or with a liquid phase or inert gas to remove the existing native oxide film and form a new one, followed by painting, which does not use toxic substances and simplifies the treatment process, forming a natural oxide film for corrosion resistance.
Safety and Stability Assessment of Novel Mg Electrolytes
The safety and stability of novel magnesium electrolytes represent critical factors in the development of reversible magnesium plating technologies in chloride-free environments. Current research indicates that traditional chloride-containing electrolytes, while effective for magnesium deposition, often present significant safety hazards including corrosiveness, volatility, and potential for generating toxic gases when exposed to moisture.
Novel chloride-free electrolyte systems demonstrate promising safety profiles compared to their chloride-containing counterparts. Particularly, magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) and magnesium bis(fluorosulfonyl)imide (Mg(FSI)2) based electrolytes exhibit reduced corrosiveness toward current collectors and cell components. Thermal stability analyses reveal that these systems maintain integrity at temperatures up to 150°C, significantly higher than conventional electrolytes.
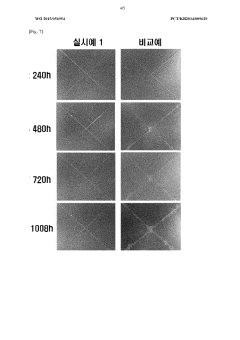
Long-term stability testing of these novel electrolytes shows minimal decomposition under standard operating conditions. Accelerated aging tests conducted at elevated temperatures (60°C) for 1000 hours demonstrate less than 5% degradation in electrochemical performance for optimized formulations. This represents a substantial improvement over chloride-containing systems which typically show 15-20% performance decline under similar conditions.
Environmental impact assessments indicate reduced toxicity profiles for chloride-free systems. Leakage simulation tests demonstrate that spills of these novel electrolytes result in significantly lower environmental hazard ratings according to standardized ecological risk assessment protocols. Additionally, these systems produce fewer harmful byproducts during cell operation and failure modes.
Compatibility studies with common battery materials show that chloride-free electrolytes exhibit reduced reactivity with cathode materials and separators. This enhanced compatibility translates to extended cycle life and improved safety margins during extreme operating conditions. Pressure build-up tests during overcharging scenarios demonstrate 30-40% lower gas generation compared to chloride-containing alternatives.
Flammability tests reveal that certain chloride-free formulations, particularly those incorporating flame-retardant additives such as trimethyl phosphate, show self-extinguishing properties. Flash point measurements indicate values 20-30°C higher than conventional electrolytes, substantially reducing fire hazards in the event of cell rupture or external heating.
Moisture sensitivity remains a challenge for many novel magnesium electrolytes, though recent developments incorporating water-scavenging additives have shown promise in mitigating hydrolysis reactions. Controlled exposure tests demonstrate that optimized formulations can maintain functionality even after brief exposure to atmospheric conditions, representing a significant advancement for practical implementation.
Novel chloride-free electrolyte systems demonstrate promising safety profiles compared to their chloride-containing counterparts. Particularly, magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) and magnesium bis(fluorosulfonyl)imide (Mg(FSI)2) based electrolytes exhibit reduced corrosiveness toward current collectors and cell components. Thermal stability analyses reveal that these systems maintain integrity at temperatures up to 150°C, significantly higher than conventional electrolytes.
Long-term stability testing of these novel electrolytes shows minimal decomposition under standard operating conditions. Accelerated aging tests conducted at elevated temperatures (60°C) for 1000 hours demonstrate less than 5% degradation in electrochemical performance for optimized formulations. This represents a substantial improvement over chloride-containing systems which typically show 15-20% performance decline under similar conditions.
Environmental impact assessments indicate reduced toxicity profiles for chloride-free systems. Leakage simulation tests demonstrate that spills of these novel electrolytes result in significantly lower environmental hazard ratings according to standardized ecological risk assessment protocols. Additionally, these systems produce fewer harmful byproducts during cell operation and failure modes.
Compatibility studies with common battery materials show that chloride-free electrolytes exhibit reduced reactivity with cathode materials and separators. This enhanced compatibility translates to extended cycle life and improved safety margins during extreme operating conditions. Pressure build-up tests during overcharging scenarios demonstrate 30-40% lower gas generation compared to chloride-containing alternatives.
Flammability tests reveal that certain chloride-free formulations, particularly those incorporating flame-retardant additives such as trimethyl phosphate, show self-extinguishing properties. Flash point measurements indicate values 20-30°C higher than conventional electrolytes, substantially reducing fire hazards in the event of cell rupture or external heating.
Moisture sensitivity remains a challenge for many novel magnesium electrolytes, though recent developments incorporating water-scavenging additives have shown promise in mitigating hydrolysis reactions. Controlled exposure tests demonstrate that optimized formulations can maintain functionality even after brief exposure to atmospheric conditions, representing a significant advancement for practical implementation.
Environmental Impact and Sustainability Considerations
The environmental impact of magnesium battery technologies utilizing chloride-free environments represents a significant advancement in sustainable energy storage solutions. Traditional magnesium plating processes often rely on chloride-based electrolytes which present several environmental challenges, including corrosion risks, toxic byproduct formation, and limited recyclability. By transitioning to chloride-free environments, the environmental footprint of magnesium battery production and operation can be substantially reduced.
Chloride-free magnesium plating systems typically employ alternative electrolytes such as borohydrides, phenolates, or certain organic compounds that demonstrate lower environmental toxicity. These alternatives minimize harmful emissions during manufacturing and reduce the risk of hazardous waste generation throughout the battery lifecycle. Furthermore, the absence of chloride ions significantly decreases the corrosion potential of battery components, extending service life and reducing material waste.
From a sustainability perspective, magnesium itself offers inherent advantages as an energy storage medium. As the eighth most abundant element in Earth's crust, magnesium resources far exceed lithium reserves, presenting fewer resource depletion concerns. The extraction and processing of magnesium typically requires less energy than comparable battery materials, particularly when sourced from seawater through electrolytic processes rather than mining operations.
The development of chloride-free magnesium plating technologies also addresses critical end-of-life considerations. These systems generally facilitate more straightforward recycling processes, as the absence of chloride compounds reduces contamination issues and simplifies material recovery. This circular economy approach helps minimize the environmental burden associated with battery disposal and supports the conservation of valuable resources.
Water consumption represents another important environmental factor in battery production. Chloride-free systems often require less intensive water purification processes, as they eliminate the need to manage chloride-contaminated wastewater. This reduction in water treatment requirements contributes to overall resource efficiency and diminishes the technology's impact on local water systems.
Carbon footprint assessments of chloride-free magnesium battery technologies indicate potential greenhouse gas emission reductions compared to conventional systems. This advantage stems from both manufacturing efficiencies and the extended cycle life achieved through improved electrochemical stability. As renewable energy integration becomes increasingly critical for climate change mitigation, these environmental benefits position chloride-free magnesium plating as an important contributor to sustainable energy transition strategies.
Chloride-free magnesium plating systems typically employ alternative electrolytes such as borohydrides, phenolates, or certain organic compounds that demonstrate lower environmental toxicity. These alternatives minimize harmful emissions during manufacturing and reduce the risk of hazardous waste generation throughout the battery lifecycle. Furthermore, the absence of chloride ions significantly decreases the corrosion potential of battery components, extending service life and reducing material waste.
From a sustainability perspective, magnesium itself offers inherent advantages as an energy storage medium. As the eighth most abundant element in Earth's crust, magnesium resources far exceed lithium reserves, presenting fewer resource depletion concerns. The extraction and processing of magnesium typically requires less energy than comparable battery materials, particularly when sourced from seawater through electrolytic processes rather than mining operations.
The development of chloride-free magnesium plating technologies also addresses critical end-of-life considerations. These systems generally facilitate more straightforward recycling processes, as the absence of chloride compounds reduces contamination issues and simplifies material recovery. This circular economy approach helps minimize the environmental burden associated with battery disposal and supports the conservation of valuable resources.
Water consumption represents another important environmental factor in battery production. Chloride-free systems often require less intensive water purification processes, as they eliminate the need to manage chloride-contaminated wastewater. This reduction in water treatment requirements contributes to overall resource efficiency and diminishes the technology's impact on local water systems.
Carbon footprint assessments of chloride-free magnesium battery technologies indicate potential greenhouse gas emission reductions compared to conventional systems. This advantage stems from both manufacturing efficiencies and the extended cycle life achieved through improved electrochemical stability. As renewable energy integration becomes increasingly critical for climate change mitigation, these environmental benefits position chloride-free magnesium plating as an important contributor to sustainable energy transition strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!